Abstract
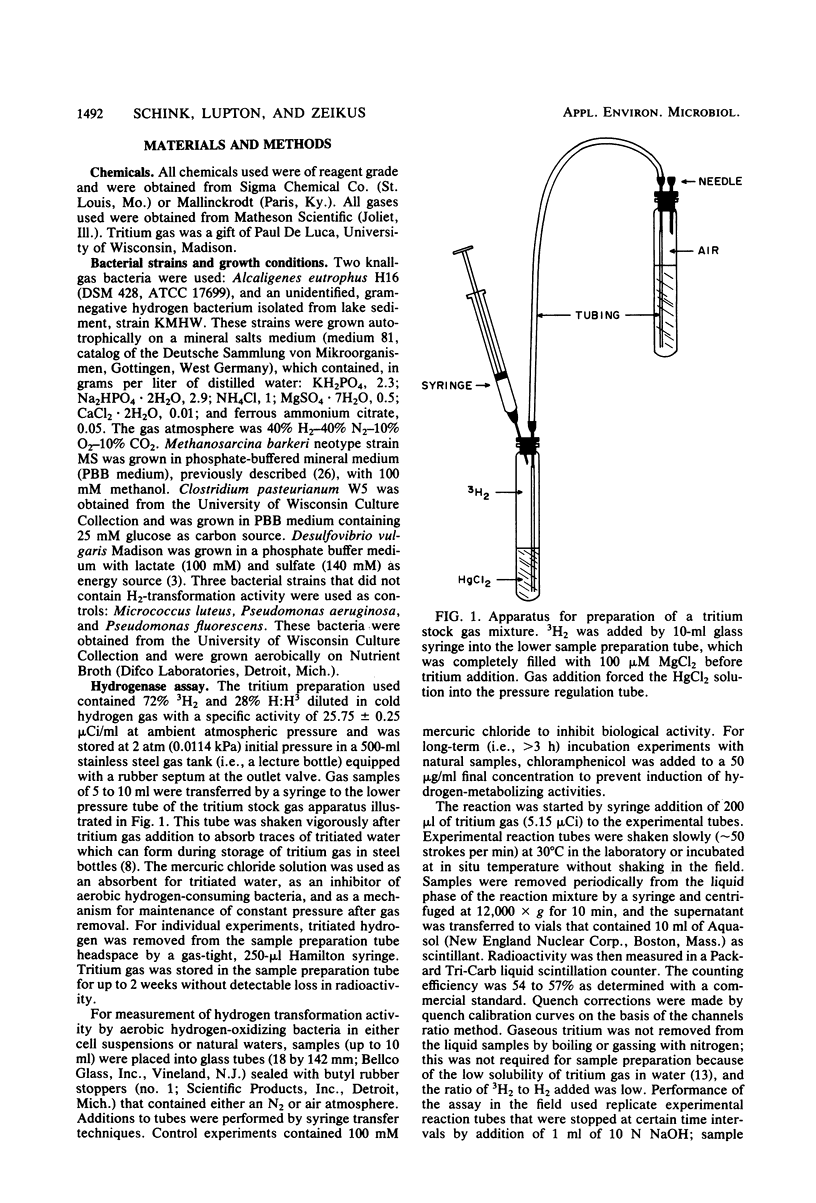
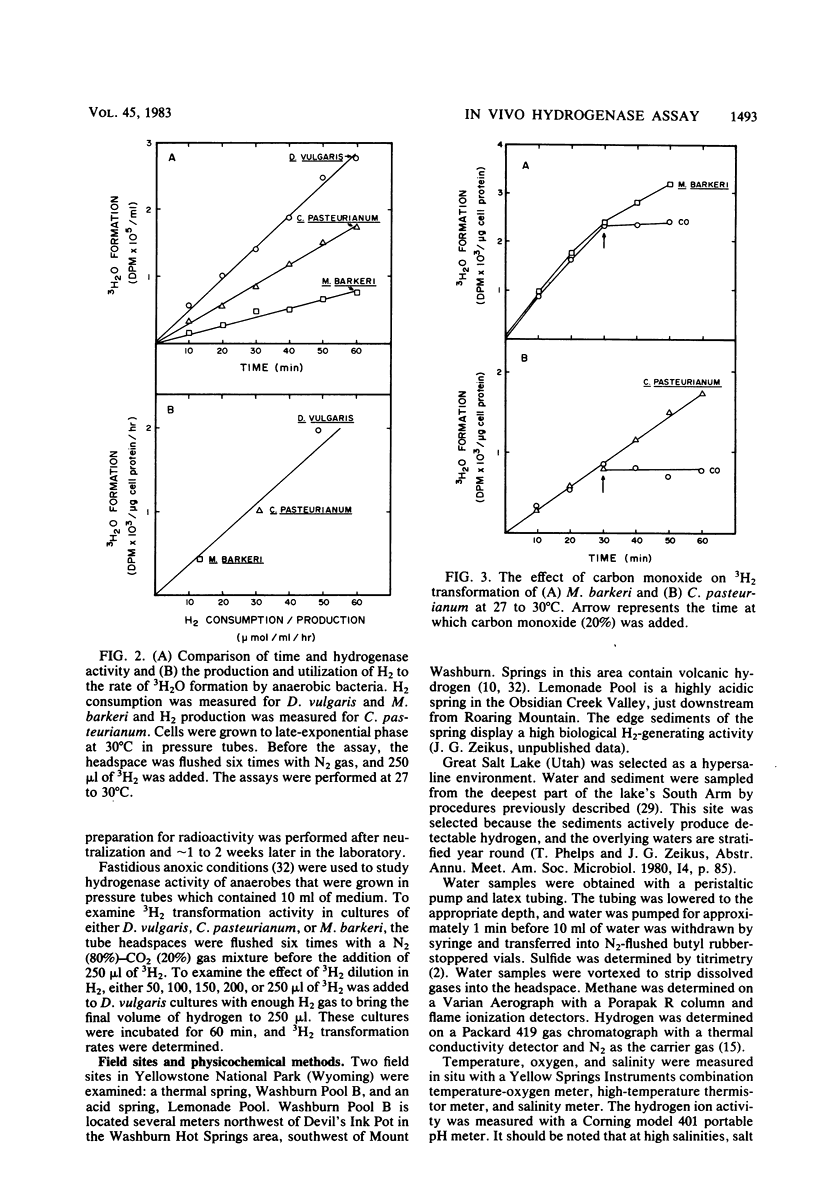
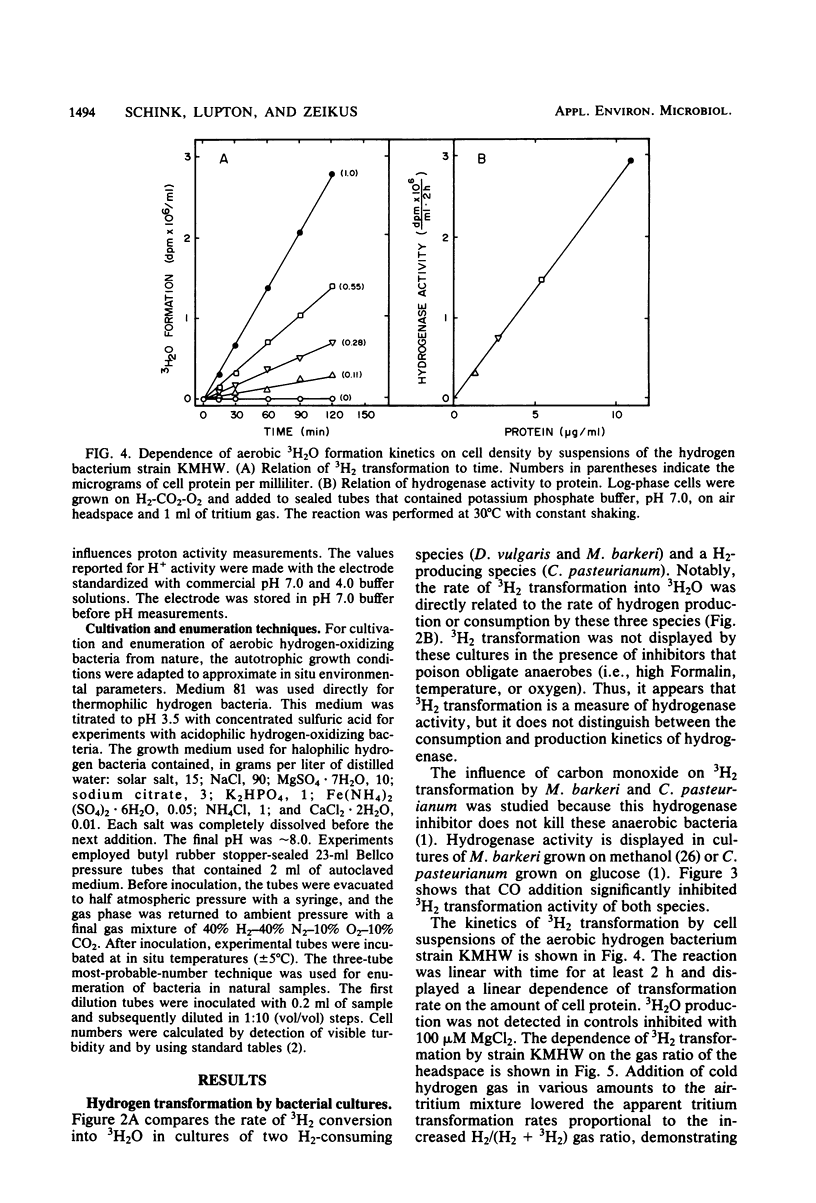
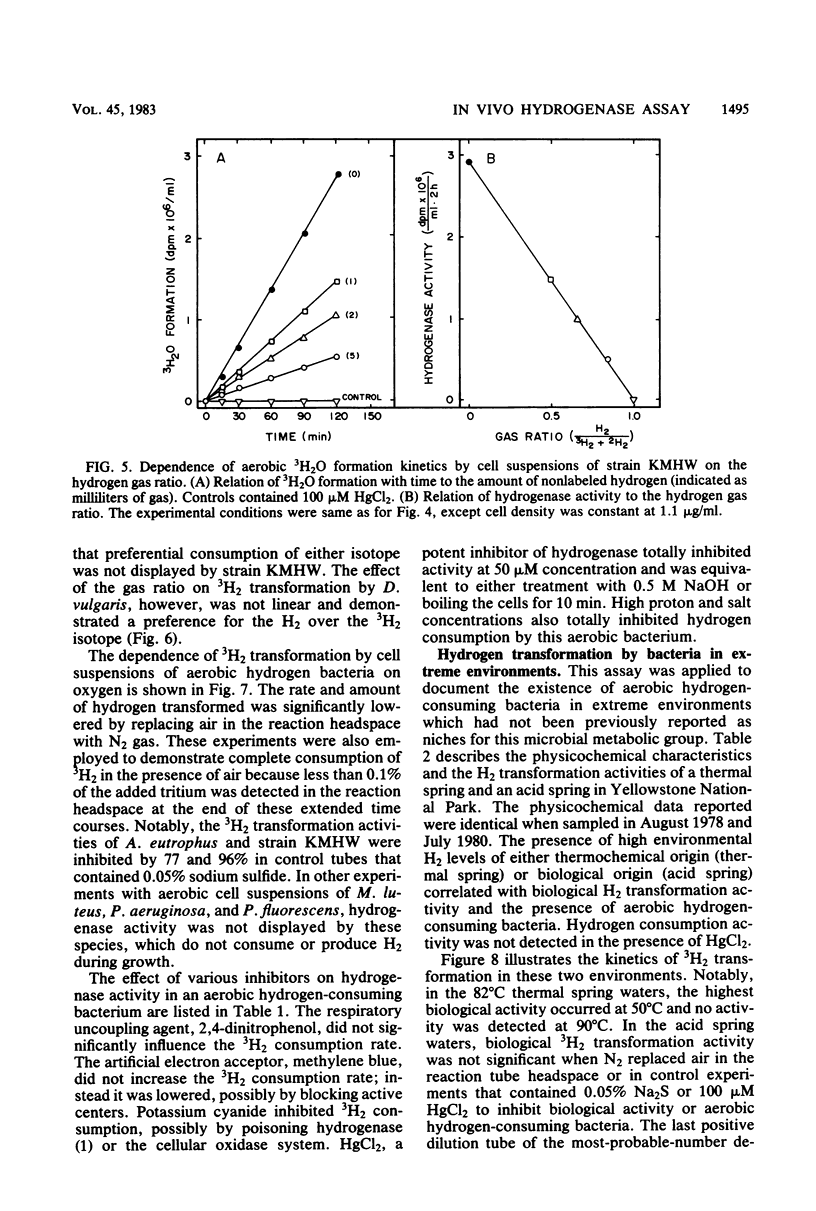
An isotopic tracer assay based on the hydrogenase-dependent formation of tritiated water from tritium gas was developed for in life analysis of microbial hydrogen transformation. This method allowed detection of bacterial hydrogen metabolism in pure cultures or in natural samples obtained from aquatic ecosystems. A differentiation between chemical-biological and aerobic-anaerobic hydrogen metabolism was established by variation of the experimental incubation temperature or by addition of selective inhibitors. Hydrogenase activity was shown to be proportional to the consumption or production of hydrogen by cultures of Desulfovibrio vulgaris, Clostridium pasteurianum, and Methanosarcina barkeri. This method was applied, in connection with measurements of free hydrogen and most-probable-number enumerations, in aerobic natural source waters to establish the activity and document the ecology of hydrogen-consuming bacteria in extreme acid, thermal, or saline environments. The utility of the assay is based in part on the ability to quantify bacterial hydrogen transformation at natural hydrogen partial pressures, without the use of artificial electron acceptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Badziong W., Thauer R. K., Zeikus J. G. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol. 1978 Jan 23;116(1):41–49. doi: 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Daniels L., Fulton G., Spencer R. W., Orme-Johnson W. H. Origin of hydrogen in methane produced by Methanobacterium thermoautotrophicum. J Bacteriol. 1980 Feb;141(2):694–698. doi: 10.1128/jb.141.2.694-698.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Nitrogenase--hydrogenase interrelationships in Rhizobia. Biochimie. 1978;60(3):233–236. doi: 10.1016/s0300-9084(78)80819-0. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- Ingvorsen K., Zeikus J. G., Brock T. D. Dynamics of bacterial sulfate reduction in a eutrophic lake. Appl Environ Microbiol. 1981 Dec;42(6):1029–1036. doi: 10.1128/aem.42.6.1029-1036.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- Powell M. R., Doebbler G. F., Hamilton R. W., Jr Serum enzyme level changes in pigs following decompression trauma. Aerosp Med. 1974 May;45(5):519–524. [PubMed] [Google Scholar]
- Schink B., Probst I. Competitive inhibition of the membrane-bound hydrogenase of Alcaligenes eutrophus by molecular oxygen. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1563–1569. doi: 10.1016/s0006-291x(80)80076-3. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. Hydrogen metabolism in aerobic hydrogen-oxidizing bacteria. Biochimie. 1978;60(3):297–305. doi: 10.1016/s0300-9084(78)80826-8. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus: II. Localization and immunological comparison with other hydrogenase systems. Antonie Van Leeuwenhoek. 1980;46(1):1–14. doi: 10.1007/BF00422224. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J. 1981 Jan 1;193(1):99–107. doi: 10.1042/bj1930099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Kinetic parameters of the conversion of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol. 1978 Aug;36(2):330–340. doi: 10.1128/aem.36.2.330-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Nelson D. R., Klevickis S. C., Zeikus J. G. Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl Environ Microbiol. 1977 Feb;33(2):312–318. doi: 10.1128/aem.33.2.312-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Anaerobic metabolism of immediate methane precursors in Lake Mendota. Appl Environ Microbiol. 1979 Feb;37(2):244–253. doi: 10.1128/aem.37.2.244-253.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


