Abstract
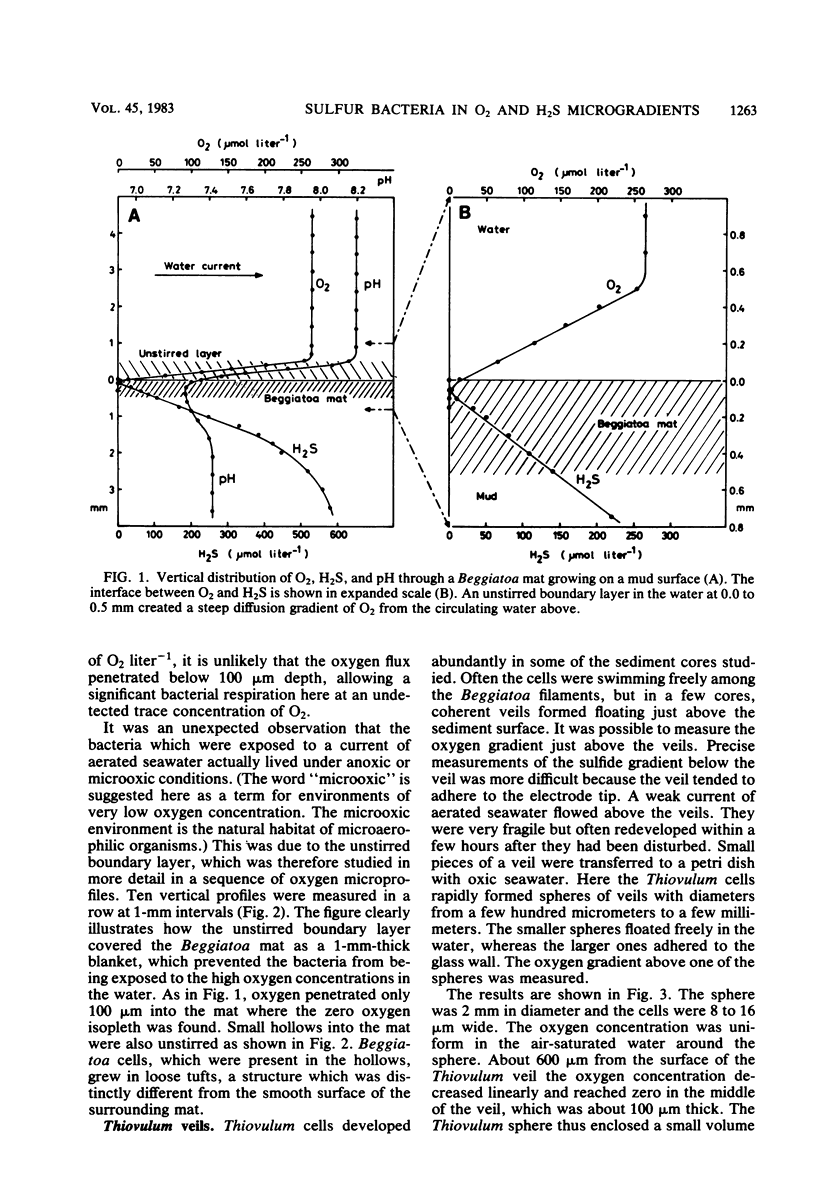
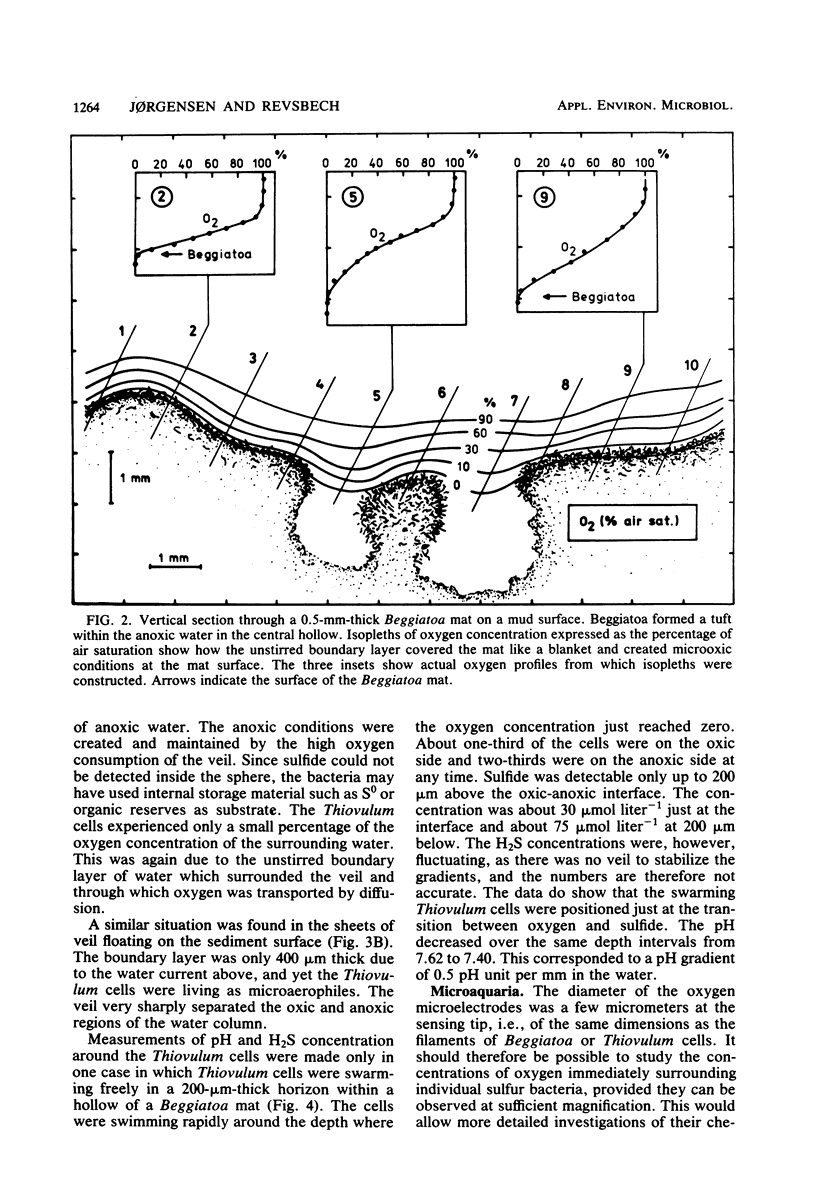
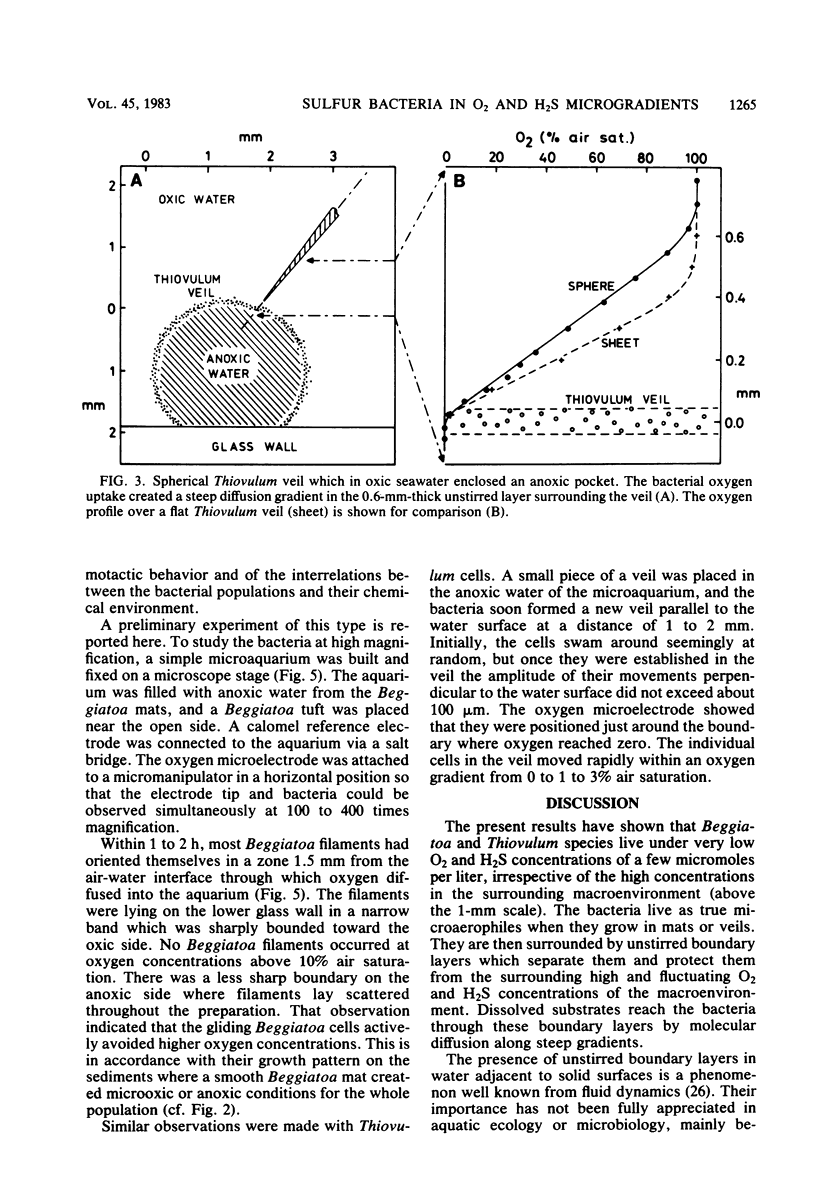
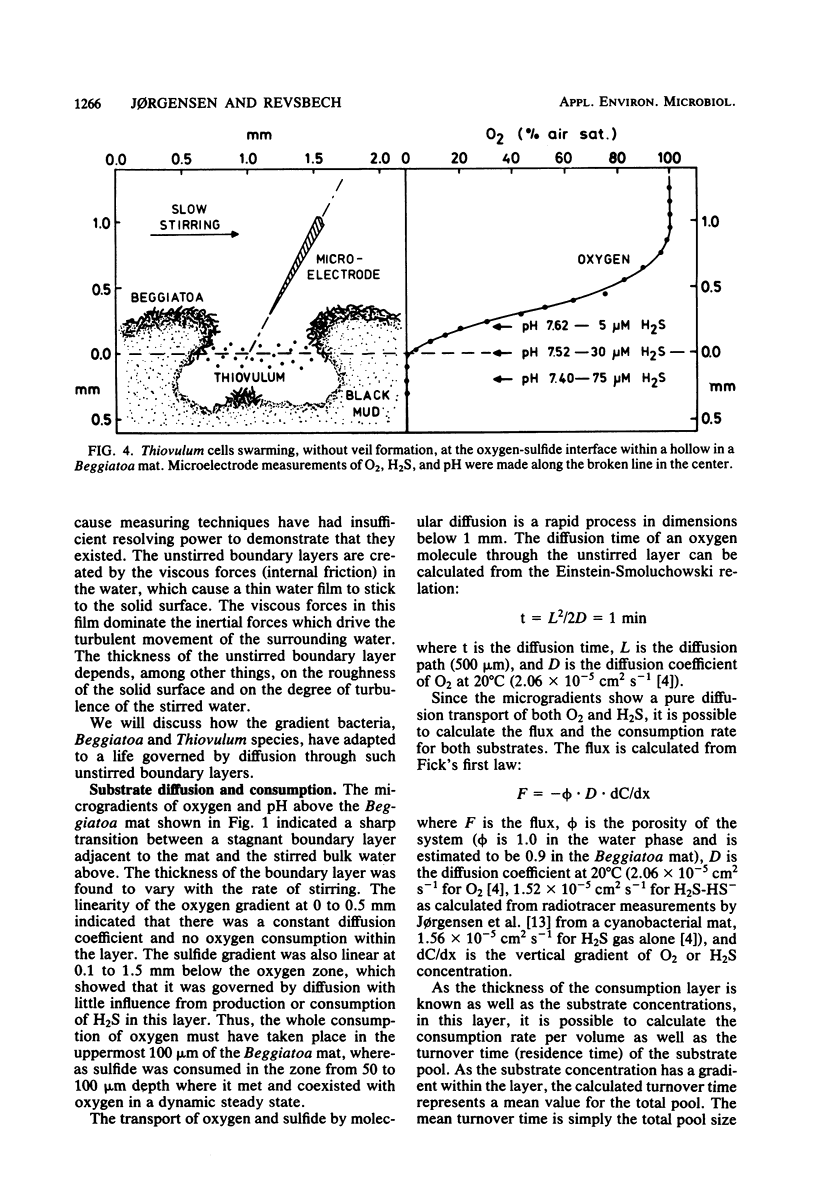
The interactions between colorless sulfur bacteria and the chemical microgradients at the oxygen-sulfide interface were studied in Beggiatoa mats from marine sediments and in Thiovulum veils developing above the sediments. The gradients of O2, H2S, and pH were measured by microelectrodes at depth increments of 50 μm. An unstirred boundary layer in the water surrounding the mats and veils prevented microturbulent or convective mixing of O2 and H2S. The two substrates reached the bacteria only by molecular diffusion through the boundary layer. The bacteria lived as microaerophiles or anaerobes even under stirred, oxic water. Oxygen and sulfide zones overlapped by 50 μm in the bacterial layers. Both compounds had concentrations in the range of 0 to 10 μmol liter−1 and residence times of 0.1 to 0.6 s in the overlapping zone. The sulfide oxidation was purely biological. Diffusion calculations showed that formation of mats on solid substrates or of veils in the water represented optimal strategies for the bacteria to achieve a stable microenvironment, a high substrate supply, and an efficient competition with chemical sulfide oxidation. The continuous gliding movement of Beggiatoa cells in mats or the flickering motion of Thiovulum cells in veils were important for the availability of both O2 and H2S for the individual bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON S. D., MORITA R. Y. EFFECT OF CATALASE AND CULTURAL CONDITIONS ON GROWTH OF BEGGIATOA. J Bacteriol. 1964 Dec;88:1755–1761. doi: 10.1128/jb.88.6.1755-1761.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güde H., Strohl W. R., Larkin J. M. Mixotrophic and heterotrophic growth of Beggiatoa alba in continuous culture. Arch Microbiol. 1981 Jul;129(5):357–360. doi: 10.1007/BF00406462. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B., Revsbech N. P., Blackburn T. H., Cohen Y. Diurnal cycle of oxygen and sulfide microgradients and microbial photosynthesis in a cyanobacterial mat sediment. Appl Environ Microbiol. 1979 Jul;38(1):46–58. doi: 10.1128/aem.38.1.46-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Kuenen J. G., Beudeker R. F. Microbiology of thiobacilli and other sulphur-oxidizing autotrophs, mixotrophs and heterotrophs. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):473–497. doi: 10.1098/rstb.1982.0093. [DOI] [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Organic nutrition of Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):236–247. doi: 10.1128/jb.147.1.236-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Use of reduced sulfur compounds by Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):140–154. doi: 10.1128/jb.147.1.140-154.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim E. G. Die Mixotrophie von Beggiatoa. Arch Mikrobiol. 1967;59(1):247–254. [PubMed] [Google Scholar]
- Strohl W. R., Cannon G. C., Shively J. M., Güde H., Hook L. A., Lane C. M., Larkin J. M. Heterotrophic carbon metabolism by Beggiatoa alba. J Bacteriol. 1981 Nov;148(2):572–583. doi: 10.1128/jb.148.2.572-583.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. R., Larkin J. M. Enumeration, isolation, and characterization of beggiatoa from freshwater sediments. Appl Environ Microbiol. 1978 Nov;36(5):755–770. doi: 10.1128/aem.36.5.755-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsen C. O., Jannasch H. W. Physiological and morphological observations on Thiovulum sp. J Bacteriol. 1978 Nov;136(2):765–774. doi: 10.1128/jb.136.2.765-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de BOER W., LA RIVIERE J. W., HOUWINK A. L. Observations on the morphology of Thiovulum majus Hinze. Antonie Van Leeuwenhoek. 1961;27:447–456. doi: 10.1007/BF02538470. [DOI] [PubMed] [Google Scholar]


