Abstract
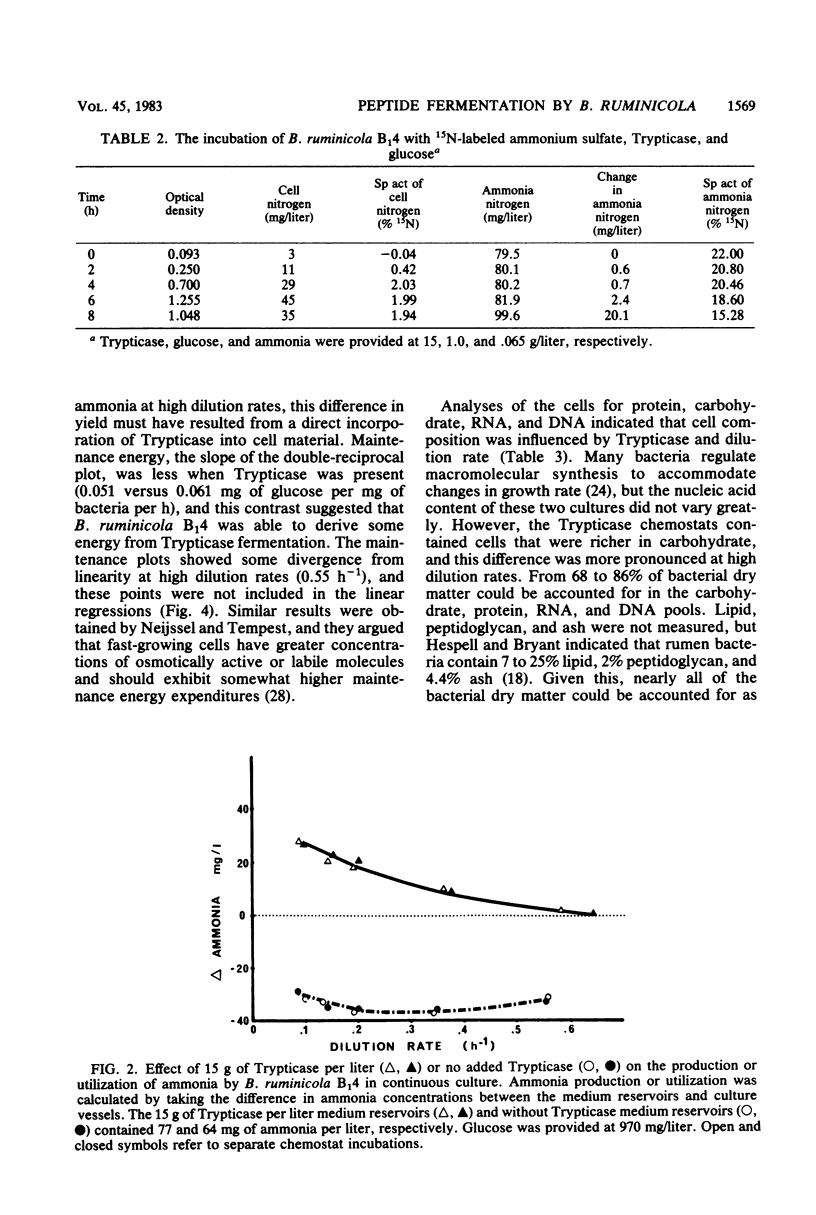
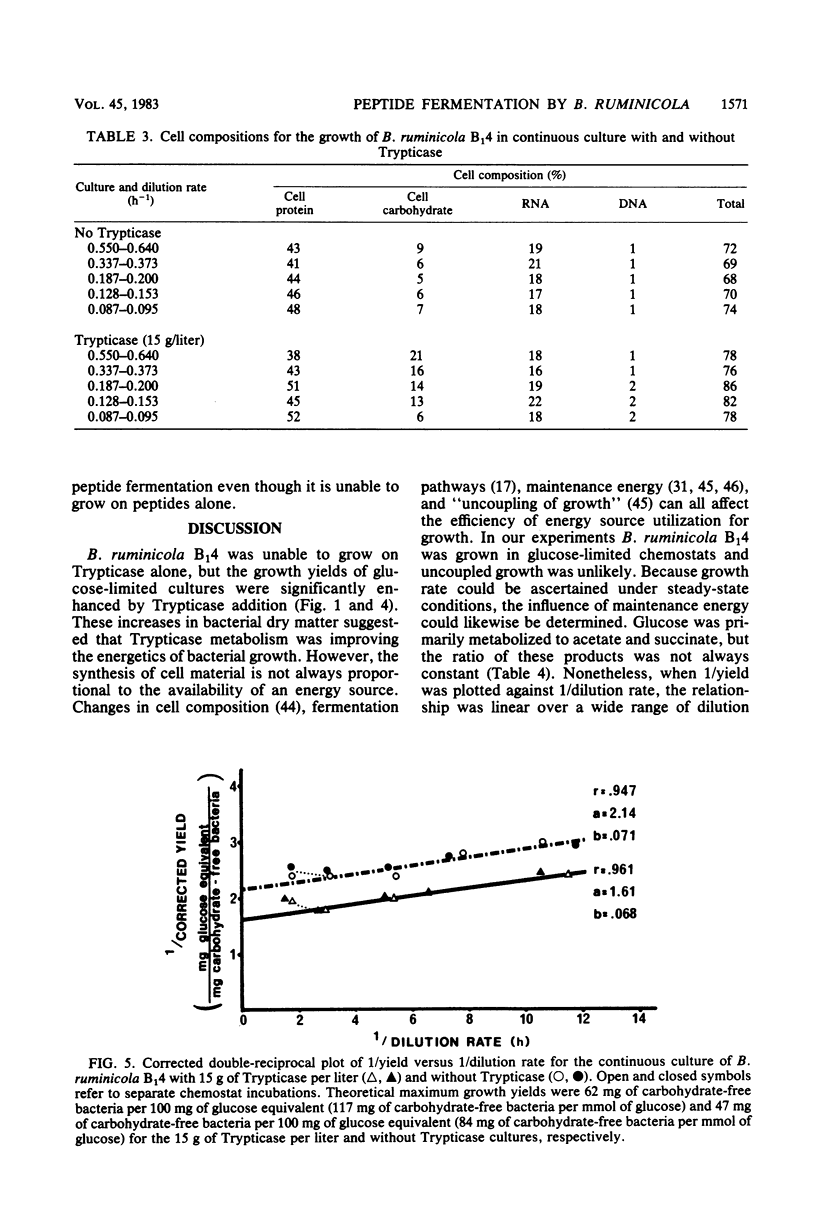
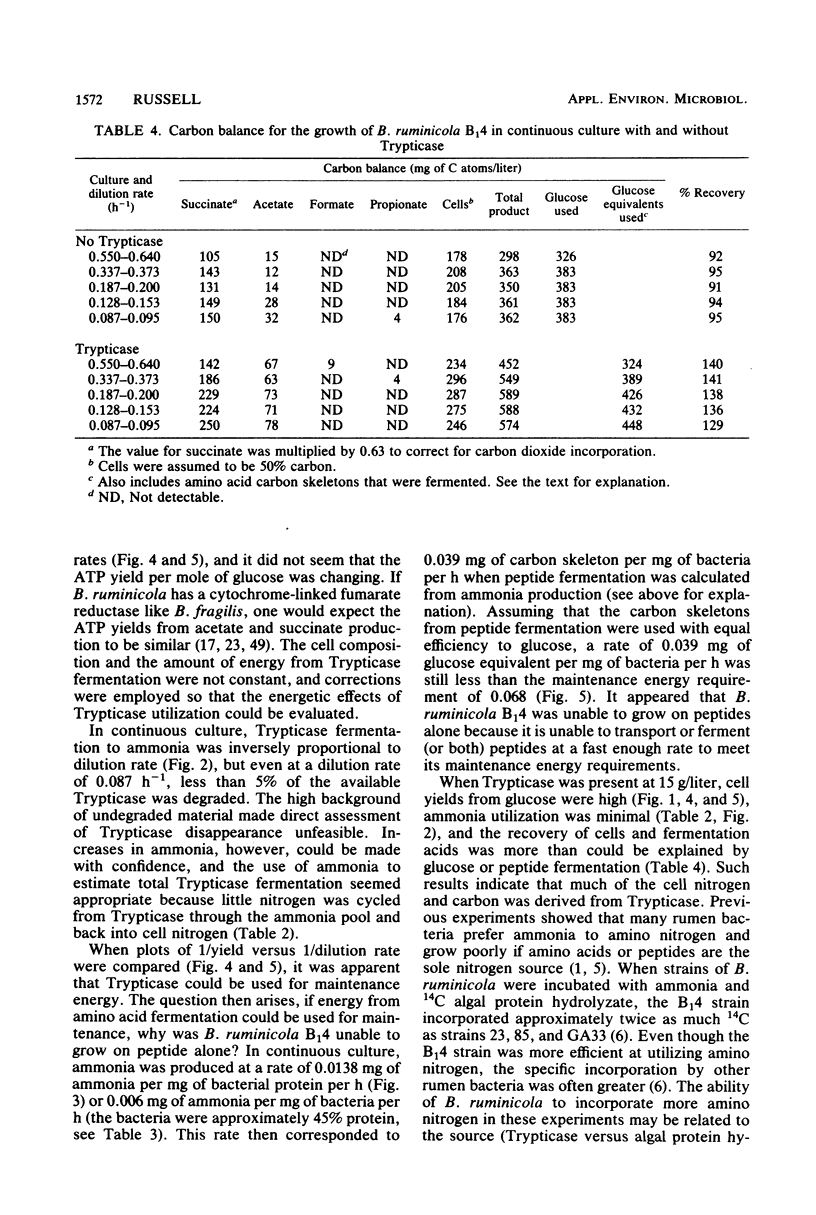
The maximum growth rate of Bacteroides ruminicola B14 was significantly improved when either Trypticase or acetate and C4-C5 fatty acids were added to defined medium containing macrominerals, microminerals, vitamins, hemin, cysteine hydrochloride, and glucose. The organism was unable to grow with peptides as the sole energy source, but growth yields from glucose were significantly improved when Trypticase was added to batch cultures containing basal medium, acetate, and C4-C5 volatile fatty acids. During periods of rapid growth, very little peptide was deaminated to ammonia, but after growth ceased there was a linear increase in ammonia. Fifteen grams of Trypticase per liter resulted in maximum ammonia production. In glucose-limited chemostats, ammonia production from peptides was inversely proportional to the dilution rate, and 87% of the variation in ammonia production could be explained by retention time in the culture vessel. Chemostats receiving Trypticase had higher theoretical maximum growth yields and lower maintenance energy expenditures than similar cultures not receiving peptide. Cells from the Trypticase cultures contained more carbohydrate, and this difference was most evident at rapid dilution rates. When corrections were made for cell composition and the amount of peptides that were fermented, it appeared that peptide carbon skeletons could be used for maintenance energy. B. ruminicola B14 was unable to grow on peptides alone because it was unable to utilize peptides at a fast enough rate to meet its maintenance requirement.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F. Nitrogen metabolism in the sheep; protein digestion in the rumen. Biochem J. 1956 Dec;64(4):705–714. doi: 10.1042/bj0640705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Studies on the Nitrogen Requirements of Some Ruminal Cellulolytic Bacteria. Appl Microbiol. 1961 Mar;9(2):96–103. doi: 10.1128/am.9.2.96-103.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Characterization of several bovine rumen bacteria isolated with a xylan medium. J Bacteriol. 1966 May;91(5):1724–1729. doi: 10.1128/jb.91.5.1724-1729.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan J. P. Quantitative aspects of nitrogen utilization in ruminants. J Dairy Sci. 1975 Aug;58(8):1164–1177. doi: 10.3168/jds.S0022-0302(75)84695-9. [DOI] [PubMed] [Google Scholar]
- Howlett M. R., Mountfort D. O., Turner K. W., Roberton A. M. Metabolism and growth yields in Bacteroides ruminicola strain b14. Appl Environ Microbiol. 1976 Aug;32(2):274–283. doi: 10.1128/aem.32.2.274-283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macy J., Probst I., Gottschalk G. Evidence for cytochrome involvement in fumarate reduction and adenosine 5'-triphosphate synthesis by Bacteroides fragilis grown in the presence of hemin. J Bacteriol. 1975 Aug;123(2):436–442. doi: 10.1128/jb.123.2.436-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDONALD I. W. The role of ammonia in ruminal digestion of protein. Biochem J. 1952 Apr;51(1):86–90. doi: 10.1042/bj0510086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink R. W., Hespell R. B. Long-term nutrient starvation of continuously cultured (glucose-limited) Selenomonas ruminantium. J Bacteriol. 1981 Nov;148(2):541–550. doi: 10.1128/jb.148.2.541-550.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Roberton A. M. Origins of fermentation products formed during growth of Bacteroides ruminicola on glucose. J Gen Microbiol. 1978 Jun;106(2):353–360. doi: 10.1099/00221287-106-2-353. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol. 1976 Mar 19;107(2):215–221. doi: 10.1007/BF00446843. [DOI] [PubMed] [Google Scholar]
- PITTMAN K. A., BRYANT M. P. PEPTIDES AND OTHER NITROGEN SOURCES FOR GROWTH OF BACTEROIDES RUMINICOLA. J Bacteriol. 1964 Aug;88:401–410. doi: 10.1128/jb.88.2.401-410.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter A. P., Hatfield E. E., Owens F. N., Garrigus U. S. Effects of aldehyde treatment of soybean meal on in vitro ammonia release, solubility and lamb performance. J Nutr. 1971 May;101(5):605–611. doi: 10.1093/jn/101.5.605. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Pittman K. A., Lakshmanan S., Bryant M. P. Oligopeptide uptake by Bacteroides ruminicola. J Bacteriol. 1967 May;93(5):1499–1508. doi: 10.1128/jb.93.5.1499-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl Environ Microbiol. 1979 Mar;37(3):537–543. doi: 10.1128/aem.37.3.537-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of substrate affinities among several rumen bacteria: a possible determinant of rumen bacterial competition. Appl Environ Microbiol. 1979 Mar;37(3):531–536. doi: 10.1128/aem.37.3.531-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter L. D., Roffler R. E. Nitrogen requirement and utilization in dairy cattle. J Dairy Sci. 1975 Aug;58(8):1219–1237. doi: 10.3168/jds.S0022-0302(75)84698-4. [DOI] [PubMed] [Google Scholar]
- Schaefer D. M., Davis C. L., Bryant M. P. Ammonia saturation constants for predominant species of rumen bacteria. J Dairy Sci. 1980 Aug;63(8):1248–1263. doi: 10.3168/jds.S0022-0302(80)83076-1. [DOI] [PubMed] [Google Scholar]
- Scheifinger C., Russell N., Chalupa W. Degradation of amino acids by pure cultures of rumen bacteria. J Anim Sci. 1976 Oct;43(4):821–827. doi: 10.2527/jas1976.434821x. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- TAGARI H., ASCARELLI I., BONDI A. The influence of heating on the nutritive value of soya-bean meal for ruminants. Br J Nutr. 1962;16:237–243. doi: 10.1079/bjn19620025. [DOI] [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallnöfer P., Baldwin R. L. Pathway of propionate formation in Bacteroides ruminicola. J Bacteriol. 1967 Jan;93(1):504–505. doi: 10.1128/jb.93.1.504-505.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



