Abstract
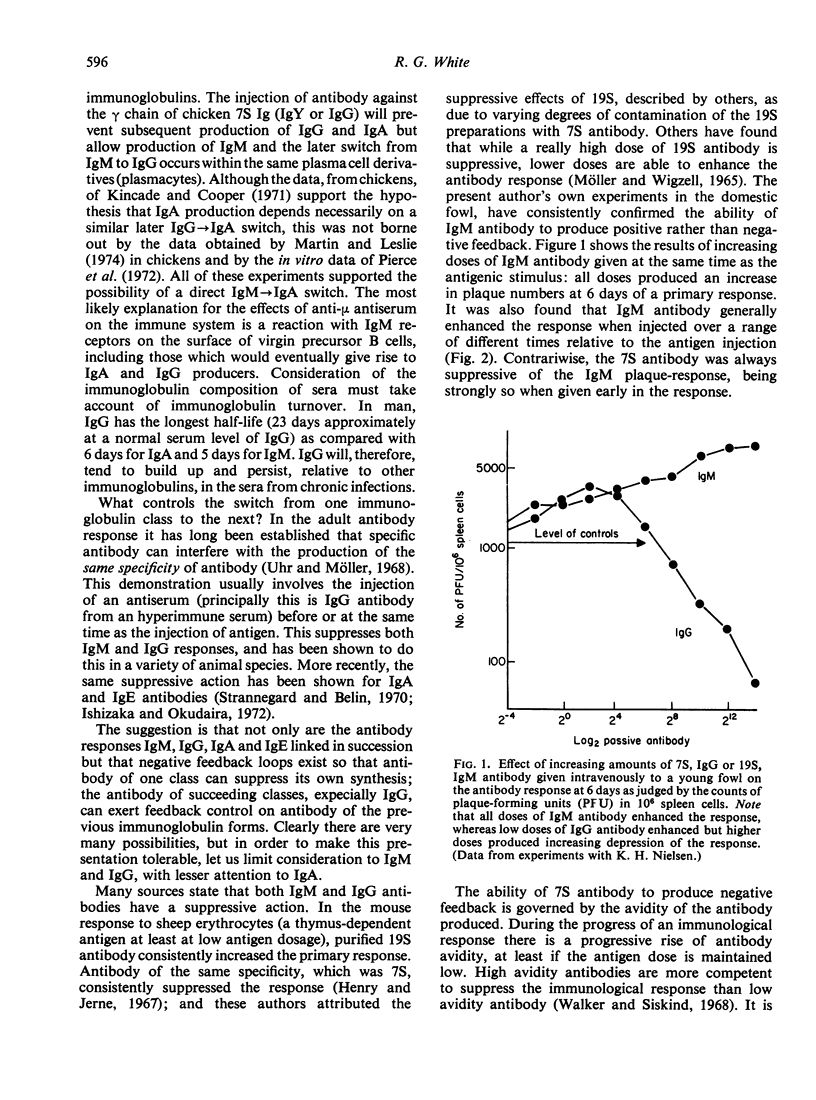
The use of a sensitive and quantitative radioimmunoassay has enabled much finer dissection of the immunoglobulin-antibody profiles for an individual immune response. The kinetics of the response are considered in relation to the switch from IgM to IgG antibody production. In the domestic fowl, the kinetics of this switch varied with different antigens: whereas the response to a thymus-dependent antigen proceeded through a brief 19S response to a declining 7S response, the response to a thymus-independent antigen failed to switch from 19S to 7S for several weeks and consisted of repeated excursions of 19S antibodies. When injected intravenously and simultaneously, Salmonella adelaide O (killed) organisms (thymus-independent) and sheep red cells (thymus-dependent) interact so that the response to the latter fails to switch from 19S to 7S and consists of repeated excursions of 19S antibody. The changed character of the sheep red cell response is interpreted as being due to lack of 7S antibody. Passive antibody to either sheep red cells or to S. adelaide produced an inhibition of the sheep red rell response so that only one excursion of 19S antibody was observed.
The use of the radio-immunoassay enables an independent measurement of all IgM, IgG and IgA antibody to the surface antigen of Brucella obortus. The test, when applied to forty-six sera from individuals with various types of brucellosis, successfully detected antibody in many instances in which conventional serological tests were negative, and such antibody (if IgM) was associated with acute or (if IgG or IgA) with chronic cases of brucellosis. The radioassay test should prove highly valuable effectively to eliminate, in individual patients, the diagnosis of brucellosis based on the inability of conventional tests to detect significant antibody levels.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. K., Jenness R., Brumfield H. P., Gough P. Brucella-Agglutinating Antibodies: Relation of Mercaptoethanol Stability to Complement Fixation. Science. 1964 Mar 20;143(3612):1334–1335. doi: 10.1126/science.143.3612.1334. [DOI] [PubMed] [Google Scholar]
- Coghlan J. D., Weir D. M. Antibodies in human brucellosis. Br Med J. 1967 Apr 29;2(5547):269–271. doi: 10.1136/bmj.2.5547.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Okudaira H. Reaginic antibody formation in the mouse. I. Antibody-mediated suppression of reaginic antibody formation. J Immunol. 1972 Jul;109(1):84–89. [PubMed] [Google Scholar]
- Kerr W. R., Coghlan J. D., Payne D. J., Robertson L. The laboratory diagnosis of chronic brucellosis. Lancet. 1966 Nov 26;2(7474):1181–1183. doi: 10.1016/s0140-6736(66)90492-2. [DOI] [PubMed] [Google Scholar]
- Kerr W. R., McCaughey W. J., Coghlan J. D., Payne D. J., Quaife R. A., Robertson L., Farrell I. D. Techniques and interpretations in the serological diagnosis of brucellosis in man. J Med Microbiol. 1968 Nov;1(2):181–193. doi: 10.1099/00222615-1-2-181. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Cooper M. D. Development and distribution of immunoglobulin-containing cells in the chicken. An immunofluorescent analysis using purified antibodies to mu, gamma and light chains. J Immunol. 1971 Feb;106(2):371–382. [PubMed] [Google Scholar]
- Kincade P. W., Lawton A. R., Bockman D. E., Cooper M. D. Suppression of immunoglobulin G synthesis as a result of antibody-mediated suppression of immunoglobulin M synthesis in chickens. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1918–1925. doi: 10.1073/pnas.67.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLER G., WIGZELL H. ANTIBODY SYNTHESIS AT THE CELLULAR LEVEL. ANTIBODY-INDUCED SUPPRESSION OF 19S AND 7S ANTIBODY RESPONSE. J Exp Med. 1965 Jun 1;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning D. D., Jutila J. W. Immunosuppression in mice injected with heterologous anti-immunoglobulin antisera. J Immunol. 1972 Jan;108(1):282–285. [PubMed] [Google Scholar]
- Manning D. D., Jutila J. W. Immunosuppression of mice injected with heterologous anti-immunoglobulin heavy chain antisera. J Exp Med. 1972 Jun 1;135(6):1316–1333. doi: 10.1084/jem.135.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. N., Leslie G. A. IgM-forming cells as the immediate precursor of IgA-producing cells during ontogeny of the immunoglobulin-producing system of the chicken. J Immunol. 1974 Jul;113(1):120–126. [PubMed] [Google Scholar]
- Murgita R. A., Mattioli C. A., Tomasi T. B., Jr Production of a runting syndrome and selective A deficiency in mice by the administration of anti-heavy chain antisera. J Exp Med. 1973 Jul 1;138(1):209–228. doi: 10.1084/jem.138.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K. H., Parratt D., White R. G. Quantitation of antibody to particulate antigens using a radiolabelled anti-immunoglobulin reagent: application to estimation of antibody in farmer's lung syndrome. J Immunol Methods. 1973 Nov;3(3):301–313. doi: 10.1016/0022-1759(73)90025-2. [DOI] [PubMed] [Google Scholar]
- Nielsen K. H., White R. G. Effect of host decomplementation on homeostasis of antibody production in fowl. Nature. 1974 Jul 19;250(463):234–236. doi: 10.1038/250234a0. [DOI] [PubMed] [Google Scholar]
- Parratt D., Nielsen K. H., White R. G. Radioimmunoassay of IgM, IgG, and IgA Brucella antibodies. Lancet. 1977 May 21;1(8021):1075–1078. doi: 10.1016/s0140-6736(77)92334-0. [DOI] [PubMed] [Google Scholar]
- Pierce C. W., Solliday S. M., Asofsky R. Immune responses in vitro. IV. Suppression of primary M, G, and A plaque-forming cell responses in mouse spleen cell cultures by class-specific antibody to mouse immunoglobulins. J Exp Med. 1972 Mar 1;135(3):675–697. doi: 10.1084/jem.135.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDIN J. L., ANDERSON R. K., JENNESS R., SPINK W. W. SIGNIFICANCE OF 7S AND MACROGLOBULIN BRUCELLA AGGLUTININS IN HUMAN BRUCELLOSIS. N Engl J Med. 1965 Jun 17;272:1263–1268. doi: 10.1056/NEJM196506172722403. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Walker J. G., Siskind G. W. Studies on the control of antibody synthesis. Effect of antibody affinity upon its ability to suppress antibody formation. Immunology. 1968 Jan;14(1):21–28. [PMC free article] [PubMed] [Google Scholar]
- White R. G., Nielsen K. H. Interactions between the immunological responses of a thymus-independent antigen (Salmonella adelaide O antigen) with a thymus-dependent antigen (sheep erythrocytes) in the adult bird. Immunology. 1975 May;28(5):959–972. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Immunoglobulin patterns of antibodies against Brucella in man and animals. J Immunol. 1966 Mar;96(3):457–463. [PubMed] [Google Scholar]



