Abstract
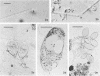
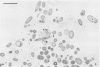
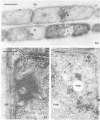
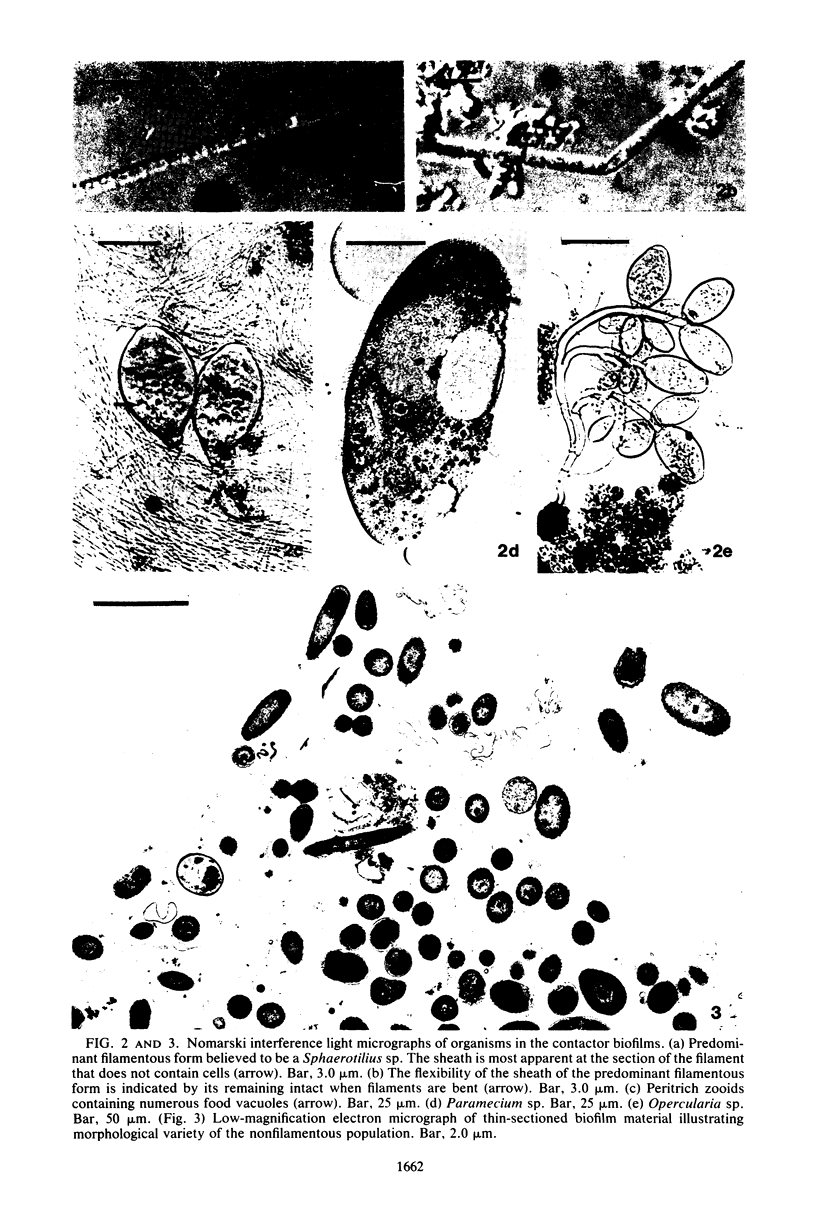
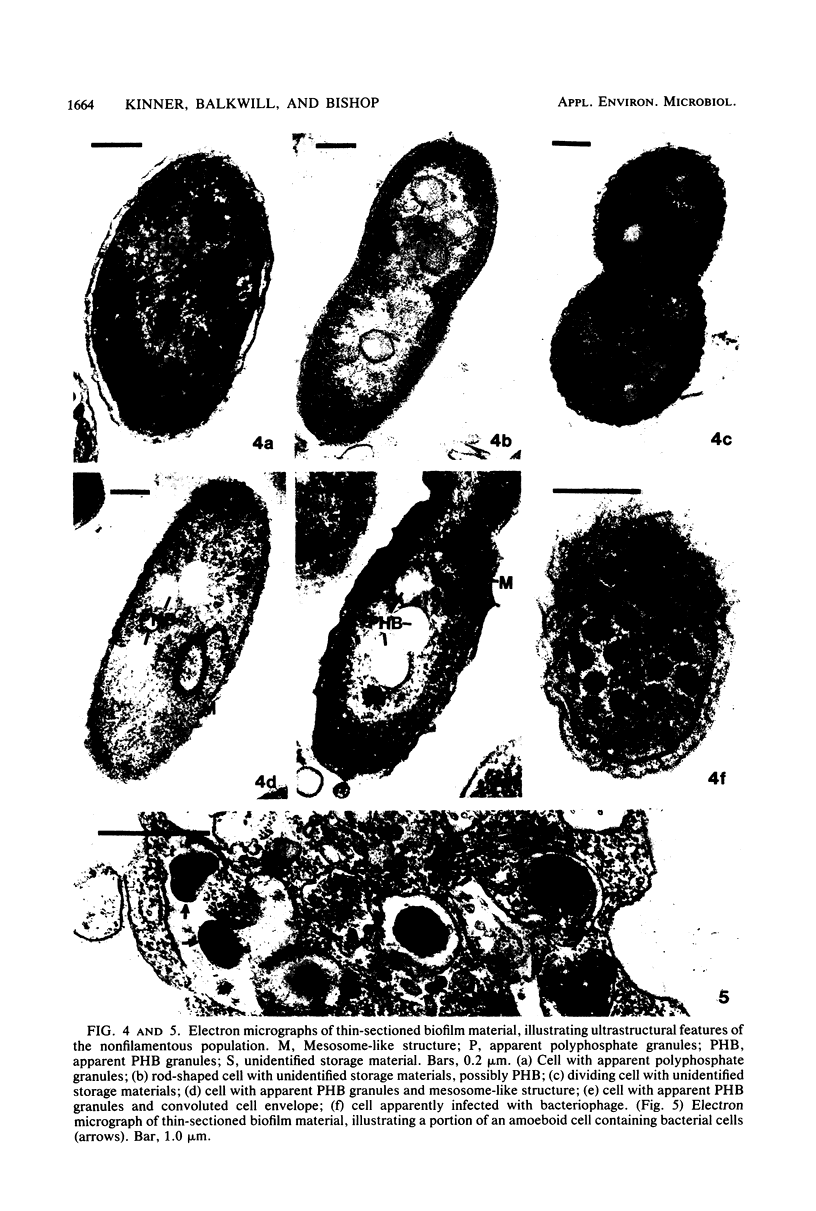
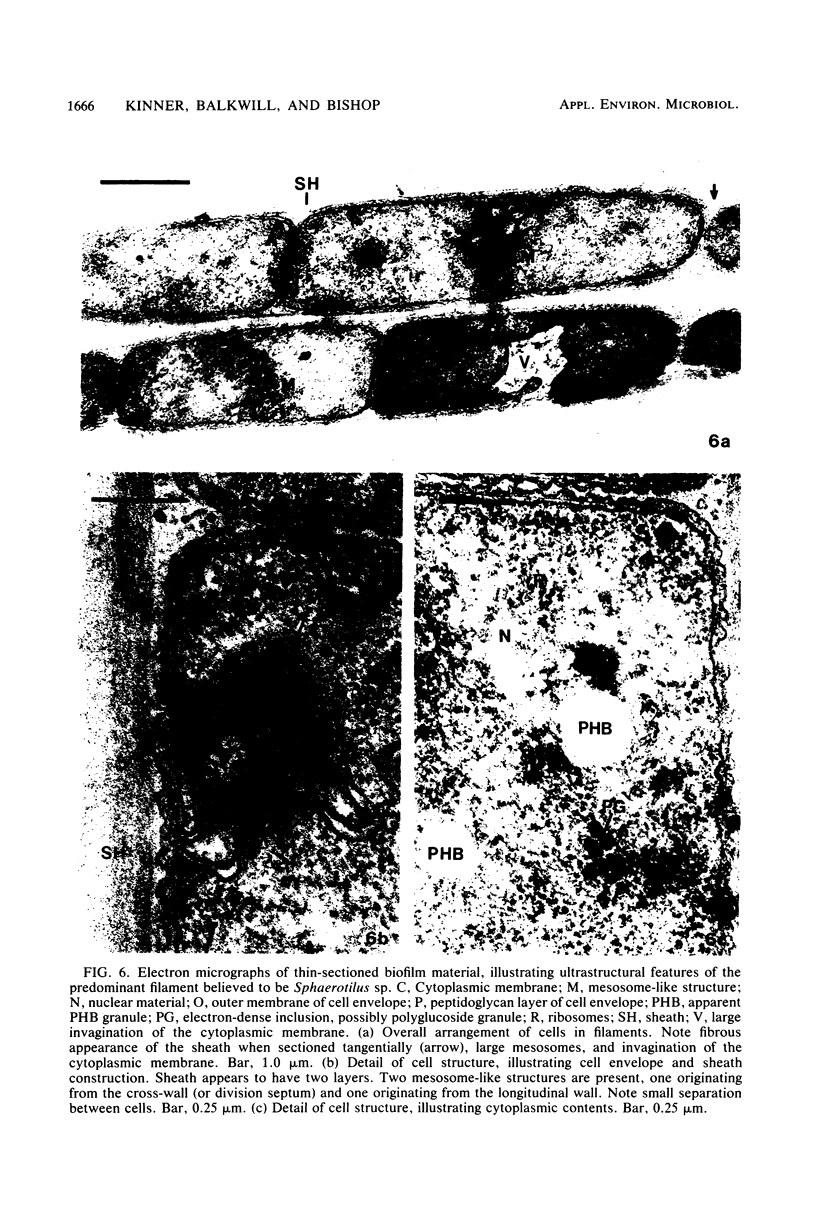
The biofilms growing in the first compartments of two rotating biological contactors used to treat municipal wastewater were examined by light and electron microscopy. The biofilms were found to contain a complex and varied microbial community that included filamentous and unicellular bacteria, protozoa, metazoa, and (possibly) bacteriophage. The predominant microorganism among these appeared to be a filamentous bacterium that was identical to Sphaerotilus in both morphological and ultrastructural characteristics. It was possible to isolate a Sphaerotilus-like bacterium from each contactor. Both the Sphaerotilus filaments and the wide variety of unicellular bacteria present tended to contain poly-β-hydroxybutyrate inclusions, a probable indication that these organisms were removing carbon from the wastewater and storing it. The microbial population of the biofilms appeared to be metabolically active, as evidenced by the presence of microcolonies and dividing cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRABTREE K., MCCOY E., BOYLE W. C., ROHLICH G. A. ISOLATION, IDENTIFICATION, AND METABOLIC ROLE OF THE SUDANOPHILIC GRANULES OF ZOOGLOEA RAMIGERA. Appl Microbiol. 1965 Mar;13:218–226. doi: 10.1128/am.13.2.218-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONDERO N. C., PHILLIPS R. A., HEUKELEKIAN H. Isolation and preservation of cultures of Sphaerotilus. Appl Microbiol. 1961 May;9:219–227. doi: 10.1128/am.9.3.219-227.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONDERO N. C. Sphaerotilus, its nature and economic significance. Adv Appl Microbiol. 1961;3:77–107. doi: 10.1016/s0065-2164(08)70507-0. [DOI] [PubMed] [Google Scholar]
- Dias F. F., Dondero N. A., Finstein M. S. Attached growth of Sphaerotilus and mixed populations in a continuous-flow apparatus. Appl Microbiol. 1968 Aug;16(8):1191–1199. doi: 10.1128/am.16.8.1191-1199.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondero N. C. The Sphaerotilus-Leptothrix group. Annu Rev Microbiol. 1975;29:407–428. doi: 10.1146/annurev.mi.29.100175.002203. [DOI] [PubMed] [Google Scholar]
- Dunlop W. F., Robards A. W. Ultrastructural study of poly- -hydroxybutyrate granules from Bacillus cereus. J Bacteriol. 1973 Jun;114(3):1271–1280. doi: 10.1128/jb.114.3.1271-1280.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T., Yoshimoto A., Matsumoto M., Hosokawa S., Saito T. Enzymatic synthesis of poly-beta-hydroxybutyrate in Zoogloea ramigera. Arch Microbiol. 1976 Nov 2;110(23):149–156. doi: 10.1007/BF00690222. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W., Whiteside T. L. Mesosomes: membranous bacterial organelles. Bacteriol Rev. 1975 Dec;39(4):405–463. doi: 10.1128/br.39.4.405-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeniger J. F., Tauschel H. D., Stokes J. L. The fine structure of Sphaerotilus natans. Can J Microbiol. 1973 Mar;19(3):309–313. doi: 10.1139/m73-051. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULDER E. G., VAN VEENW INVESTIGATIONS ON THE SPHAEROTILUSLEPTOTHRIX GROUP. Antonie Van Leeuwenhoek. 1963;29:121–153. doi: 10.1007/BF02046045. [DOI] [PubMed] [Google Scholar]
- Parsons A. B., Dugan P. R. Production of extracellular polysaccharide matrix by Zoogloea ramigera. Appl Microbiol. 1971 Apr;21(4):657–661. doi: 10.1128/am.21.4.657-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitprez M., Leclerc H. Les baéries du group Sphaerotilus-Leptothrix. Ann Inst Pasteur Lille. 1969;20:115–140. [PubMed] [Google Scholar]
- Petitprez M., Petitprez A., Leclerc H., Vivier E. Quelques aspects structuraux de Sphaerotilus natans. Ann Inst Pasteur Lille. 1969;20:103–113. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUF M. A., STOKES J. L. Isolation and identification of the sudanophilic granules of Sphaerotilus natans. J Bacteriol. 1962 Feb;83:343–347. doi: 10.1128/jb.83.2.343-347.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKES J. L. Studies on the filamentous sheathed iron bacterium Sphaerotilus natans. J Bacteriol. 1954 Mar;67(3):278–291. doi: 10.1128/jb.67.3.278-291.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Beech G. A., Ritchie G. A., Dawes E. A. The role of oxygen limitation in the formation of poly- -hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem J. 1972 Aug;128(5):1193–1201. doi: 10.1042/bj1281193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J. 1973 May;134(1):225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stokes J. L., Parson W. L. Role of poly-beta-hydroxybutyrate in survival of Sphaerotilus discophorus during starvation. Can J Microbiol. 1968 Jul;14(7):785–789. doi: 10.1139/m68-130. [DOI] [PubMed] [Google Scholar]
- Taber W. A. Wastewater microbiology. Annu Rev Microbiol. 1976;30:263–277. doi: 10.1146/annurev.mi.30.100176.001403. [DOI] [PubMed] [Google Scholar]
- Torpey W. N., Heukelekian H., Kaplowsky A. J., Epstein R. Rotating disks with biological growths prepare wastewater for disposal or reuse. J Water Pollut Control Fed. 1971 Nov;43(11):2181–2188. [PubMed] [Google Scholar]
- van Veen W. L., Mulder E. G., Deinema M. H. The Sphaerotilus-Leptothrix group of bacteria. Microbiol Rev. 1978 Jun;42(2):329–356. doi: 10.1128/mr.42.2.329-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]