Abstract
Cell–cell recognition and patterning of cell contacts have a critical role in mediating reversible assembly of a variety of transcellular complexes in the nervous system. This study provides evidence for regulation of cell interactions through modulation of ankyrin binding to neurofascin, a member of the L1CAM family of nervous system cell adhesion molecules. The phosphorylation state of the conserved FIGQY tyrosine in the cytoplasmic domain of neurofascin regulates ankyrin binding and governs neurofascin-dependent cell aggregation as well as cell sorting when neurofascin is expressed in neuroblastoma cells. These findings suggest a general mechanism for the patterning of cell contact based on external signals that regulate tyrosine phosphorylation of L1CAM members and modulate their binding to ankyrin.
The L1CAM family of nervous system cell adhesion molecules (vertebrate L1, neurofascin, NrCAM, NgCAM, and Drosophila neuroglian) participate in diverse activities involving cell–cell recognition, including axon fasciculation, myelination, and synaptogenesis (1). These proteins have variable ectodomains that engage in homo- and heterophilic cell–cell interactions, and share a conserved cytoplasmic domain that associates with the membrane-skeletal protein ankyrin (2–4). Ankyrin-binding activity of neurofascin requires a cytoplasmic sequence, FIGQY, present in all L1CAM family members (5). Tyrosine-phosphorylation at the FIGQY site both abolishes ankyrin binding and reduces coupling of neurofascin to the cytoskeleton (5). This study demonstrates that neurofascin expressed in neuroblastoma cells directs cell–cell adhesion and cell sorting that is dependent on ankyrin-binding activity of the cytoplasmic domain. Nerve growth factor (NGF) promotes rapid disassociation of neurofascin-dependent cell aggregates and regulates cell sorting by a mechanism involving phosphorylation of the FIGQY tyrosine of neurofascin. These findings suggest a general mechanism for the patterning of cell contact based on external signals that regulate tyrosine phosphorylation of L1CAM family members and modulate their ankyrin-binding activity.
METHODS
Construct Generation and Transfection of Neuroblastoma Cells.
cDNA constructs encoding native neurofascin, neurofascin with a FIGQF mutation, neurofascin with a truncated cytoplasmic domain and neurofascin with a FIGQY deletion (Fig. 1A) were prepared and transfected into B104 neuroblastoma cells as described (5). All neurofascin constructs contained a HA-epitope in the extodomain N-terminal to the first Ig domain.
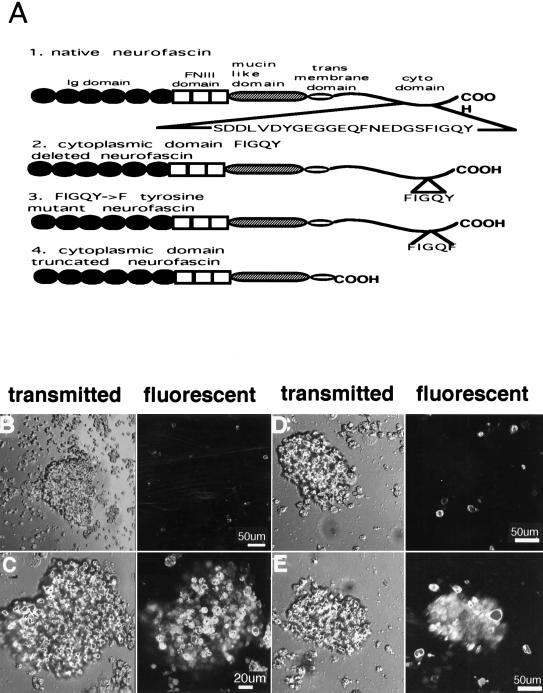
Figure 1.
Neuroblastoma cells expressing native neurofascin segregate in the presence of neuroblastoma cells expressing either no neurofascin or cytoplasmic-domain truncated neurofascin. B104 neuroblastoma cells were transfected with HA epitope-tagged neurofascin cDNAs (A) and cell segregation experiments were performed as described in the text. Cells expressed all neurofascin constructs to approximately the same level based on immunofluorescence and immunoblot analysis (see Methods). Left panels are images obtained by transmitted light, and right panels are fluorescent images revealing DiA-labeled cells. (B) DiA-labeled cells that were untransfected and express no neurofascin were mixed with unlabeled cells expressing native neurofascin. (C) DiA-labeled cells expressing native neurofascin were mixed with unlabeled cells that express no neurofascin. (D) DiA-labeled cells expressing cytoplasmic domain-truncated neurofascin were mixed with unlabeled cells transfected with native neurofascin. (E) DiA-labeled cells expressing native neurofascin were mixed with unlabeled cells expressing cytoplasmic domain-truncated neurofascin.
The level of cell surface expression of neurofascin constructs in neuroblastoma transfected cells was evaluated using a Zeiss confocal microscope equipped with an image analysis system to quantitate the immnofluorescnce levels of the transfected cell lines. Cells were first labeled on the cell surface though a hemagglutinin (HA) epitope-tagged neurofascin using an HA-specific mAb (1:1,000, Babco, Richmond, CA) and then labeled with fluoroscein isothiocyanate-conjugated goat anti-mouse antibody (1:500, Jackson ImmunoResearch). Cells (n = 40) of each cell line were measured and no statistical difference in the energy content, between the different cell lines was found (P > 0.05 by Student’s t test). The total level of neurofascin expression was also determined by immunoblots of cell extracts using HA-specific mAb and 125I-labeled protein A, and phosphoimaging to measure 125I. Briefly, SDS/PAGE was performed by loading the same concentration of protein extracts of cultured cells followed by transfer of resolved proteins to nitrocellulose. Nitrocellulose was incubated overnight at 4°C with the HA monoclonal primary antibody (1:1,000), washed, and then incubated with 125I-radiolabeled protein A for 2 hr at 4°C. Blots were developed in a PhosphorImager, and no statistical difference between the different cell lines was found (P > 0.05).
Cell Aggregation and Segregation Experiments.
Cultured cells were scraped from plastic plates and carefully pipetted until a single cell suspension was achieved. Cells were incubated for 30 min at room temperature in the presence of 1 μg/ml 4-[4-(dihexadecylamino)styryl]-N-methylpyridinium iodide (DiA; Molecular Probes) (excitation, 488 nm; emission, 590 nm), and then washed twice at 1,000 rpm for 5 min to remove excess DiA. DiA-labeled and unlabeled cells, were mixed in a 1:1 ratio (5 × 105 cells/ml). The single cell suspensions were incubated, in a DMEM buffer containing 10 mM Hepes, while shaking (100 rpm) for 30 min at 37°C. Transmitted light and fluorescent pictures were taken simultaneously with an LSM 410 Zeiss confocal microscope.
Quantification of cell aggregation was performed with 10-ml single cell suspensions at 5 × 105 cells/ml, in a DMEM buffer containing 10 mM Hepes, while shaking (100 rpm) for 120 min at 37°C. Aliquots were taken every 15 min and OD at 595 nm was determined. Percent change in OD was calculated by dividing the OD at each time point by the initial OD at 0 min and subtracting changes in OD due to sedimentation of a single cell suspension as function of time.
Immunoprecipitation of Epitope-Tagged Neurofascin.
Epitope-tagged neurofascin was immunoprecipitated from 250 μg of crude cell lysate by using an HA-specific mAb (Babco) as described (5).
RESULTS
Neurofascin Mediates Cell Segregation.
The ability of neurofascin to mediate cell–cell interactions was evaluated by mixing neuroblastoma cells transfected with neurofascin in a 1:1 ratio with untransfected neuroblastoma cells that do not express neurofascin. Each type of cell was labeled in separate experiments with DiA, a vital dye (Fig. 1 B and C). Cells transfected with neurofascin assembled into aggregates up to 0.2–0.25 mm in diameter within 30 min in suspension. Aggregates of neurofascin-transfected cells excluded DiA-labeled fluorescent cells lacking neurofascin (Fig. 1B). Conversely, cell aggregates were comprised of uniformly labeled cells when neurofascin-transfected cells were labeled with DiA (Fig. 1 C). The cells out of the optical plane section appear less bright, but were equally labeled. These results demonstrate the ability of neurofascin to direct segregation of cells by a cell–cell interaction requiring neurofascin on both cell surfaces.
The Cytoplasmic Domain of Neurofascin Contributes to Cell Sorting.
The role of the cytoplasmic domain of neurofascin in formation of cell–cell contacts was evaluated by mixing neuroblastoma cells transfected with native neurofascin with neuroblastoma cells transfected with cytoplasmic domain-deleted neurofascin (Fig. 1 D and E). All cell lines exhibited similar levels of neurofascin expression on the cell surface (see Methods). DiA-labeled cells expressing native neurofascin assembled into DiA-labeled aggregates, while cells expressing cytoplasmic domain-deleted neurofascin (Fig. 1A) were excluded from these aggregates (Fig. 1D). DiA-staining of cell aggregates was not evident when cells expressing cytoplasmic domain-deleted neurofascin were DiA labeled (Fig. 1E). The cytoplasmic domain of neurofascin therefore contributes to extracellular interactions resulting in cell sorting.
Ankyrin-Binding Activity of Neurofascin Promotes Cell–Cell Interactions.
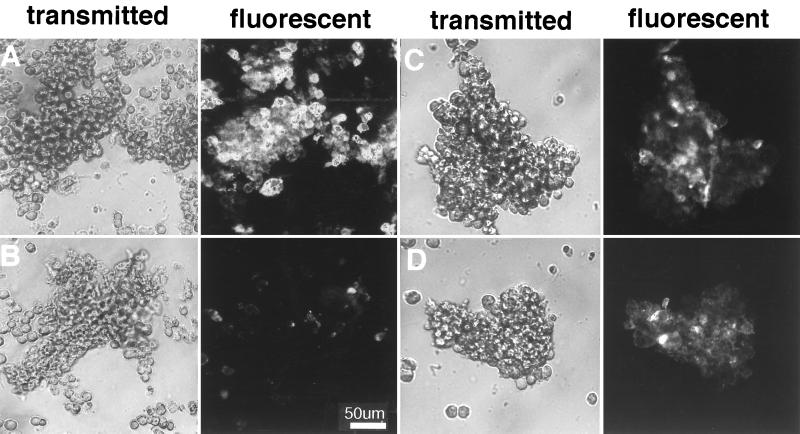
The role of ankyrin binding in the activity of neurofascin in promoting cell segregation and cell adhesion was evaluated using neuroblastoma cells expressing a form of neurofascin that binds ankyrin weakly. Ankyrin binding is reduced >80% by deletion of FIGQY residues from the cytoplasmic domain of neurofascin (5). Native neurofascin, as well as cytoplasmic domain-deleted and FIGQY-deleted forms of neurofascin (Fig. 1A), were evaluated for ankyrin-binding activity, as determined by co-immunoprecipitation of ankyrin (Fig. 2A). Neurofascin missing either the entire cytoplasmic domain or the FIGQY residues exhibits no detectable co-immunoprecipitation with ankyrin isoforms expressed in neuroblastoma cells. Neuroblastoma cells expressing native, cytoplasmic domain-deleted, and FIGQY-deleted forms of neurofascin were labeled with DiA and mixed with unlabeled cells that expressed no neurofascin (Fig. 2 B–D). Cells expressing either cytoplasmic domain-deleted (Fig. 2B) or FIGQY-deleted neurofascin (Fig. 2C) both exhibited much smaller aggregates than cells expressing native neurofascin (Fig. 2D) in the presence of untransfected neuroblastoma cells.
Figure 2.
Ankyrin-binding activity of the cytoplasmic domain of neurofascin is required for neurofascin-directed cell segregation and aggregation. Native neurofascin, or neurofascin lacking either the entire cytoplasmic domain or FIGQY residues within the cytoplasmic domain (Fig. 1A) were expressed in neuroblastoma cells and evaluated for ankyrin-binding activity (A), ability to direct cell segregation (B–D), and cell aggregation activity (E). (A) HA epitope-tagged neurofascin constructs were immunoprecipitated from crude cell lysates, resolved by SDS/PAGE, and analyzed by immunoblotting with a brain ankyrin-specific polyclonal antibody (Methods). In a separate experiment, cells expressing either native neurofascin or FIGQY→F mutant of neurofascin were treated with NGF (100 ng/ml) as described in Fig. 4, followed by the same immunoprecipitation and immunoblotting protocol. Cell segregation (B–D) was observed by the same method as in Fig. 1. DiA-labeled cells expressing neurofascin with a truncated cytoplasmic domain (B), cells expressing neurofascin missing FIGQY (C), or cells expressing native neurofascin (D) were mixed with unlabeled cells that were untransfected and express no neurofascin. (E) Cell aggregation was measured as a function of time.
Neuroblastoma cells expressing native neurofascin also aggregated faster and to a greater extent than cells expressing either cytoplasmic domain-deleted or FIGQY-deleted neurofascin (Fig. 2E). Cells expressing native neurofascin aggregated at an initial rate of 1.4% per min; after 1 hr ≈65% of cells were in aggregates and 73% were aggregated after 4 hr. In contrast, cells expressing either cytoplasmic domain-deleted or FIGQY-deleted neurofascin exhibited an initial aggregation rate of 0.4% per min and after 1 hr just 22% of cells were in aggregates and 25% after 4 hr. The size of the aggregates and the number of cells involved was also dramatically different when aggregates formed from native neurofascin transfected cells are compared with aggregates formed from cells expressing cytoplasmic domain-deleted and FIGQY-deleted neurofascin. The average cross-sectional area of aggregates observed with neurofascin-transfected neuroblastoma cells was 5 × 104 ± 650 μm2 (n = 52), where n is the number of aggregates measured, and corresponds to ≈300–400 cells (a cell diameter is ≈12 μm) per aggregate. The size of aggregates of cells transfected with cytoplasmic domain-deleted neurofascin was 1.2 × 103 ± 600 μm2 (n = 30) and with FIGQY-deleted neurofascin was 1.4 × 103 ± 1 × 103 μm2 (n = 32). In both cases aggregates contained 8–10 cells.
These comparisons demonstrate that the cytoplasmic domain has a major effect on ability of neurofascin to form cell aggregates, and enables cells expressing neurofascin to segregate in the presence of cells lacking neurofascin. The activity of the cytoplasmic domain in promoting cell interactions is completely lost with deletion of FIGQY residues, which also is required for ankyrin-binding activity. These results suggest the hypothesis that ankyrin is a critical cofactor for cell–cell interactions of neurofascin.
The Phosphorylation State of the FIGQY Tyrosine of Neurofascin Determines Cell Sorting.
Phosphorylation of the FIGQY tyrosine abolishes ankyrin-binding activity of neurofascin (5) (Fig. 2A), suggesting the possibility that phosphorylation of neurofascin could also affect cell aggregation behavior. NGF promotes phosphorylation of the FIGQY tyrosine of native neurofascin in transfected neuroblastoma cells (5) (Fig. 3A), and was used to manipulate the extent of FIGQY tyrosine phosphorylation. Exposure of cells to NGF abolished association of ankyrin with native neurofascin, but had no effect on association of ankyrin with the FIGQY→F mutant form of neurofascin (Fig. 2A), which is resistant to phosphorylation (Fig. 3A).
Figure 3.
NGF reverses neurofascin-dependent cell segregation and inhibits neurofascin-dependent cell aggregation. Neuroblastoma cells expressing either native neurofascin or the FIGQY→F mutant of neurofascin were incubated with NGF and evaluated for tyrosine phosphorylation of neurofascin (A), ability to direct cell segregation (B and C), and cell aggregation activity (E). (A) HA epitope-tagged neurofascin or FIGQY→F mutant neurofascin were immunoprecipitated from extracts of cells incubated 30 min in the presence or absence of NGF (100 ng/ml). Immunoprecipitates were resolved by SDS/PAGE, and analyzed for phosphotyrosine immunoreactivity by immunoblotting with an antiphosphotyrosine polyclonal antibody (10). (B and C) Unlabeled cells expressing either native neurofascin (B) or FIGQY→F-mutated neurofascin (C) were mixed in a 1:1 ratio with DiA-labeled untransfected cells and incubated for 30 min resulting in aggregates as in Fig. 1. Cells were then further incubated in the absence or presence of NGF (100 ng/ml) for 30 min. (D) Measurement of cell aggregation as a function of time in the presence and absence of NGF (100 ng/ml) for cells expressing native or the FIGQY→F mutant neurofascin (Fig. 2E).
Addition of NGF to neuroblastoma cells expressing native neurofascin resulted in a rapid disassociation of aggregates comprised of cells expressing native neurofascin (Fig. 3B). In contrast, cells expressing the FIGQY→F mutant form of neurofascin remained aggregated following addition of NGF (Fig. 3C). Addition of NGF to cells expressing native neurofascin reduced the rate and extent of aggregation to levels observed with cells expressing cytoplasmic domain-deleted and FIGQY-deleted forms of neurofascin (Fig. 3D). NGF treatment of neurofascin-expressing neuroblastoma cells thus promotes disassociation of neurofascin-dependent cell complexes once they have formed, and reduces initial neurofascin-dependent cell aggregation.
The experiments in Fig. 3 indicate that NGF disassociates neurofascin-dependent cell aggregates by promoting phosphorylation of the FIGQY tyrosine of neurofascin that, in turn, results in inhibition of ankyrin-binding activity. Tyrosine phosphorylation of neurofascin may have other consequences in addition to loss of ankyrin binding that could also affect cell aggregation. The possibility that neuroblastoma cells expressing neurofascin would segregate according to the state of tyrosine phosphorylation of neurofascin was evaluated by mixing neurofascin-transfected neuroblastoma cells that were either untreated, or treated with NGF and vanadate to promote tyrosine phosphorylation (Fig. 4). Neurofascin-expressing neuroblastoma cells that were untreated associated into aggregates that excluded neurofascin-expressing cells that had been treated with NGF and vanadate (Fig. 4 A and B). In contrast, neuroblastoma cells expressing the FIGQY→F mutant neurofascin did not segregate following treatment with NGF and vanadate (Fig. 4 C and D). These results demonstrate that the tyrosine-phosphorylation state of neurofascin can determine cell sorting.
Figure 4.
Neuroblastoma cells expressing native neurofascin segregate according to the state of FIGQY tyrosine phosphorylation of neurofascin. Neuroblastoma cells expressing either native or the FIGQY→F mutant neurofascin were either untreated or incubated for 30 min with 100 ng/ml NGF + 1 μM sodium vanadate. Each cell population (treated or untreated) was pipetted to obtain a single cell suspension, labeled with DiA, and mixed in a 1:1 ratio with an unlabeled cell population followed by incubation 30 min. (Left) Images obtained by transmitted light, and (Right) fluorescent images revealing DiA-labeled cells. (A) Unlabeled cells expressing native neurofascin and incubated with NGF/sodium vanadate were mixed with DiA-labeled cells expressing native neurofascin that were untreated. Aggregates contain labeled cells. (B) DiA-labeled cells expressing native neurofascin incubated with NGF/sodium vanadate were mixed with unlabeled cells expressing native neurofascin that were untreated. Aggregates contain only unlabeled cells. (C and D) The same experiments in B and C were performed with cells transfected with FIGQY→F deleted neurofascin. Note that the aggregates in C and D contain both labeled and unlabeled cells.
DISCUSSION
This study provides evidence for regulation of cell–cell interactions of neurofascin by phosphorylation of the FIGQY tyrosine conserved in the cytoplasmic domains of all L1CAM family members. Experiments also are presented demonstrating that ankyrin-binding activity is required for cell–cell interactions of neurofascin, and is inhibited by phosphorylation of the FIGQY tyrosine. A likely mechanism for regulation of cell adhesion activity of neurofascin by tyrosine phosphorylation is through inhibition of ankyrin-binding activity, although additional consequences of tyrosine phosphorylation cannot be excluded. These observations raise the question of how ankyrin binding in the cytoplasmic domain of neurofascin could effect interactions in the extracellular domain located 40 nm away. One possibility is that ankyrin promotes lateral oligomerization of neurofascin in the plane of the plasma membrane, thereby increasing the local concentration of neurofascin on the cell surface. Ankyrin is multivalent with respect to neurofascin (6), and could stabilize dimers of neurofascin in the plasma membrane. Spectrin tetramers have two binding sites for ankyrin, located within 20 nm of each other (7, 8). The combination of association of neurofascin with ankyrin to form neurofascin dimers, and of two ankyrin molecules with spectrin would result in two sets of neurofascin dimers in close proximity (Fig. 5). Oligomerization of neurofascin would allow multiple contacts between cells and enhance the stability of intercellular neurofascin complexes, especially if interactions of monomeric neurofascin were of low affinity (Fig. 5). Similar considerations could explain the ability of ankyrin to promote association of CD44 with hyaluronic acid (9).
Figure 5.
Schematic model depicting possible roles of ankyrin and spectrin in oligomerization of neurofascin in the plane of the membrane. Spectrin tetramers, ankyrin, and neurofascin are not drawn to scale. The axonal form of ankyrin with a C-terminal tail domain is depicted, although the same principles would apply to forms of ankyrin lacking this domain.
Cytoplasmic domains and their association with cytoplasmic proteins have been observed to participate in adhesive functions of many different types of cell adhesion molecules (10). Viewed in this broad context, the results of this study are not novel. However, our results do differ from those of earlier studies of L1CAM family members L1 (11) and neuroglian (12) that concluded that homotypic adhesion occurs in the absence of a cytoplasmic domain. Several considerations could explain the apparent discrepancy. A trivial explanation is that neurofascin differs from L1 and neuroglian, and that these L1CAM family members have much higher affinity in homotypic interactions that do not require a cytoplasmic domain. Another possibility is use of different host cells, which may not express the appropriate ankyrin isoforms. Interpretation of data also may be a factor.
Protein tyrosine phosphorylation-based regulation of cell–cell contacts and cell segregation mediated by neurofascin and possibly other L1 family members adds significantly to the currently known repertoire of cell interactions in the nervous system. A population of neurons and/or glial cells all expressing the same complement of L1 CAMs could segregate based on local cell–cell signaling and/or gradients of soluble signaling ligands. Examples of localized cell signaling pathways directly involving regulation of protein tyrosine phosphorylation in the nervous system include the Eph kinases, which are activated by ligands on adjacent cell surfaces (13), and receptor protein tyrosine phosphatases (14, 15). Two physiological situations where local regulation of transcellular contacts could be important are selective fasciculation within a population of axons during development of topographic maps, and regulation of synaptic contacts as a consequence of learning and/or developmental remodeling. It is of interest in this regard that NgCAM, a L1CAM family member, is a substrate for Cek5, an Eph kinase family member expressed in retinal neurons during embryonic development (16).
Modulation of the interaction between ankyrin and L1 cell adhesion molecules may play a critical role in reversible assembly of a variety of transcellular complexes in the developing and adult brain. Mutations in L1 of humans cause mental retardation and hydrocephalus (ref. 17, L1CAM Mutation web page: http://hgins.uia.ac.be/dnalab/l1/). One missense mutation of L1, Y1229H in the FIGQY site, noted in a family with the same clinical presentation as other L1 mutations (18), would be expected to result in a form of L1 that is resistant to tyrosine phosphorylation and equivalent to the FIGQY→F mutant of neurofascin. A prediction based on this study is that axons expressing the Y1229H mutated L1 exhibit abnormal axon sorting during fasciculation in the developing nervous system, and that this defect in axonal segregation is the cellular basis for mental retardation.
Acknowledgments
Qun Ren and Fen Zhang are gratefully acknowledged for preparation of cDNA constructs and transfection of B104 cells. Oxana Tsygankova is thanked for subcloning transfected cells and gratefully acknowledged for the initial observation that neurofascin is tyrosine phosphorylated.
ABBREVIATIONS
- NGF
nerve growth factor
- HA
hemagglutinin
- DiA
4-[4-(dihexadecylamino)styryl]-N-methylpyridinium iodide
References
- 1.Hortsch M. Neuron. 1996;17:587–593. doi: 10.1016/s0896-6273(00)80192-0. [DOI] [PubMed] [Google Scholar]
- 2.Davis J Q, McLaughlin T, Bennett V. J Cell Biol. 1993;232:121–133. doi: 10.1083/jcb.121.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis J Q, Bennett V. J Biol Chem. 1994;262:27163–27166. [PubMed] [Google Scholar]
- 4.Dubreuil R R, MacVicar S, Dissanayake C, Liu C, Homer D, Hortsch M. J Cell Biol. 1996;133:647–655. doi: 10.1083/jcb.133.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garver T D, Ren Q, Tuvia S, Bennett V. J Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaely P, Bennett V. J Biol Chem. 1995;270:31298–31302. doi: 10.1074/jbc.270.52.31298. [DOI] [PubMed] [Google Scholar]
- 7.Tyler J M, Hargreaves W R, Branton D. Proc the Natl Acad Sci USA. 1979;76:5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis J Q, Bennett V. J Biol Chem. 1984;259:13550–13559. [PubMed] [Google Scholar]
- 9.Lokeshwar V B, Fregien N, Bourguignon L Y. J Cell Biol. 1994;126:1099–1109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumbiner B M. Neuron. 1993;11:551–564. doi: 10.1016/0896-6273(93)90068-3. [DOI] [PubMed] [Google Scholar]
- 11.Wong E V, Chenk G, Ross Payne H, Lemmon V. Neurosci Lett. 1995;200:155–158. doi: 10.1016/0304-3940(95)12100-i. [DOI] [PubMed] [Google Scholar]
- 12.Hortsch M, Wang Y M, Marikar Y, Bieber A J. J Biol Chem. 1995;270:18809–18817. doi: 10.1074/jbc.270.32.18809. [DOI] [PubMed] [Google Scholar]
- 13.Friedman G C, O’Leary D D M. Curr Opin Neurobiol. 1996;6:127–133. doi: 10.1016/s0959-4388(96)80018-3. [DOI] [PubMed] [Google Scholar]
- 14.Krueger N X, Van Vactor D, Wan H I, Gelbart W M, Goodman C S, Saito H. Cell. 1996;84:611–622. doi: 10.1016/s0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- 15.Desai C J, Gindhart J G, Goldstein L S B, Zinn K. Cell. 1996;84:599–609. doi: 10.1016/s0092-8674(00)81035-1. [DOI] [PubMed] [Google Scholar]
- 16.Zisch A H, Stallcup A B, Chong L D, Dahlin-Huppe K, Voshol J, Schachner M, Pasquale E B. J Neurosci Res. 1997;47:655–665. doi: 10.1002/(sici)1097-4547(19970315)47:6<655::aid-jnr12>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Kenwrick S, Jouet M, Donnai D. J Med Genet. 1996;33:59–65. doi: 10.1136/jmg.33.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanCamp G, Fransen E, Vits L, Raes G, Willems P. Hum Mutat. 1996;8:391. doi: 10.1002/(SICI)1098-1004(1996)8:4<391::AID-HUMU19>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]