Abstract
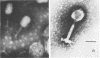
Two Aeromonas hydrophila bacteriophages, Aeh1 and Aeh2, were isolated from sewage. Both phages showed binal symmetry. The dimensions of A. hydrophila phages Aeh1 and Aeh2 differed from those of the other Aeromonas phages. Also, phage Aeh2 was the largest Aeromonas phage studied to date. Phage Aeh1 formed small, clear plaques, and phage Aeh2 formed turbid plaques with clear centers. Both phages were sensitive to chloroform treatment, being totally inactivated after treatment for 1 h at 60°C at pH 3 and 11. However, the infectivity of Aeh1 phage stocks increased by approximately fivefold after they were treated at pH 10 for 1 h at 22°C. Phages Aeh1 and Aeh2 were serologically unrelated and had latent periods of 39 and 52 min, respectively. The average burst sizes of phages Aeh1 and Aeh2 were 17 and 92 PFU per cell, respectively. Phage Aeh1 infected 13 of 22 A. hydrophila strains tested, whereas phage Aeh2 infected only its original host. Phage Aeh1 infected some A. hydrophila strains only at or below 37°C. Neither phage infected the two A. (Plesiomonas) shigelloides strains used in this study.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann H. W., Audurier A., Berthiaume L., Jones L. A., Mayo J. A., Vidaver A. K. Guidelines for bacteriophage characterization. Adv Virus Res. 1978;23:1–24. doi: 10.1016/s0065-3527(08)60096-2. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMAN H. A., WANG S. S. Sensitivity of various viruses to chloroform. Proc Soc Exp Biol Med. 1961 Apr;106:736–738. doi: 10.3181/00379727-106-26459. [DOI] [PubMed] [Google Scholar]
- Gibbs E. L., Gibbs T. J., Van Dyck P. C. Rana pipiens: health and disease. Lab Anim Care. 1966 Apr;16(2):142–160. [PubMed] [Google Scholar]
- Hazen T. C., Fliermans C. B., Hirsch R. P., Esch G. W. Prevalence and distribution of Aeromonas hydrophila in the United States. Appl Environ Microbiol. 1978 Nov;36(5):731–738. doi: 10.1128/aem.36.5.731-738.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouf M. A., Rigney M. M. Growth temperatures and temperature characteristics of Aeromonas. Appl Microbiol. 1971 Oct;22(4):503–506. doi: 10.1128/am.22.4.503-506.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R. Der Nachweis von Aeromonaden der "Hydrophila-Punctata-Gruppe" im Rahmen der hygienischen Trinkwasserbeurteilung. Zentralbl Bakteriol Orig B. 1976 Mar;161(5-6):482–497. [PubMed] [Google Scholar]
- Shotts E. B., Jr, Gaines J. L., Jr, Martin L., Prestwood A. K. Aeromonas-induced deaths among fish and reptiles in an eutrophic inland lake. J Am Vet Med Assoc. 1972 Sep 15;161(6):603–607. [PubMed] [Google Scholar]
- Von Graevenitz A., Mensch A. H. The genus aeromonas in human bacteriology report of 30 cases and review of the literature. N Engl J Med. 1968 Feb 1;278(5):245–249. doi: 10.1056/NEJM196802012780504. [DOI] [PubMed] [Google Scholar]