Abstract
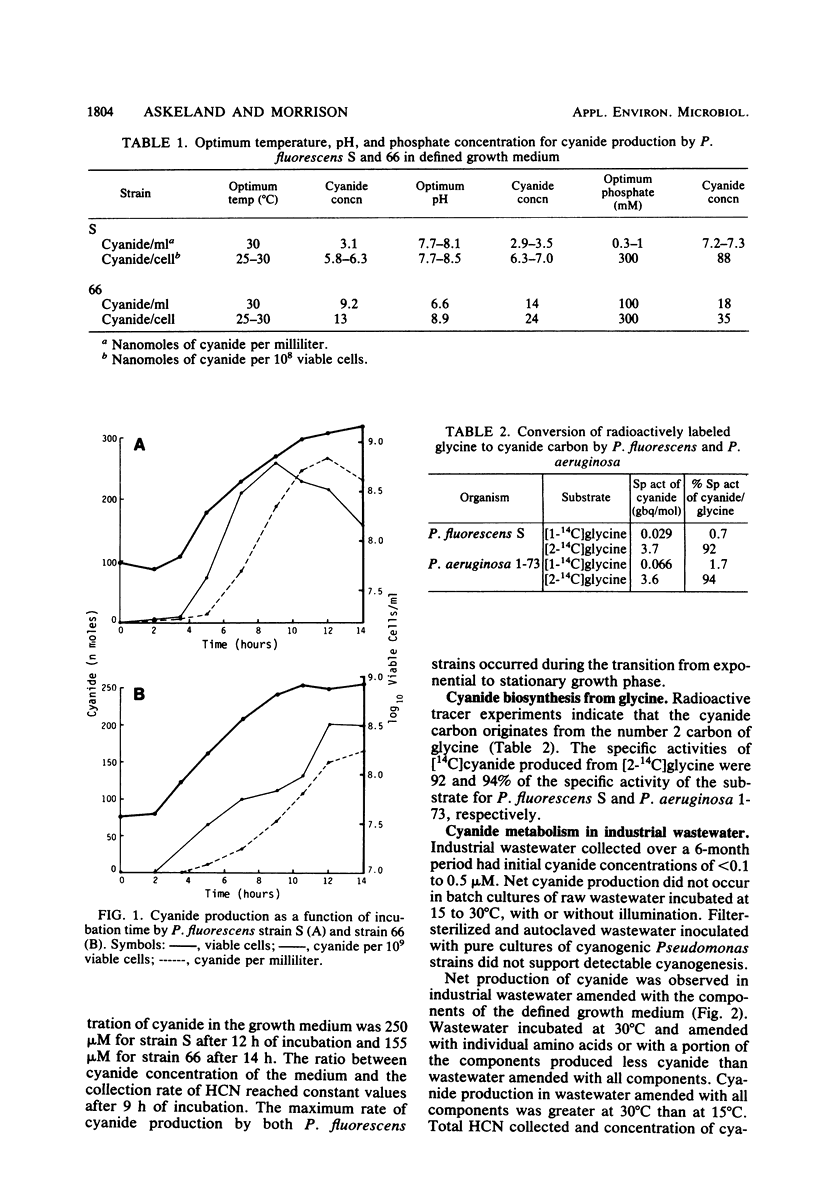
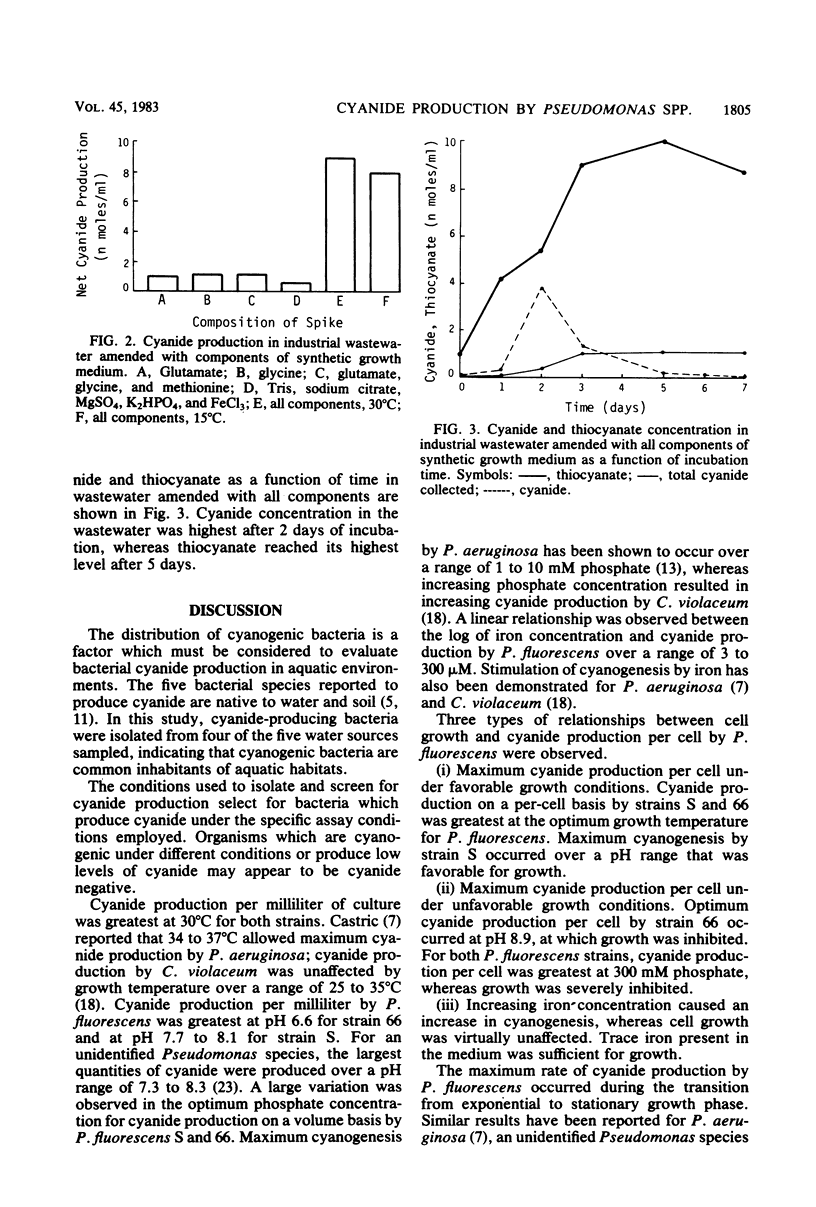
Of 200 water isolates screened, five strains of Pseudomonas fluorescens and one strain of Pseudomonas aeruginosa were cyanogenic. Maximum cyanogenesis by two strains of P. fluorescens in a defined growth medium occurred at 25 to 30 degrees C over a pH range of 6.6 to 8.9. Cyanide production per cell was optimum at 300 mM phosphate. A linear relationship was observed between cyanogenesis and the log of iron concentration over a range of 3 to 300 microM. The maximum rate of cyanide production occurred during the transition from exponential to stationary growth phase. Radioactive tracer experiments with [1-14C]glycine and [2-14C]glycine demonstrated that the cyanide carbon originates from the number 2 carbon of glycine for both P. fluorescens and P. aeruginosa. Cyanide production was not observed in raw industrial wastewater or in sterile wastewater inoculated with pure cultures of cyanogenic Pseudomonas strains. Cyanide was produced when wastewater was amended by the addition of components of the defined growth medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BU'LOCK J. D. Intermediary metabolism and antibiotic synthesis. Adv Appl Microbiol. 1961;3:293–342. doi: 10.1016/s0065-2164(08)70514-8. [DOI] [PubMed] [Google Scholar]
- Brysk M. M., Lauinger C., Ressler C. Biosynthesis of cyanide from [2-14C-15N]glycine in Chromobacterium violaceum. Biochim Biophys Acta. 1969 Sep 2;184(3):583–588. doi: 10.1016/0304-4165(69)90272-4. [DOI] [PubMed] [Google Scholar]
- Brysk M. M., Ressler C. Gamma-cyano-alpha-aminobutyric acid. A new product of cyanide fixation in Chromobacterium violaceum. J Biol Chem. 1970 Mar 10;245(5):1156–1160. [PubMed] [Google Scholar]
- Castric P. A. Glycine metabolism by Pseudomonas aeruginosa: hydrogen cyanide biosynthesis. J Bacteriol. 1977 May;130(2):826–831. doi: 10.1128/jb.130.2.826-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castric P. A. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol. 1975 May;21(5):613–618. doi: 10.1139/m75-088. [DOI] [PubMed] [Google Scholar]
- Knowles C. J. Microorganisms and cyanide. Bacteriol Rev. 1976 Sep;40(3):652–680. doi: 10.1128/br.40.3.652-680.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAELS R., CORPE W. A. CYANIDE FORMATION BY CHROMOBACTERIUM VIOLACEUM. J Bacteriol. 1965 Jan;89:106–112. doi: 10.1128/jb.89.1.106-112.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganathan R., Castric P. A. The effect of inorganic phosphate on cyanogenesis by Pseudomonas aeruginosa. Arch Microbiol. 1977 Jul 26;114(1):51–54. doi: 10.1007/BF00429629. [DOI] [PubMed] [Google Scholar]
- Michaels R., Hankes L. V., Corpe W. A. Cyanide formation from glycine by nonproliferating cells of Chromobacterium violaceum. Arch Biochem Biophys. 1965 Jul;111(1):121–125. doi: 10.1016/0003-9861(65)90329-2. [DOI] [PubMed] [Google Scholar]
- Pistorius E. K., Jetschmann K., Voss H., Vennesland B. The dark respiration of Anacystis nidulans. Production of HCN from histidine and oxidation of basic amino acids. Biochim Biophys Acta. 1979 Jul 18;585(4):630–642. doi: 10.1016/0304-4165(79)90195-8. [DOI] [PubMed] [Google Scholar]
- SNEATH P. H. Cultural and biochemical characteristics of the genus Chromobacterium. J Gen Microbiol. 1956 Aug;15(1):70–98. doi: 10.1099/00221287-15-1-70. [DOI] [PubMed] [Google Scholar]



