Abstract
Large-scale genetic screens for mutations affecting early neurogenesis of vertebrates have recently been performed with an aquarium fish, the zebrafish. Later stages of neural morphogenesis have attracted less attention in small fish species, partly because of the lack of molecular markers of developing structures that may facilitate the detection of discrete structural alterations. In this context, we report the characterization of Ol-Prx 3 (Oryzias latipes-Prx 3). This gene was isolated in the course of a large-scale screen for brain cDNAs containing a highly conserved DNA binding region, the homeobox helix-three. Sequence analysis revealed that this gene belongs to another class of homeobox genes, together with a previously isolated mouse ortholog, called OG-12 [Rovescalli, A. C., Asoh, S. & Nirenberg, M. (1996) Proc. Natl. Acad. Sci. USA 93, 10691–10696] and with the human SHOX gene [Rao, E., Weiss, B., Fukami, M., Rump, A., Niesler, B., et al. (1997) Nat. Genet. 16, 54–62], thought to be involved in the short-stature phenotype of Turner syndrome patients. These three genes exhibit a moderate level of identity in the homeobox with the other genes of the paired-related (PRX) gene family. Ol-Prx 3, as well as the PRX genes, are expressed in various cartilaginous structures of head and limbs. These genes might thus be involved in common regulatory pathways during the morphogenesis of these structures. Moreover, this paper reports a complex and monophasic pattern of Ol-Prx 3 expression in the central nervous system, which differs markedly from the patterns reported for the PRX genes, Prx 3 excluded: this gene begins to be expressed in a variety of central nervous system territories at late neurula stage. Strikingly, it remains turned on in some of the derivatives of each territory during the entire life of the fish. We hope this work will thus help identify common features for the PRX 3 family of homeobox genes.
The Japanese medaka, Oryzias latipes, is a daily spawning aquarium fish studied for more than seven decades, with cancer biology, sex determination, and reproductive processes as major foci (1). Classical genetic work in the medaka has given rise to a unique collection of highly inbred lines and natural mutant strains (2). Like the zebrafish, the medaka has a short life cycle and produces numerous transparent embryos. Its genome is smaller, and its genetic map has reached high resolution (3). The number of genes and induced mutants isolated in the medaka is still far smaller than in the zebrafish, but studies of medaka gene function and regulation will benefit from a unique method of gene transfer by nuclear injection (4), recent progress on the long-term culture of embryonic stem cells (5, 6), and the discovery of an apparently intact transposable element inserted at various positions in the tyrosinase gene of medaka albinos (7).
However, few medaka developmental genes have been cloned to date. Our interest in the development of the vertebrate central nervous system (CNS) led us to undergo a search for new types of homeobox genes expressed in the medaka brain. Most homeobox genes have been isolated in vertebrates by homology cloning on the basis of a strong conservation of the whole homeobox among distantly related species. We have chosen to screen an adult medaka brain cDNA library with a mixture of oligodeoxynucleotide probes corresponding to the third α-helix of most homeoboxes [amino acid residues 46–52 (8)]. Such a strategy was used in various species and has already led to the isolation of previously unknown families of homeobox genes (refer, for example, to refs. 9 and 10). One among the genes resulting from this screen has retained all our attention, because its sequence shows (i) a strong homology in the whole open reading frame with the mouse OG-12 gene (10), the original member of a recently discovered class of animal homeobox genes, expressed in the brain and other tissues of the mouse following a so-far-undescribed pattern, and (ii) a strong homology restricted to the homeobox with a family of paired-related genes (PRX) identified in higher vertebrates, whose members are clearly involved in head skeleton and limb morphogenesis (11). Because of the small size of medaka brain, we were able to characterize the expression in the CNS from early embryonic stages of development to adulthood. Moreover, the expression pattern was thoroughly described, using a recently established medaka brain atlas (12). This study thus provides comparative data concerning the domains of expression of the PRX 3 vertebrate genes. Interestingly, a human homolog of Ol-Prx 3 (currently named SHOX) is thought to be involved in the short-stature phenotype of Turner syndrome patients (13). It was also shown to be expressed in various human tissues, including brain. We thus believe that analysis of the role of vertebrate PRX 3 genes in different developmental processes will benefit from various approaches allowed by the use of several biological models.
MATERIALS AND METHODS
Fish Strains and Embryo Collection.
Medaka embryos and adults of an Orange-Red strain (kindly provided by A. Shima, Tokyo University) were used in most experiments. Albino embryos of an inbred line (i3BR1, gift of Y. Hyodo-Taguchi, National Institute of Radiological Sciences, Chiba, Japan) were also used for unambiguous identification of otherwise pigmented structures. Fish were raised in 20-liter tanks at 25°C, with a 12-h day/12-h night cycle (standard regime), or 16-h light/8-h darkness (reproduction regime). Eggs were incubated in Petri dishes in Yamamoto’s embryo rearing medium (14) at 28°C. Embryos were staged according to Iwamatsu (15).
Construction of Brain cDNA Libraries.
Adult fish (3 to 12 months old) were killed by immersion in ice-cold water. Two hundred brains were dissected in chilled PBS and immediately frozen in liquid nitrogen. Total mRNAs were extracted according to Chomczynski and Sacchi (16). Poly(A)+ RNAs were purified by two passages on an oligo(dT) column (Quick Push, Stratagene). Two micrograms of poly(A)+ RNA was reverse-transcribed by using either oligo(dT) or random hexamers. After addition of EcoRI linkers, cDNAs were inserted in a λ ZAPII vector and encapsidated by using Gigapack Gold (Stratagene). Thus, one library with 500- to 1,000-bp inserts (“short cDNA” library) and another bearing putative full-length cDNAs were constructed using a commercial kit (Pharmacia Biotech). Each library contained 6 × 105 independent clones and was thereafter amplified.
Library Screening.
A 1,024-fold degenerate oligonucleotide referred to as HB-1 in Bürglin et al. (8) was used to screen the short cDNA library, using conditions indicated by the authors. Of 6 × 105 clones, 20 positive clones were detected on a set of duplicated filters. One clone in a Bluescript plasmid was rescued from λ ZAP (called pSHOM) and shown to bear an insert of 724 bp (the other 19 clones are still under analysis). Radiolabeled probes were synthesized from this insert and used to screen the full-length cDNA library under stringent conditions (17). Five clones were isolated and further analyzed (see Results and Discussion).
DNA Sequencing.
Sequencing on both strands of plasmid DNA was performed directly on one cDNA clone and on deleted forms of this clone obtained by using a commercial kit (CLONTECH). T7 DNA polymerase (Pharmacia) was used and radiolabeled probes were electrophoresed on polyacrylamide gels (17). The Wisconsin Sequence Analysis Package (Genetics Computer Group, Madison, WI) was used for DNA segment assembly.
In Situ Hybridization.
Two digoxigenin-UTP probes (SHOM, 3′HOM) were generated from the pSHOM insert containing the homeobox, and from a cDNA fragment downstream of the homeobox, respectively. This latter fragment (847 bp) was cut out from a 4.3-kb cDNA clone by using Tth111I, filling with the Klenow fragment of DNA polymerase, and SacI, and inserted into the SmaI–SacI sites of Bluescript KS (+) (Stratagene). After appropriate linearization, plasmids were transcribed with T3/T7 phage RNA polymerases (Promega) to obtain sense and antisense probes. Specimens were fixed overnight at 4°C with 4% paraformaldehyde in PBS (pH 7.4). Embryos were dechorionated with fine forceps and stored dehydrated in methanol at −20°C. Following rehydration, specimens were processed according to Joly et al. (18). Overnight detection was usually performed. Whole adult brains were processed the same way, except that proteinase K treatment was extended up to 30 min and detection to 3 days.
Histological Methods.
For in situ detection of transcripts in adult thick CNS sections, young medakas (1–2 months; body length <3 cm) were killed by immersion into chilled water and fixed for 24 h at 4°C in paraformaldehyde. Brains were dissected, quickly embedded in albumin/gelatin and sectioned at 75 μm with a Leica vibrating microtome. Sections were processed as the embryos and were mounted on slides in glycerol/PBS.
After in situ hybridizations, embryos and brains were postfixed in paraformaldehyde. Some were subsequently cleared with graded glycerol/PBS baths, whereas others were embedded in paraffin, cut at 8 μm in the transverse, sagittal, or horizontal plane, and counterstained with nuclear fast red. Whole-mount embryos and sections were photographed with a Leica M 10 stereomicroscope and a Leica DMRD photomicroscope, respectively.
RESULTS AND DISCUSSION
cDNA Cloning and Characterization.
Using a pool of degenerate oligonucleotides corresponding to the most conserved region of the homeobox [Fig. 1A (8)], we screened a cDNA library of short brain cDNAs. One clone, with a 700-bp insert, was isolated and shown to contain a homeobox. This insert was used to screen another brain library containing presumably full-length cDNAs.
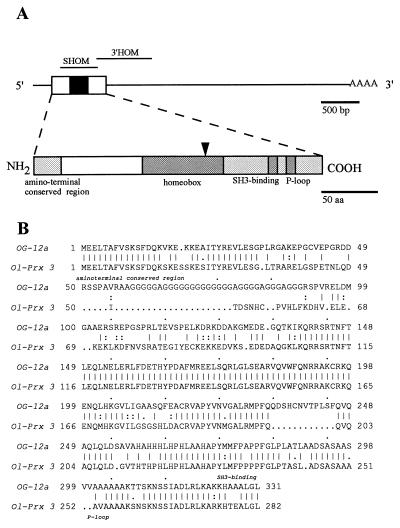
Figure 1.
(A) Schematic representations of Ol-Prx 3 cDNA (Upper, clone E) and putative protein structures (Lower). The open reading frame (box), homeobox (black box), and poly(A) tail locations are given in the cDNA drawing. Probes used for Northern blotting and in situ hybridizations are shown above the cDNA structure. Shaded boxes in the protein drawing show regions conserved with OG-12. Names of the conserved amino-terminal, homeodomain, SH3-binding, and P-loop domains are given below their respective locations. The oligonucleotide involved in library screening is indicated by an arrow. (B) Comparison of the amino acid sequence encoded by medaka Ol-Prx 3 and that of mouse OG-12. The amino-terminal, SH3-binding, and P-loop domains are named below. Double dots indicate conservative amino acid changes in OG-12 vs. Ol-PRX 3 proteins.
Five positive clones, with inserts of approximately 4.3 kb, were isolated and mapped with restriction enzymes. This analysis suggested that all five clones originated from mRNAs with identical structures. Sequences of the insert ends and of the homeoboxes were determined.
One of the cDNA clones, Ol-Prx 3E (4,329 bp), contains an 849-bp open reading frame encoding a 283 amino acid putative protein (Fig. 1A). We propose that the first AUG located 9 bp after the last in-frame stop codon is the methionine start codon. An adenine, found 3 nucleotides upstream of the AUG codon, was found to have a dominant effect on the initiation of translation of other vertebrate genes (19). Moreover, this methionine precedes a conserved stretch of 18 amino acids found in both medaka Ol-PRX 3 protein and its mouse homolog encoded by the OG-12 gene (see below). Such conserved amino-terminal regions are found in most homeodomain proteins (20), but their functions are still unclear.
Ol-Prx 3 cDNA contains an unusually long 3′ untranslated region of more than 3 kb, which is not found in OG-12 cDNAs isolated from 12-day-old mouse embryos (10). It was already reported that brain mRNAs have such an unusually long 3′ untranslated region (UTR) (21). Translational efficiency was found to be positively regulated by the length of 3′ UTR (22). A putative stabilizing effect of this region remains also to be assayed.
Comparison Between Medaka Ol-PRX 3 and Mouse OG-12 Proteins.
Strikingly, all amino acids in the Ol-PRX 3 homeodomain are identical to those found in the homeodomain encoded by the OG-12 gene (Fig. 2). This gene was isolated by a screening strategy (using the same oligonucleotidic probe) similar to that reported in this paper (10). In contrast, no closely related homeobox gene was reported in Drosophila. The aristaless gene [Fig. 2 (23)] has the most nearly identical homeobox, but a primary structure highly divergent outside the homeobox and nonhomologous expression domains. Therefore, the question of the evolutionary conservation of OG-12 genes outside the vertebrate phylum remains open.
Figure 2.
Alignments between the putative homeodomain of medaka Ol-PRX 3 and mouse OG-12 (two first lines) and other mouse (upper frame) and Drosophila (lower frame) homeodomains. The amino acid sequence of the previously isolated mouse OG-12, of Prx gene-encoded proteins, and of other classes of homeodomain are presented. Amino acids are in single-letter code. The Ol-Prx 3 sequence is given in the top line and only residues diverging from the Ol-Prx 3 sequence are represented in the following lines. Gene names are given on the left (italics) and percentages of identity on the right (boldface numbers).
Except for the presence of a long polyglycine repeat in the mouse protein, OG-12 proteins exhibit a strikingly high structural similarity (Fig. 1B). No alternative form with 12 additional amino acids such as those found in mice (10) was identified. Similarly, a second type of transcript encoding a shorter protein in the carboxyl-terminal domain was identified in humans (13). No such transcript was detected in medaka. Repeats of such amino acids were found scattered over a large number of homeodomain proteins with apparently no correlation with the species or the family of homeodomain protein (20).
When the mouse protein, with its polyglycine tail deleted, is compared with the fish protein, 72.5% of the amino acids are found to be identical (Fig. 1B). Such a high conservation is not unexpected in homeodomain proteins, and certainly corresponds to the presence of domains with conserved functions. Rovescalli et al. (10) identified sequences forming a P-loop domain (24) and a domain related to SH3-binding domains (25) in the carboxyl-terminal region of the proteins that are also found in the Ol-PRX 3 protein (Fig. 1B).
Ol-PRX 3 Homeodomain Compared with paired-related Homeodomains.
The homeodomain encoded is clearly not a hox/Antp type homeodomain but is related to the fly paired homeodomain (Fig. 2). When examined in the amino-terminal half of the homeodomain, Ol-PRX 3 homeodomain is most closely related to the Pax-3 gene homeodomain (76% amino acids in common). However, in the recognition helix at the carboxyl-terminal part of the homeodomain, a glutamine is found at position 50 instead of a serine in the canonical paired-type genes. This substitution is characteristic of a subclass of paired-type homeobox genes, called paired-related (PRX) genes (26). Only 43 or 44 amino acids of the Ol-PRX 3 homeodomain are identical to the mouse PRX 2 (26) and Phox2 genes (27), respectively. These two genes are the PRX subclass homologs with the highest identity to Ol-PRX 3 in the homeodomain. However, no homology between Ol-PRX 3 and other PRX proteins is found outside of the homeodomain. In conclusion, Ol-Prx 3 belongs to a new class of homeobox genes with loose identity, but still common structural features.
Ol-Prx 3 Expression Pattern in the Adult CNS.
To study the expression of the Ol-Prx 3 gene, two probes (described in Materials and Methods) were used, one containing the homeobox and the other not. They provided identical hybridization patterns at all stages (data not shown). To test for the specificity of the homeobox-containing probe, Northern hybridization was performed. A single transcript size (≈4 kb; as attested from the cDNA lengths) was observed after hybridization of hatching embryo or adult brain total RNAs with the homeobox-containing probe (not shown).
Analysis of serial Vibratome and paraffin-embedded sections of medaka CNS hybridized with digoxigenin probes allowed the identification of most Ol-Prx 3-expressing structures. A description of the main features follows (from rostral to caudal). No staining was ever observed in the telencephalon (including olfactory bulb; Fig. 3 B and I). In the dorsal and medial diencephalon, several nuclei were labeled, most notably the thalamic nuclei centralis posterioris and dorsalis posterioris (Fig. 3 B and C). Some cells expressed Ol-Prx 3 in the hypothalamic nucleus diffusus of the inferior lobe (not shown). The most prominent Ol-Prx 3 expression was found in the dorsal midbrain [periventricular gray zone layer 3 and central zone of the tectum, and layer 2 of the torus semicircularis; Fig. 3 B, D, H, and I]. Other mesencephalic structures were also labeled, albeit less intensively, such as scattered cells in the torus lateralis (Fig. 3C) or in the periventricular central gray (Fig. 3 B and D). Strong expression was also seen in the rhombencephalon, dorsally in scattered cells of the nucleus of the solitary fascicle (Fig. 3 B, F, and I) and ventrally in the reticular formation (Fig. 3 B, E, and F). No expression was detected in the cerebellum (Fig. 3 B, D, and I).
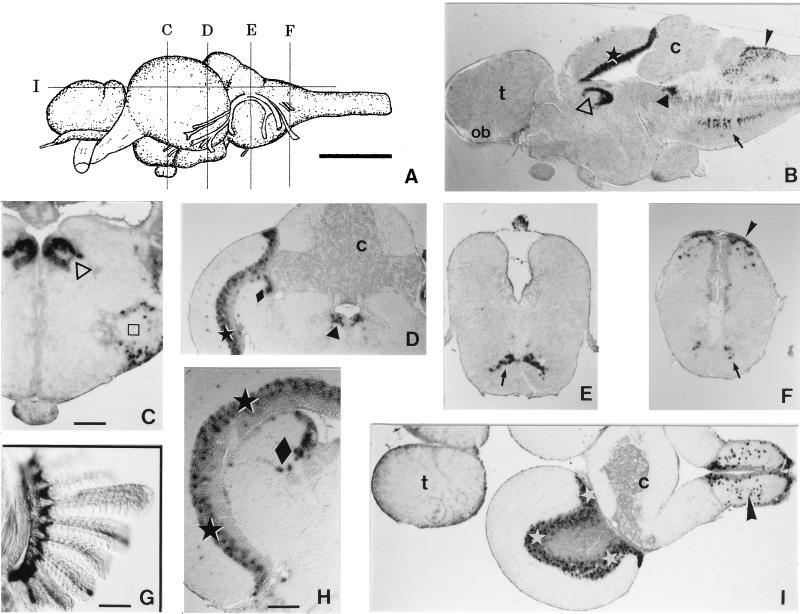
Figure 3.
Localization of Ol-Prx 3 RNA in adults. (A) Lateral view of an adult medaka brain. The approximative levels of sections C–G are indicated. (B) Midsagittal thick (75-μm) Vibratome section of medaka brain, showing the main Ol-Prx 3 expression domains: dorsal thalamus [nuclei centralis posterioris and dorsalis posterioris (▵)], dorsal midbrain [layer PGZ3 of optic tectum ★) and central gray (▴)], dorsal hindbrain [nucleus of the solitary fascicle (➤)], and ventral hindbrain [reticular formation (➞)]. Note that no cell in the telencephalon (t), olfactory bulb (ob), or cerebellum (c) expresses Ol-Prx 3. (C–F) Thin (8-μm) paraffin-embedded sections of adult medaka brain (whole-mount hybridized, cut thereafter and counterstained with nuclear fast red). (C) Expression is detected in the nuclei centralis posterioris and dorsalis posterioris of the dorsal thalamus (▵) and the torus lateralis (□). (D) Expression is seen in the periventricular gray zone layer 3 (PGZ3) and central zone layers of the tectum (★), layer 2 of the torus semicircularis (⧫), and the periventricular central gray (▴). There is no expression in the cerebellum (c). (E) Neurons of the reticular formation express Ol-Prx 3 (➞). (F) Expression is detected in the reticular formation (➞) and the nucleus of the solitary fascicle (➤). (G) Ol-Prx 3 expression persists in elements of the skeletal axis of the adult gill (but not in its epithelium), as seen in this thick Vibratome section. (H) Transverse paraffin-embedded section of the dorsal midbrain, showing the territories where Ol-Prx 3 expression is strongest: patches of cells in the PGZ3 of the tectum (★), and layer 2 of the torus semicircularis (⧫). (I) Horizontal paraffin-embedded section of an adult medaka brain. Expression is strong in the PGZ3 of the tectum (⋆) and the nucleus of the solitary fascicle (➤). The telencephalon (t) and cerebellum (c) show no Ol-Prx 3 expression. (Scale bar in A = 1 mm; in C, 100 μm; D–F same magnification as C; in G, 100 μm; and in H, 60 μm.)
To our knowledge, this expression pattern in medaka CNS is not reminiscent of that of any known homeobox gene of the PRX family. It would therefore be of particular interest to have more data about the expression patterns of Ol-Prx 3 homologs in higher vertebrates.
Ol-Prx 3 Expression Pattern During CNS Development.
By whole-mount in situ hybridization (same probes as described above), we followed Ol-Prx 3 expression in medaka embryos from neurula onwards. The first signal was detected at stage 24 (15 somites; heart beating) in the hindbrain as three bilateral masses of cells (not shown). Quickly after, at stage 25, five (Fig. 4 A, E, and H, arrowheads) such masses of cells located in the laterodorsal aspect of the neural tube, in close vicinity to the otic vesicles, are strongly labeled. This area remains Ol-Prx 3-positive from this stage to the adult, and therefore most likely overlaps the presumptive territory of the nucleus of the solitary fascicle.
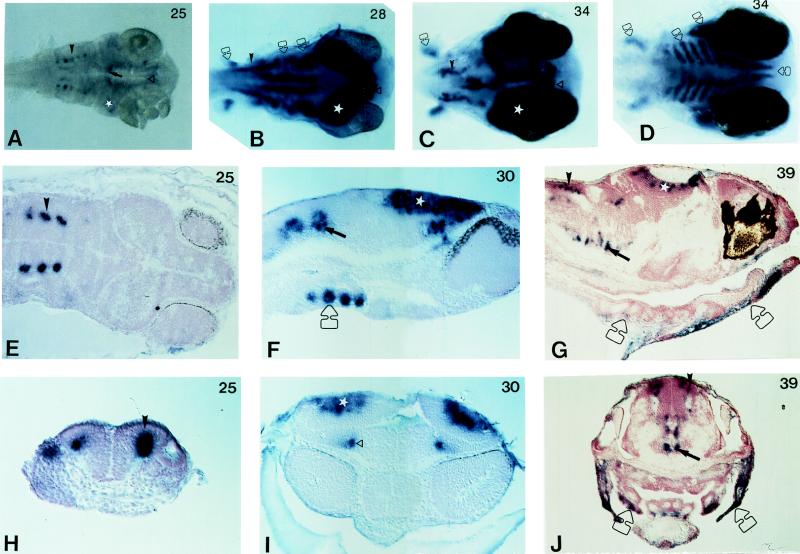
Figure 4.
Localization of Ol-Prx 3 RNA in embryos. Cells expressing Ol-Prx 3 appear in dark blue after an alkaline phosphatase chromogenic reaction. Sections were counterstained with nuclear red and photographed using interference contrast optics. Developmental stages according to Iwamatsu (15) are indicated at top right of each picture. In all panels, corresponding domains of expression are pointed out by a symbol, bilateral structures being marked on only one side of the embryo. ▵, Anterior domain of expression in the diencephalon. ⋆, Prominent staining in the optic tectum. ➞, Ventral CNS signal. ➤, Hindbrain positive cells. Open arrow, chondrifying structures. (A–C) Dorsal views of the anterior regions of whole-mounted embryos, cleared in glycerol/PBS, and from which yolk and yolk sac were dissected. (D) Ventral view from an embryo at the same stage as in C. From left to right, indicated by an open arrow: pectoral fins, branchial arches, opercle precursors, and inferior jaw anlage. (E) Horizontal section. Only three of the five pairs of stained cells are visible on this section, due to the plane of section. (F and G) Parasagittal sections of anterior regions of late somitogenesis and hatching embryos. (H) Transverse section through hindbrain; neural keel is V-shaped. (I) Frontal section. Both anterior domains of expression in the diencephalon are visible. (J) Transverse section through otic vesicles, visible on both sides of the embryo.
At stage 25–26, other domains of Ol-Prx 3 expression become detectable: (i) two stripes in the ventral aspect of the rhombencephalon/caudal mesencephalon (Fig. 4, arrows), which might represent the developing reticular formation; (ii) a strongly positive domain in the dorsal midbrain area (Fig. 4, stars). We suggest that this latter domain corresponds to the developing tectum and torus semicircularis. Interestingly, at this and later embryonic stages, the label encompasses most of the thickness of the tectum (Fig. 4 F and I). Therefore, Ol-Prx 3 could provide an useful marker to follow the ontogenesis of the dorsal mesencephalon, especially the neuronal migrations in this area. (iii) Two patches of strongly labeled cells are found in the dorsal thalamic territory, between the optic vesicles (Fig. 4). They probably correspond to the developing nuclei centralis posterioris and dorsalis posterioris.
From stage 28 onwards (and up to adult stage), Ol-Prx 3 expression remains strong and strictly limited to these areas. No transient domain of expression was ever detected.
To summarize, the expression of this gene is essentially monophasic, several CNS domains being turned on one after the other, and remaining positive up to the adult stage. This type of expression is rather surprising, because most vertebrate homeobox genes have several phases of expression in the CNS [for example, refer to the even-skipped family of homeobox genes, (28)]. The molecular mechanisms at work to sustain such long waves of Ol-Prx 3 expression in medaka brain remain to be elucidated. Genes involved in the long-term maintenance of homeobox gene expression in Drosophila have been isolated (29), and it would be particularly interesting to test whether any vertebrate homologs of these gene families are also found to act during medaka CNS development.
Given the paucity of data on late stages of neural development in teleost fishes on one hand, and the continuity of its expression pattern on the other hand, Ol-Prx 3 can thus allow molecular identification of specific neuronal territories in a variety of experimental situations.
Ol-Prx 3 Expression Pattern Outside the CNS.
By whole-mount in situ hybridization at various embryonic stages, Ol-Prx 3 was detected outside the CNS in a variety of chondrifying structures, identified by comparison with previous descriptions of these structures (30). Most prominent is its expression in developing head cartilage: axis of branchial arches, opercles, and mandible (Fig. 4, open arrows). Thus, we propose that Ol-Prx 3 could also be used as a molecular marker to follow chondrification and differentiation of part of the head skeleton in medaka. Outside the head, the only domain of expression was found to be cartilaginous structures in the pectoral fin anlagen (open arrows in Fig. 3 B, C, and D). We have not analyzed in detail adult Ol-Prx 3 expression outside the CNS, but partial results indicate that it persists at least in the axis of the gills (Fig. 3G).
Whereas Ol-Prx 3 expression in the CNS does not resemble that of other known PRX homeobox genes, its pattern in nonneural structures, and notably in cartilages of the branchial arches and pectoral fins, is reminiscent of that of the PRX family (26). Together with the fact that homeoboxes of these genes are homologous, this indicates that this latter family and the OG-12/Ol-Prx 3 family of homeobox genes might be implicated in common regulatory pathways during the development of the head/fin cartilage. Indeed, it is clear from knock-out data in mice, that MHox, one member of the PRX family, is involved in these developmental events (31). In contrast, these genes appear to be involved in the ontogenesis of wholly different structures in the neuraxis. These speculative points of course await the availability of loss- or gain-of-function mutants in different vertebrate model species. In this respect, it is interesting to note that a recently isolated class of zebrafish mutants (32, 33) exhibits defects both in head/fin cartilaginous structures and in dorsal midbrain [detected by aberrant axonal migration (34) or tectum defects (32)]. It will be of major interest to test whether some of these lines bear mutations at loci corresponding to the OG-12/Ol-Prx 3 gene family.
Acknowledgments
We acknowledge Prof. A. Shima and Y. Hyodo-Taguchi for the kind gift of medaka strains, Vincent Galy for help with sequence analysis, and Pascal Lafaux and Marc Vandeputte for skillful maintenance of the fish facility. This work was supported by the Institut National de la Recherche Agronomique, by the Institut National de la Santé et de la Recherche Médicale, and by the European Commission (Biotechnology Program, Contract BI02-CT93-0430).
ABBREVIATION
- CNS
central nervous system
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF001393).
References
- 1.Ozato K, Wakamatsu Y. Dev Growth Differ. 1994;36:437–443. doi: 10.1111/j.1440-169X.1994.00437.x. [DOI] [PubMed] [Google Scholar]
- 2.Hyodo-Taguchi Y, Sakaizumi M. Fish Biol J Medaka. 1993;5:29–30. [Google Scholar]
- 3.Wada H, Naruse K, Shimada A, Shima A. Mol Mar Biol Biotech. 1995;4:269–274. [PubMed] [Google Scholar]
- 4.Ozato K, Kondoh H, Inohara H, Iwamatsu T, Wakamatsu Y, Okada T S. Cell Differ. 1986;19:237–244. doi: 10.1016/0045-6039(86)90100-4. [DOI] [PubMed] [Google Scholar]
- 5.Hong Y, Schartl M. Mol Mar Biol Biotech. 1996;5:93–104. [Google Scholar]
- 6.Hong Y, Winkler C, Schartl M. Mech Dev. 1996;60:33–44. doi: 10.1016/s0925-4773(96)00596-5. [DOI] [PubMed] [Google Scholar]
- 7.Koga A, Suzuki M, Inagaki H, Bessho Y, Hori H. Nature (London) 1996;383:30. doi: 10.1038/383030a0. [DOI] [PubMed] [Google Scholar]
- 8.Bürglin T R, Finney M, Coulson A, Ruvkun G. Nature (London) 1989;341:239–243. doi: 10.1038/341239a0. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg B, Wright C V E, De Robertis E M, Cho K W Y. Science. 1991;253:194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- 10.Rovescalli A C, Asoh S, Nirenberg M. Proc Natl Acad Sci USA. 1996;93:10691–10696. doi: 10.1073/pnas.93.20.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brickell P M. BioEssays. 1995;17:750–753. doi: 10.1002/bies.950170903. [DOI] [PubMed] [Google Scholar]
- 12.Anken R H, Bourrat F. Brain Atlas of the Medakafish, Oryzias latipes. Paris: Institut National de la Recherche Agronomique; 1997. [Google Scholar]
- 13.Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, Muroya K, Binder G, Kirsch S, Winkelmann M, Nordsiek G, Heinrich U, Breuning M H, Ranke M B, Rosenthal A, Ogata T, Rappold G A. Nat Genet. 1997;16:54–62. doi: 10.1038/ng0597-54. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T. Medaka (Killifish), Biology and Strains. Tokyo: Keigaku; 1975. [Google Scholar]
- 15.Iwamatsu T. Zool Sci. 1994;11:825–839. [Google Scholar]
- 16.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 18.Joly J S, Joly C, Schulte-Merker S, Boulekbache H, Condamine H. Development. 1993;119:1261–1275. doi: 10.1242/dev.119.4.1261. [DOI] [PubMed] [Google Scholar]
- 19.Kozak M. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 20.Bürglin T R. In: Guidebook to the Homeobox Genes. Duboule D, editor. Oxford: Oxford Univ. Press; 1994. pp. 27–71. [Google Scholar]
- 21.Sutcliffe J G. Annu Rev Neurosci. 1988;11:157–198. doi: 10.1146/annurev.ne.11.030188.001105. [DOI] [PubMed] [Google Scholar]
- 22.Tanguay R L, Gallie D R. Mol Cell Biol. 1996;16:146–156. doi: 10.1128/mcb.16.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneitz K, Spielmann P, Noll M. Genes Dev. 1993;7:114–129. doi: 10.1101/gad.7.1.114. [DOI] [PubMed] [Google Scholar]
- 24.Saraste M, Sibbald P R, Wittinghofer A. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 25.Ren R, Mayer B, Cicchetti P, Baltimore D. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 26.Leussink B, Brouwer A, El Khattabi M, Poelmann R E, Gittenberg-de Groot A C, Meijlink F. Mech Dev. 1995;52:1–14. doi: 10.1016/0925-4773(95)00389-i. [DOI] [PubMed] [Google Scholar]
- 27.Valarche I, Tissier-Seta J P, Hirsch M R, Martinez S, Goridis C, Brunet J F. Development. 1993;119:881–896. doi: 10.1242/dev.119.3.881. [DOI] [PubMed] [Google Scholar]
- 28.Bastian H, Gruss P. EMBO J. 1990;9:1839–1852. doi: 10.1002/j.1460-2075.1990.tb08309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moazed D, O’Farrell P H. Development. 1992;116:805–810. doi: 10.1242/dev.116.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langille R M, Hall B K. J Morphol. 1987;193:135–158. doi: 10.1002/jmor.1051930203. [DOI] [PubMed] [Google Scholar]
- 31.Martin J F, Bradley A, Olson E N. Genes Dev. 1995;9:1237–1249. doi: 10.1101/gad.9.10.1237. [DOI] [PubMed] [Google Scholar]
- 32.Neuhauss S C, Solnica K L, Schier A F, Zwartkruis F, Stemple D L, Malicki J, Abdelilah S, Stainier D Y, Driever W. Development. 1996;123:357–367. doi: 10.1242/dev.123.1.357. [DOI] [PubMed] [Google Scholar]
- 33.Schilling T F, Piotrowski T, Grandel H, Brand M, Heisenberg C P, Jiang Y J, Beuchle D, Hammerschmidt M, Kane D A, Mullins M C, van Eeden E F, Kelsh R N, Furutani S M, Granato M, Haffter P, Odenthal J, Warga R M, Trowe T, Nüsslein-Volhard C. Development. 1996;123:329–344. doi: 10.1242/dev.123.1.329. [DOI] [PubMed] [Google Scholar]
- 34.Karlstrom R O, Trowe T, Klostermann S, Baier H, Brand M, Crawford A D, Grunewald B, Haffter P, Hoffmann H, Meyer S U, Muller B K, Richter S, van Eeden E F, Nüsslein-Volhard C, Bonhoeffer F. Development. 1996;123:427–438. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]