Abstract
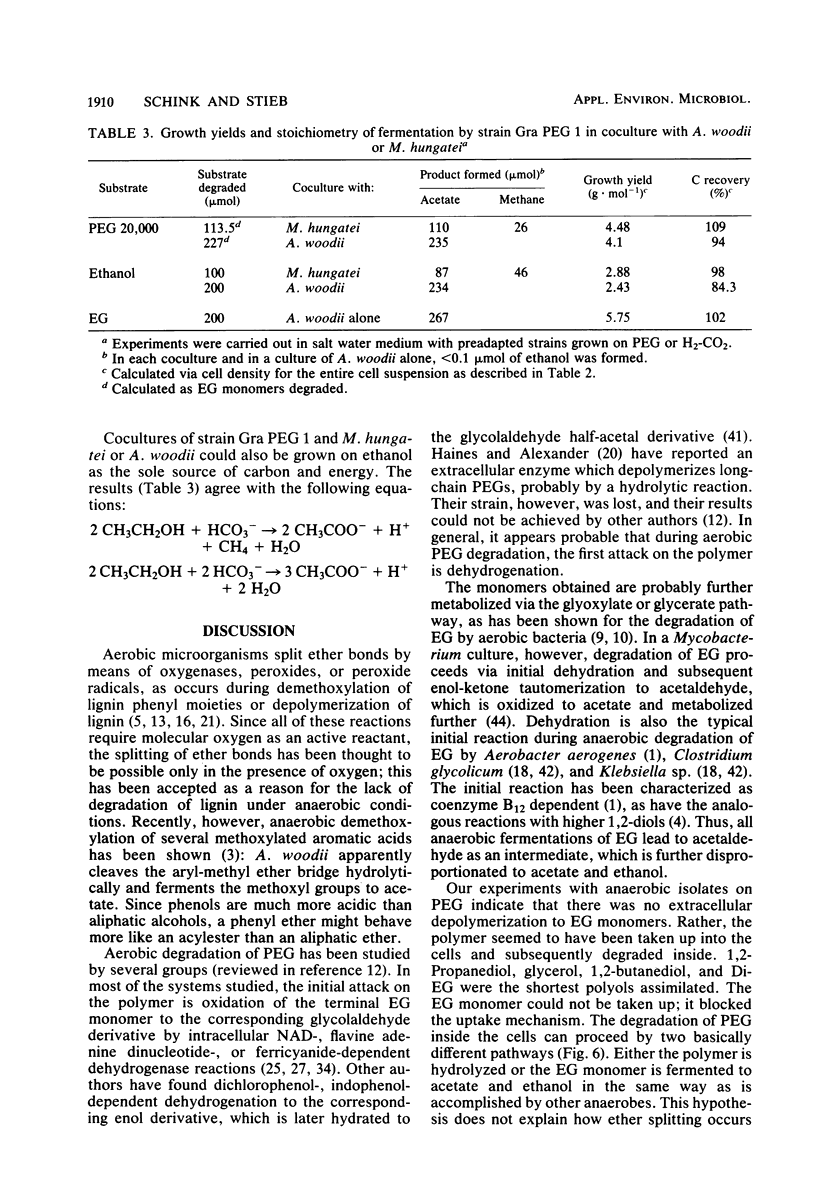
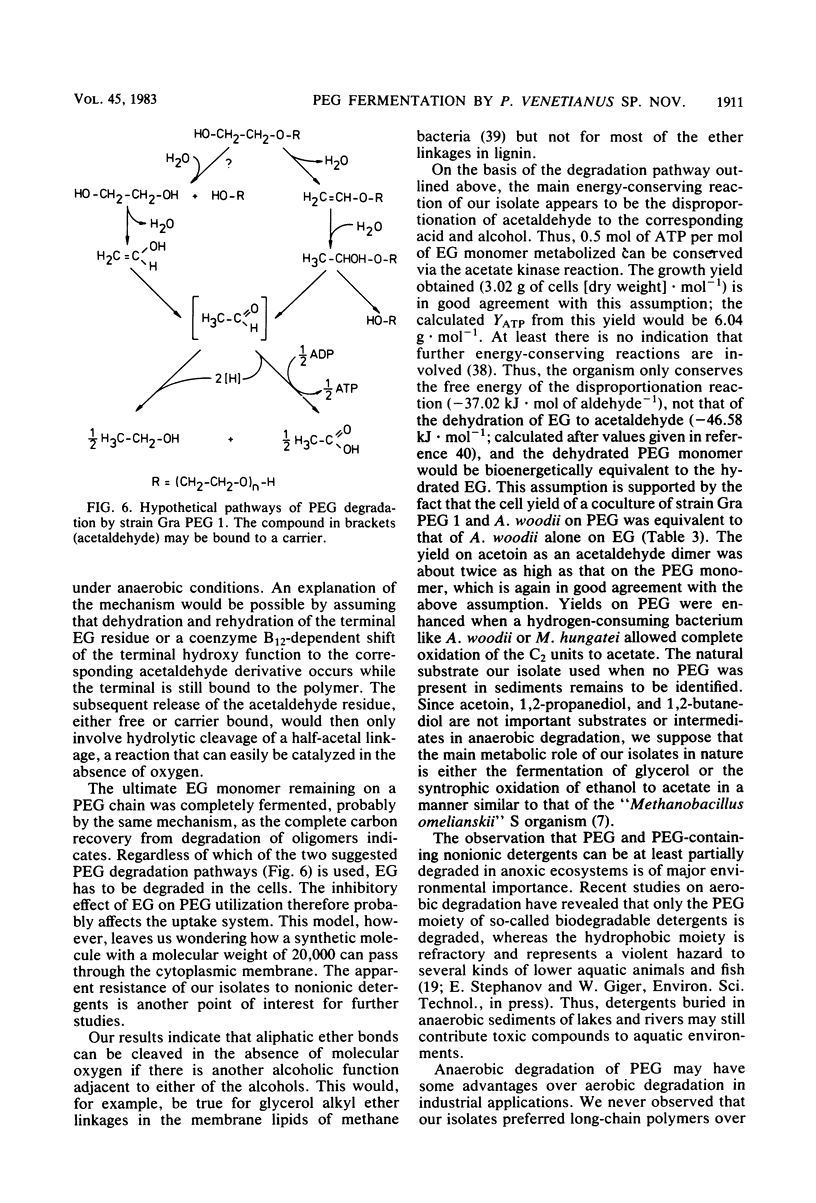
The synthetic polyether polyethylene glycol (PEG) with a molecular weight of 20,000 was anaerobically degraded in enrichment cultures inoculated with mud of limnic and marine origins. Three strains (Gra PEG 1, Gra PEG 2, and Ko PEG 2) of rod-shaped, gram-negative, nonsporeforming, strictly anaerobic bacteria were isolated in mineral medium with PEG as the sole source of carbon and energy. All strains degraded dimers, oligomers, and polymers of PEG up to a molecular weight of 20,000 completely by fermentation to nearly equal amounts of acetate and ethanol. The monomer ethylene glycol was not degraded. An ethylene glycol-fermenting anaerobe (strain Gra EG 12) isolated from the same enrichments was identified as Acetobacterium woodii. The PEG-fermenting strains did not excrete extracellular depolymerizing enzymes and were inhibited by ethylene glycol, probably owing to a blocking of the cellular uptake system. PEG, some PEG-containing nonionic detergents, 1,2-propanediol, 1,2-butanediol, glycerol, and acetoin were the only growth substrates utilized of a broad variety of sugars, organic acids, and alcohols. The isolates did not reduce sulfate, sulfur, thiosulfate, or nitrate and were independent of growth factors. In coculture with A. woodii or Methanospirillum hungatei, PEGs and ethanol were completely fermented to acetate (and methane). A marine isolate is described as the type strain of a new species, Pelobacter venetianus sp. nov. Its physiology and ecological significance, as well as the importance and possible mechanism of anaerobic polyether degradation, are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., LEE H. A., Jr An intramolecular oxidation-reduction requiring a cobamide coenzyme. J Biol Chem. 1961 Aug;236:2347–2350. [PubMed] [Google Scholar]
- Barker H. A. Corrinoid-dependent enzymic reactions. Annu Rev Biochem. 1972;41:55–90. doi: 10.1146/annurev.bi.41.070172.000415. [DOI] [PubMed] [Google Scholar]
- Bernhardt F. H., Staudinger H., Ullrich V. Eigenschaften einer p-Anisat-O-Demethylase im zellfreien Extrakt von Pseudomonas species. Hoppe Seylers Z Physiol Chem. 1970 Apr;351(4):467–478. [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Caskey W. H., Taber W. A. Oxidation of ethylene glycol by a salt-requiring bacterium. Appl Environ Microbiol. 1981 Jul;42(1):180–183. doi: 10.1128/aem.42.1.180-183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child J., Willetts A. Microbial metabolism of aliphatic glycols. Bacterial metabolism of ethylene glycol. Biochim Biophys Acta. 1978 Jan 18;538(2):316–327. doi: 10.1016/0304-4165(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Cox D. P. The biodegradation of polyethylene glycols. Adv Appl Microbiol. 1978;23:173–194. doi: 10.1016/s0065-2164(08)70068-6. [DOI] [PubMed] [Google Scholar]
- De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M. I., Dagley S. Bacterial degradation of 3,4,5-trimethoxycinnamic acid with production of methanol. J Bacteriol. 1981 Aug;147(2):471–476. doi: 10.1128/jb.147.2.471-476.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHER E. L., PAYNE W. J. Bacterial utilization of ether glycols. Appl Microbiol. 1962 Nov;10:542–547. doi: 10.1128/am.10.6.542-547.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Pankratz H. S. Ultrastructural Localization of Hydrogen Peroxide Production in Ligninolytic Phanerochaete chrysosporium Cells. Appl Environ Microbiol. 1982 Sep;44(3):732–736. doi: 10.1128/aem.44.3.732-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASTON L. W., STADTMAN E. R. Fermentation of ethylene glycol by Clostridium glycolicum, sp. n. J Bacteriol. 1963 Feb;85:356–362. doi: 10.1128/jb.85.2.356-362.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J. R., Alexander M. Microbial degradation of polyethylene glycols. Appl Microbiol. 1975 May;29(5):621–625. doi: 10.1128/am.29.5.621-625.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger R., Azaz E., Donbrow M. Autoxidation of polyoxyethylenic non-ionic surfactants and of polyethylene glycols. Pharm Acta Helv. 1975;50(1-2):10–17. [PubMed] [Google Scholar]
- Hollaus F., Sleytr U. On the taxonomy and fine structure of some hyperthermophilic saccharolytic Clostridia. Arch Mikrobiol. 1972;86(2):129–146. doi: 10.1007/BF00413368. [DOI] [PubMed] [Google Scholar]
- Jones N., Watson G. K. Ethylene glycol and polyethylene glycol catabolism by a sewage bacterium. Biochem Soc Trans. 1976;4(5):891–892. doi: 10.1042/bst0040891. [DOI] [PubMed] [Google Scholar]
- Kawai F., Kimura T., Fukaya M., Tani Y., Ogata K., Ueno T., Fukami H. Bacterial oxidation of polyethylene glycol. Appl Environ Microbiol. 1978 Apr;35(4):679–684. doi: 10.1128/aem.35.4.679-684.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee C. M., Rodeheaver G., Edgerton M. T., Edlich R. F. A more reliable gram staining technic for diagnosis of surgical infections. Am J Surg. 1975 Sep;130(3):341–346. doi: 10.1016/0002-9610(75)90398-0. [DOI] [PubMed] [Google Scholar]
- Payne W. J., Todd R. L. Flavin-linked dehydrogenation of ether glycols by cell-free extracts of a soil bacterium. J Bacteriol. 1966 Apr;91(4):1533–1536. doi: 10.1128/jb.91.4.1533-1536.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Honda S., Fukui S. Fermentation of 1,2-propanediol with 1,2-ethanediol by some genera of Enterobacteriaceae, involving coenzyme B12-dependent diol dehydratase. J Bacteriol. 1979 Jul;139(1):39–47. doi: 10.1128/jb.139.1.39-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]