Abstract
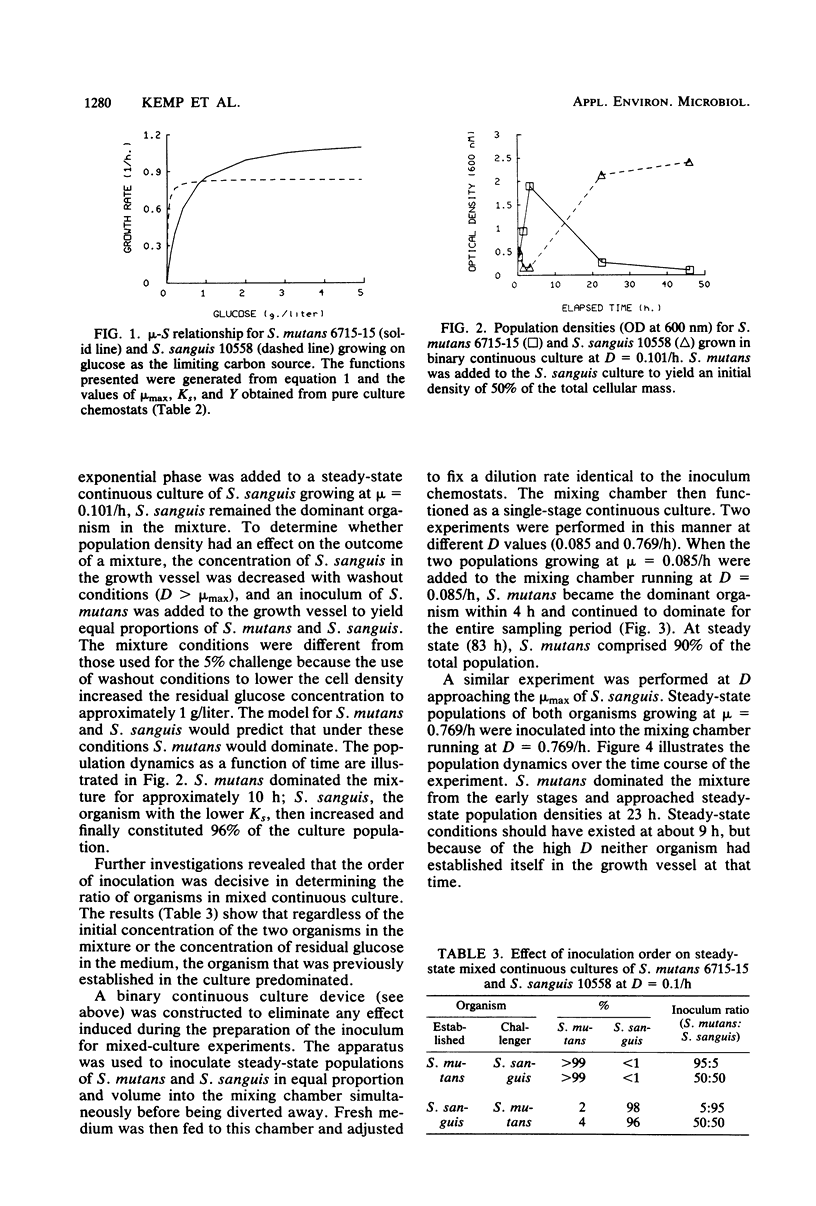
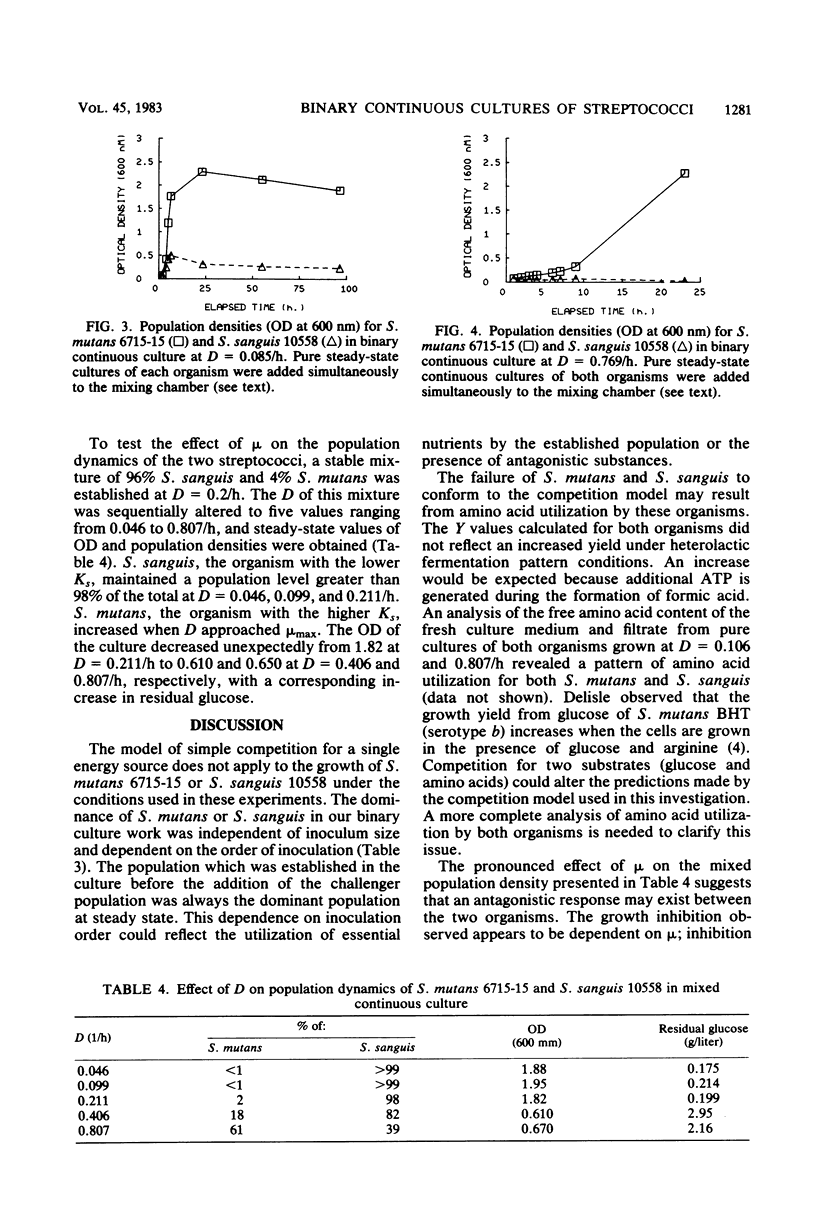
Streptococcus mutans 6715-15 and Streptococcus sanguis 10558 were grown together in continuous culture with glucose as the limiting carbon source. The relationship of growth rate to substrate concentration was determined for pure cultures of each organism in continuous and batch cultures. A model based on competition for a growth-limiting substrate (glucose) was used to predict the proportions of each organism when grown in binary cultures. The results indicate that interactions other than competition for glucose carbon exist between S. mutans and S. sanguis grown under these conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen W. H. Effects of foods on oral bacterial populations in man and animals. J Dent Res. 1970 Nov-Dec;49(6):1276–1282. doi: 10.1177/00220345700490061701. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Delisle A. L., Weaver D. S. Effects of high concentrations of L-arginine on acid production, cell yield and viability of the oral bacterium Streptococcus mutans BHT. Arch Oral Biol. 1981;26(8):693–696. doi: 10.1016/0003-9969(81)90168-0. [DOI] [PubMed] [Google Scholar]
- Griffith C. J., Melville T. H. Growth of oral streptococci in a chemostat. Arch Oral Biol. 1974 Jan;19(1):87–90. doi: 10.1016/0003-9969(74)90230-1. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W. Competitive elimination of Enterobacteriaceae from seawater. Appl Microbiol. 1968 Oct;16(10):1616–1618. doi: 10.1128/am.16.10.1616-1618.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W. Enrichments of aquatic bacteria in continous culture. Arch Mikrobiol. 1967;59(1):165–173. doi: 10.1007/BF00406328. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W. Estimations of bacterial growth rates in natural waters. J Bacteriol. 1969 Jul;99(1):156–160. doi: 10.1128/jb.99.1.156-160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W. Growth characteristics of heterotrophic bacteria in seawater. J Bacteriol. 1968 Feb;95(2):722–723. doi: 10.1128/jb.95.2.722-723.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Kemp C. W., Bowen W. H. Use of extractable adenosine triphosphate to estimate the viable cell mass in dental plaque samples obtained from monkeys. Appl Environ Microbiol. 1978 Apr;35(4):743–749. doi: 10.1128/aem.35.4.743-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. A., Bowen W. H., Little W. A., Kuzmiak-Jones H. M., Gomez I. M. Simultaneous implantation of five serotypes of Streptococcus mutans in gnotobiotic rats. Caries Res. 1979;13(1):9–17. doi: 10.1159/000260376. [DOI] [PubMed] [Google Scholar]


