Abstract
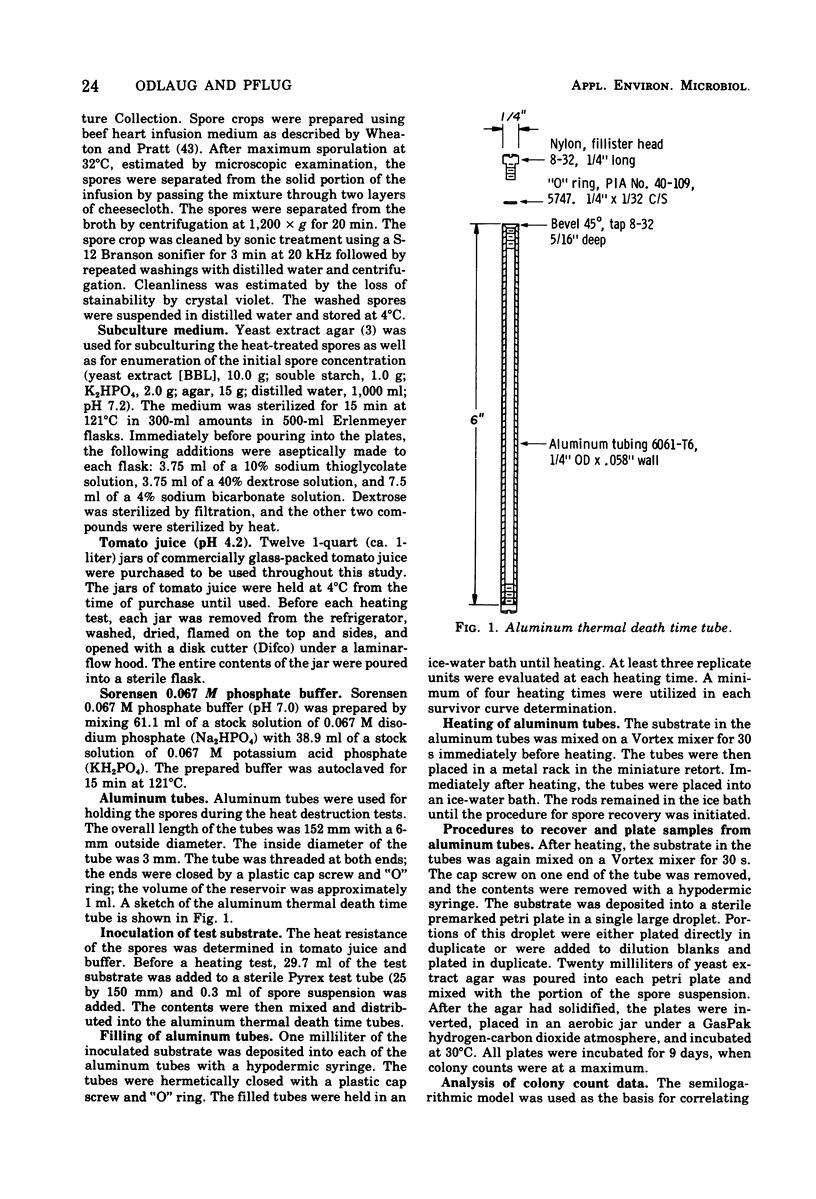
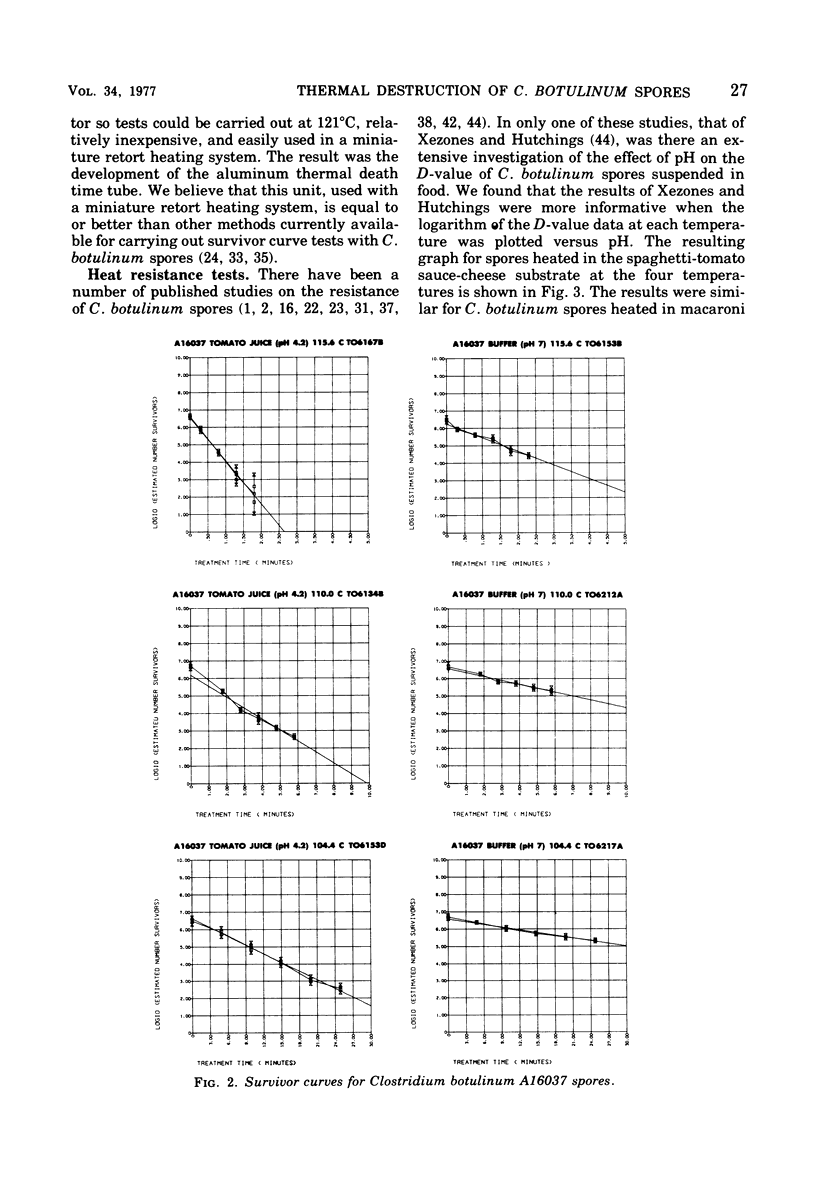
The heat destruction characteristics of Clostridium botulinum spores suspended in tomato juice and phosphate buffer were determined by the survivor curve method with aluminum thermal death time tubes. Two type A strains of C. botulinum and a type B strain were evaluated. Strains A16037 and B15580 were implicated in outbreaks of botulism involving home-canned tomato products. Strain A16037 had a higher heat resistance than either 62A or B15580. The mean thermal resistance (D-values) for A16037 in tomato juice (pH 4.2) were: 115.6 degrees C, 0.4 min; 110.0 degrees C, 1.6 min; and 104.4 degrees C, 6.0 min. The mean D-values for A16037 in Sorensen 0.067 M phosphate buffer (pH 7) were: 115.6 degrees C, 1.3 min; 110.0 degrees C, 4.4 min; and 104.4 degrees C, 17.6 min. At each test temperature, the D-values were approximately three times higher in buffer than in tomato juice. The z-value for C. botulinum A16037 spores in tomato juice was 9.4 degrees C, and in buffer the z-value was 9.9 degrees C. The use of aluminum thermal death time tubes in a miniature retort system makes it possible to determine survivor curves for C. botulinum spores at 121.1 degrees C. This is possible because the lag correction factor for the aluminum tubes is only about 0.2 min, making possible heating times as short as 0.5 min.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderton G., Chen J. K., Ito K. A. Effect of lysozyne on the recovery of heated Clostridium botulinum spores. Appl Microbiol. 1974 Mar;27(3):613–615. doi: 10.1128/am.27.3.613-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton G., Ito K. A., Chen J. K. Chemical manipulation of the heat resistance of Clostridium botulinum spores. Appl Environ Microbiol. 1976 Apr;31(4):492–498. doi: 10.1128/aem.31.4.492-498.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin J. A., Pflug I. J. Recovery patterns of spores of putrefactive anaerobe 3679 in various subculture media after heat treatment. Appl Microbiol. 1967 Mar;15(2):266–276. doi: 10.1128/am.15.2.266-276.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecz N., Tang T. Relation of dipicolinic acid to heat resistance of bacterial spores. J Gen Microbiol. 1970 Nov;63(3):303–310. doi: 10.1099/00221287-63-3-303. [DOI] [PubMed] [Google Scholar]
- Huhtanen C. N., Naghski J., Custer C. S., Russell R. W. Growth and toxin production by Clostridium botulinum in moldy tomato juice. Appl Environ Microbiol. 1976 Nov;32(5):711–715. doi: 10.1128/aem.32.5.711-715.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. A., Chen J. K., Lerke P. A., Seeger M. L., Unverferth J. A. Effect of acid and salt concentration in fresh-pack pickles on the growth of Clostridium botulinum spores. Appl Environ Microbiol. 1976 Jul;32(1):121–124. doi: 10.1128/aem.32.1.121-124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiman W. J., Geers J. M. Simple and accurate technique for the determination of heat resistance of bacterial spores. J Appl Bacteriol. 1975 Apr;38(2):185–189. doi: 10.1111/j.1365-2672.1975.tb00519.x. [DOI] [PubMed] [Google Scholar]
- TSUJI K., PERKINS W. E. Sporulation of Clostridium botulinum. I. Selection of an aparticulate sporulation medium. J Bacteriol. 1962 Jul;84:81–85. doi: 10.1128/jb.84.1.81-85.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lagarde A., Beerens H. Contribution a l'étude de la formation de toxine botulique dans les conserves de fruits. Ann Inst Pasteur Lille. 1970;21:231–253. [PubMed] [Google Scholar]



