Abstract
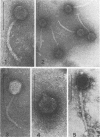
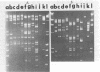
Bacteriophages isolated from culture supernatants of Pseudomonas syringae pv. syringae and from sewage were identified. The DNA from each phage was isolated and digested with the restriction endonuclease EcoRI. Eight isolates were determined to be different, with two phage isolates from sewage having restriction patterns identical to two phages from culture supernatants. The sizes of the phage DNA ranged from 24 to49 kilobases for isolates from sewage and from 39 to 52.5 kilobases for the isolates from culture supernatants. Buoyant densities of phage particles in CsCl varied from 1.498 to 1.507 g/cm3 for isolates from sewage and from 1.506 to 1.516 g/cm3 for isolates from culture supernatants. Electron microscopy revealed four morphological types. Based on plaque-forming ability of culture supernatants, 31 out of 47 strains of P. syringae are probably lysogenic.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auling G., Bernard U., Hüttermann A., Mayer F. Characterization and comparison of the DNAs of the three closely related bacteriophages gd, ge and gf with the genome DNA of the hydrogen-oxidizing host strain Pseudomonas pseudoflava GA3. J Gen Virol. 1980 Jul;49(1):51–59. doi: 10.1099/0022-1317-49-1-51. [DOI] [PubMed] [Google Scholar]
- BRADLEY D. E. The structure of some Staphylococcus and Pseudomonas phages. J Ultrastruct Res. 1963 Jun;8:552–565. doi: 10.1016/s0022-5320(63)80055-6. [DOI] [PubMed] [Google Scholar]
- Bouché J. P. The effect of spermidine on endonuclease inhibition by agarose contaminants. Anal Biochem. 1981 Jul 15;115(1):42–45. doi: 10.1016/0003-2697(81)90519-4. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen R. O., Currier T. C. Generalized Transduction in the Phytopathogen Pseudomonas syringae. Appl Environ Microbiol. 1983 Jun;45(6):1884–1889. doi: 10.1128/aem.45.6.1884-1889.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Buckner S. Typing of fluorescent phytopathogenic pseudomonads by bacteriocin production. Can J Microbiol. 1978 Jan;24(1):14–18. doi: 10.1139/m78-003. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967 Nov;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]