Abstract
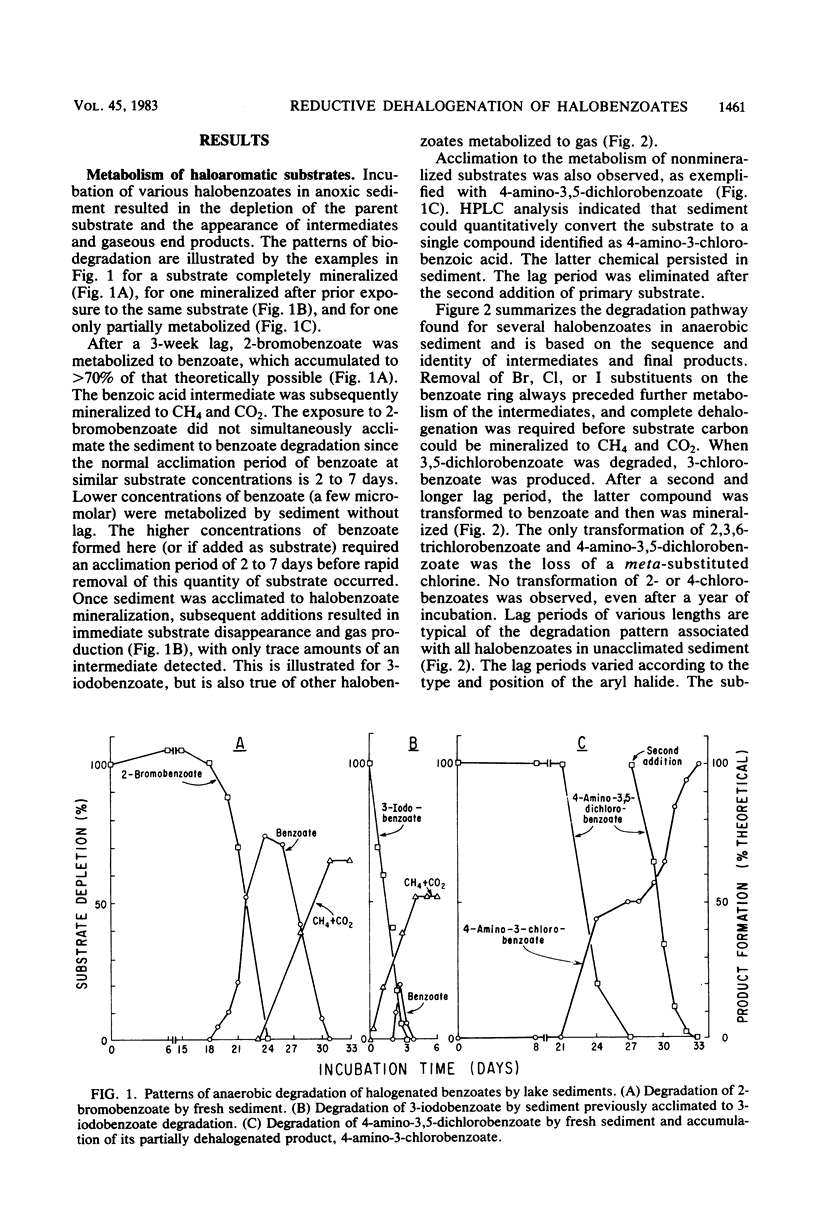
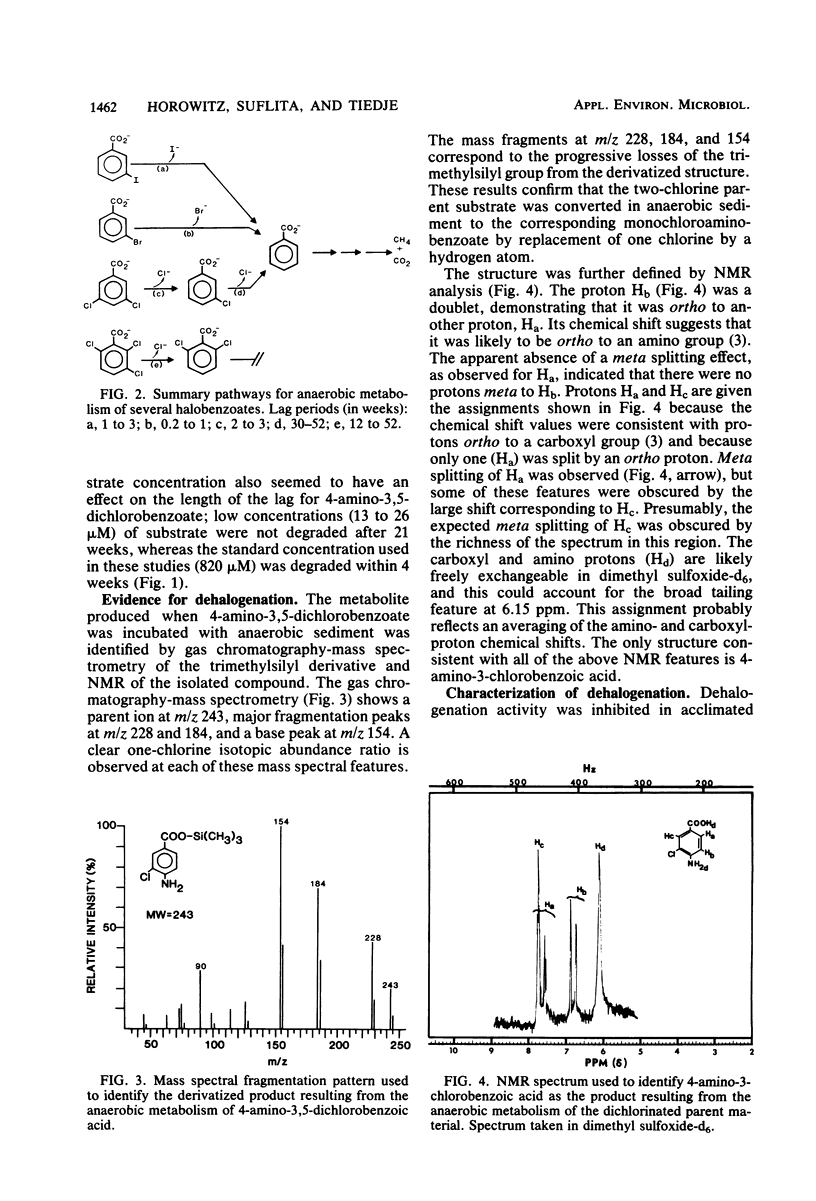
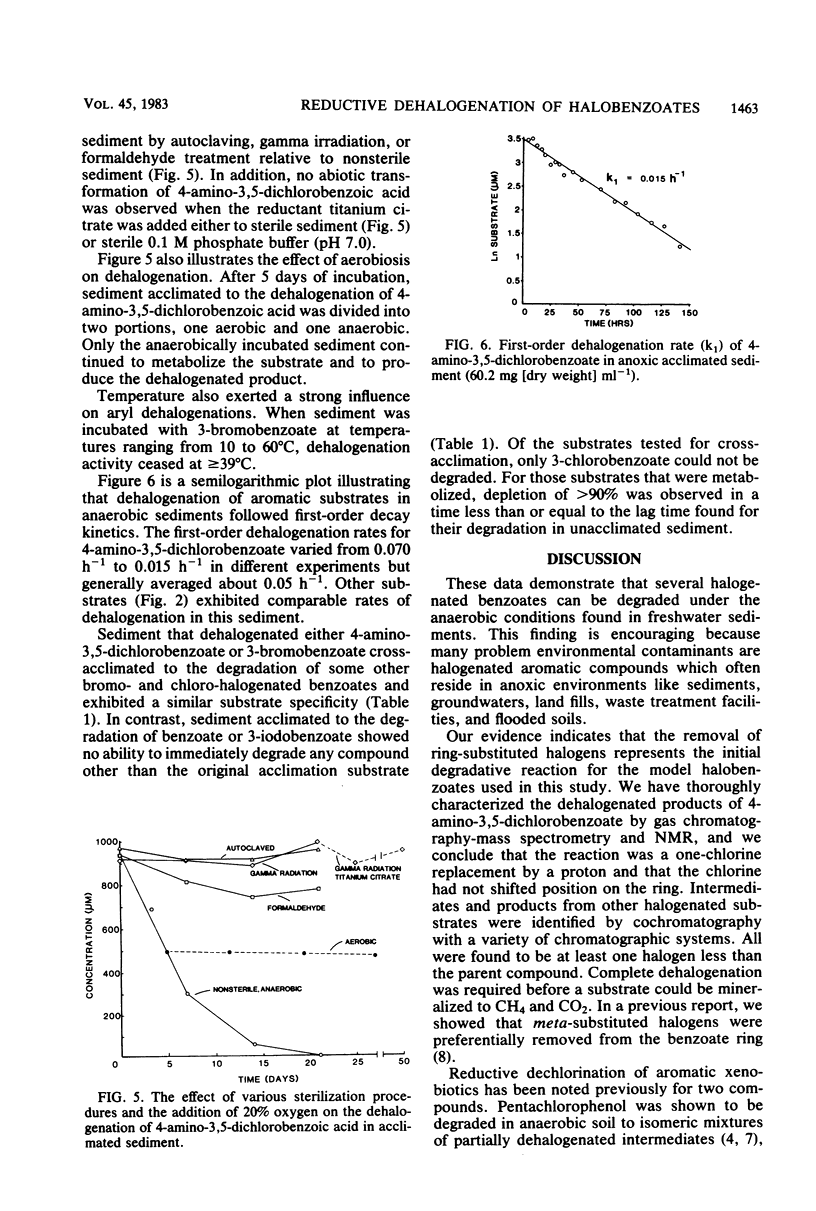
Methane-producing freshwater lake sediment was found to dehalogenate chloro-, bromo-, and iodobenzoates by a reductive reaction in which the halogen was replaced by a hydrogen atom. The identity of the dehalogenated products was confirmed by mass spectrometry, nuclear magnetic resonance, or cochromatography. Removal of the halogens to produce benzoate was necessary before mineralization to CH4 + CO2 could occur. The dehalogenation occurred after a lag period which lasted from 1 week to more than 6 months, depending on the chemical. Dehalogenation was not observed in the absence of CH4 production, and it was inhibited by the addition of 20% O2. Once sediment was acclimated to halobenzoate dehalogenation, new additions of the halobenzoate were degraded without lag. Acclimation was observed regardless of whether the parent substrates were eventually mineralized to CH4 + CO2. Sediment acclimated to bromo- and chlorobenzoate degradation generally metabolized bromo- and chlorobenzoates, but sediment acclimated to iodobenzoate degradation only metabolized iodobenzoate. Prior acclimation of sediment to benzoate decomposition did not alter the pattern of dehalogenation, and sediment acclimated to dehalogenation was not concurrently acclimated to benzoate degradation. The presence of this apparent specificity, the lag period, and subsequent acclimation, together with our findings of the absence of dehalogenation in sterile sediments and by sediments previously incubated at ≥39°C, suggests that this reaction was biologically catalyzed. Apparently, a pathway for the reductive dehalogenation of aryl halides is present in anaerobic microorganisms of this methanogenic sediment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick D., Biggs S. R., Conway B., Finn C. M., Hawkins D. R., Honda T., Ishida M., Powell G. P. Metabolism of N-(2,3-dichlorophenyl)-3,4,5,6-tetrachlorophthalamic acid (techlofthalam) in paddy soil and rice. J Agric Food Chem. 1981 Nov-Dec;29(6):1149–1153. doi: 10.1021/jf00108a012. [DOI] [PubMed] [Google Scholar]
- Murthy N. B., Kaufman D. D., Fries G. F. Degradation of pentachlorophenol (PCP) in aerobic and anaerobic soil. J Environ Sci Health B. 1979;14(1):1–14. doi: 10.1080/03601237909372110. [DOI] [PubMed] [Google Scholar]
- Suflita J. M., Horowitz A., Shelton D. R., Tiedje J. M. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science. 1982 Dec 10;218(4577):1115–1117. doi: 10.1126/science.218.4577.1115. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoro J. A., Hunter J. M., Eglinton G., Ware G. C. Degradation of p,p'-DDT in reducing environments. Nature. 1974 Jan 25;247(5438):235–237. doi: 10.1038/247235a0. [DOI] [PubMed] [Google Scholar]



