Abstract
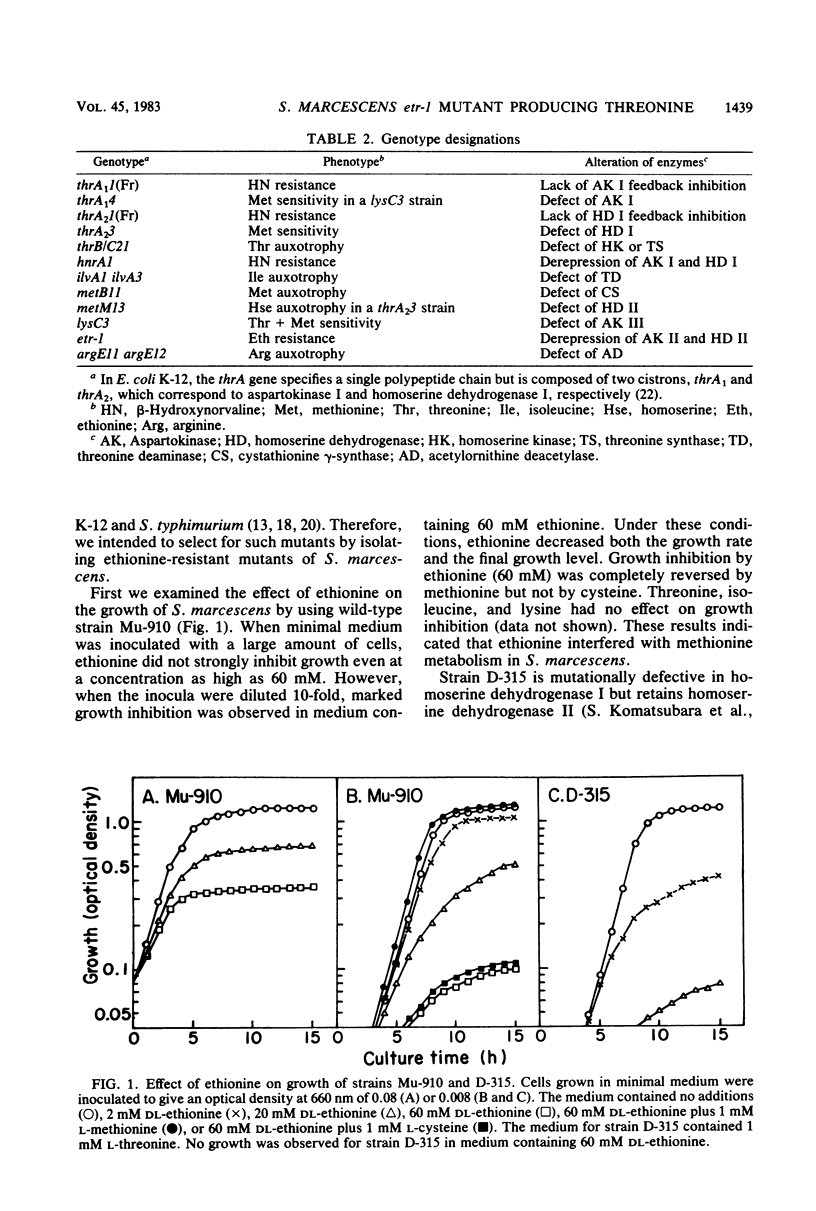
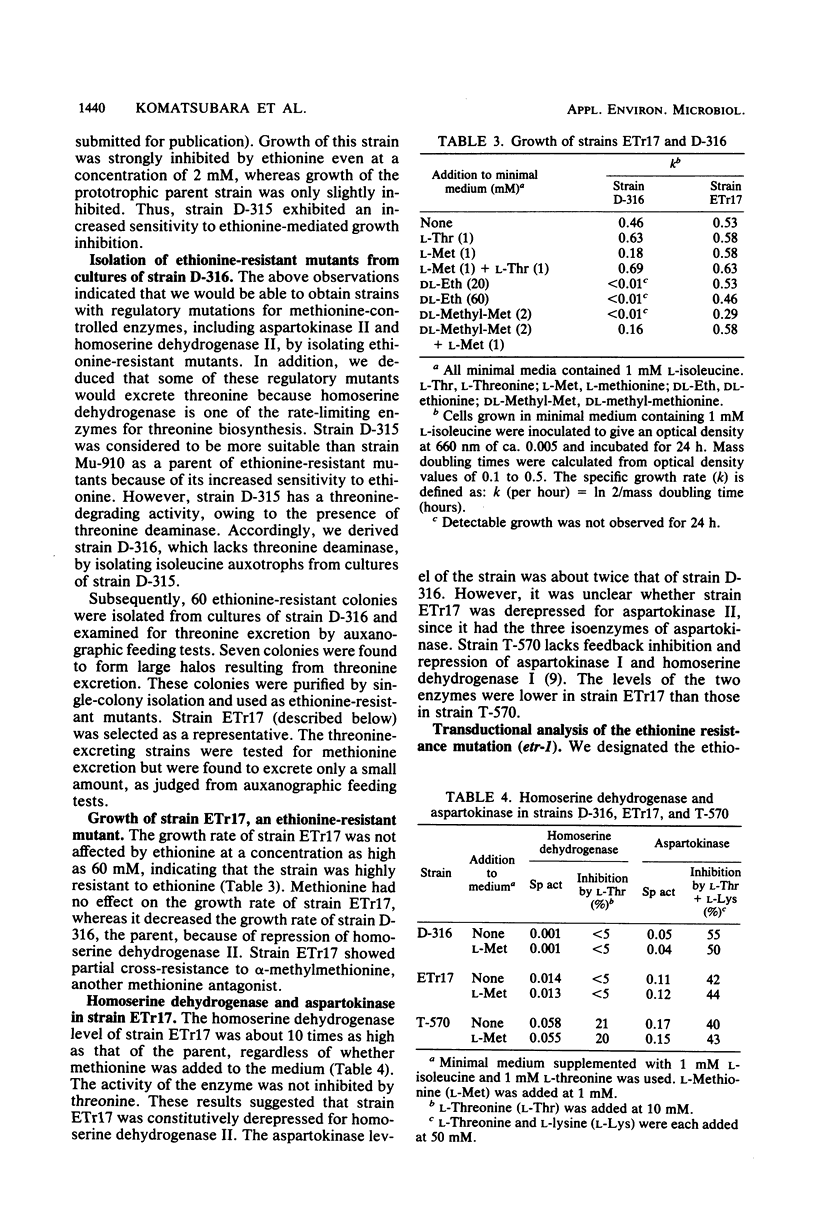
Ethionine reduced both the growth rate and the final growth level of Serratia marcescens Sr41. Growth inhibition was completely reversed by methionine. Strain D-315, defective in homoserine dehydrogenase I, was more sensitive to ethionine-mediated growth inhibition than was the wild-type strain. Ethionine-resistant mutants were isolated from cultures of strain D-316, which was derived from strain D-315 as a threonine deaminase-deficient mutant. Of 60 resistant colonies, 7 excreted threonine on minimal agar plates. One threonine-excreting strain, ETr17, was highly resistant to ethionine and, moreover, insensitive to methionine-mediated growth inhibition, whereas the parent strain was sensitive. When cultured in minimal medium with or without excess methionine, strain ETr17 had a higher homoserine dehydrogenase level than did strain D-316. The homoserine dehydrogenase activity was not inhibited by threonine or methionine. Transductional analysis revealed that the ethionine-resistant (etr-1) mutation carried by strain ETr17 was located in the metBM-argE region and caused the derepressed synthesis of homoserine dehydrogenase II. Strain ETr17 had a higher aspartokinase level than did the parent strain. By transductional cross with the argE+ marker, the etr-1 mutation was transferred into strain D-562 which was derived from D-505, a strain defective in aspartokinases I and III. The constructed strain had a higher aspartokinase level than did strain D-505 in medium with or without excess methionine, indicating that the etr-1 mutation led to the derepressed synthesis of aspartokinase II. Strain ETr17 produced about 8 mg of threonine per ml of medium containing sucrose and urea.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferata R. L., Freundlich M. Evidence for channeling of homoserine in Salmonella typhimurium. Biochim Biophys Acta. 1970 Dec 29;222(3):671–674. doi: 10.1016/0304-4165(70)90196-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Enhancement of isoleucine hydroxamate-mediated growth inhibition and improvement of isoleucine-producing strains of Serratia marcescens. Appl Environ Microbiol. 1977 Dec;34(6):647–653. doi: 10.1128/aem.34.6.647-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Chibata I. Participation of lysine-sensitive aspartokinase in threonine production by S-2-aminoethyl cysteine-resistant mutants of Serratia marcescens. Appl Environ Microbiol. 1979 Nov;38(5):777–782. doi: 10.1128/aem.38.5.777-782.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Chibata I. Transductional construction of a threonine-hyperproducing strain of Serratia marcescens: lack of feedback controls of three aspartokinases and two homoserine dehydrogenases. Appl Environ Microbiol. 1983 May;45(5):1445–1452. doi: 10.1128/aem.45.5.1445-1452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Chibata I. Transductional construction of a threonine-producing strain of Serratia marcescens. Appl Environ Microbiol. 1979 Dec;38(6):1045–1051. doi: 10.1128/aem.38.6.1045-1051.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Chibata I. Transductional construction of an isoleucine-producing strain of Serratia marcescens. J Gen Microbiol. 1980 Jul;119(1):51–61. doi: 10.1099/00221287-119-1-51. [DOI] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Murata K., Chibata I. Threonine production by regulatory mutants of Serratia marcescens. Appl Environ Microbiol. 1978 May;35(5):834–840. doi: 10.1128/aem.35.5.834-840.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Murata K., Kisumi M., Chibata I. Threonine degradation by Serratia marcescens. J Bacteriol. 1978 Aug;135(2):318–323. doi: 10.1128/jb.135.2.318-323.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Hosogaya S., Suzuki K., Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975 Feb;19(1):35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T., Hosogaya S. A generalized transducing phage of Serratia marcescens. Jpn J Microbiol. 1973 Nov;17(6):473–479. doi: 10.1111/j.1348-0421.1973.tb00933.x. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Rowbury R. J., Lawrence D. A., Smith D. A. Regulation of the methionine-specific aspartokinase and homoserine dehydrogenase of Salmonella typhimurium. J Gen Microbiol. 1968 Dec;54(3):337–342. doi: 10.1099/00221287-54-3-337. [DOI] [PubMed] [Google Scholar]
- Smith D. A. S-amino acid metabolism and its regulation in Escherichia coli and Salmonella typhimurium. Adv Genet. 1971;16:141–165. doi: 10.1016/s0065-2660(08)60357-0. [DOI] [PubMed] [Google Scholar]
- Thèze J., Margarita D., Cohen G. N., Borne F., Patte J. C. Mapping of the structural genes of the three aspartokinases and of the two homoserine dehydrogenases of Escherichia coli K-12. J Bacteriol. 1974 Jan;117(1):133–143. doi: 10.1128/jb.117.1.133-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Saint-Girons I. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol. 1974 Jun;118(3):990–998. doi: 10.1128/jb.118.3.990-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]


