Abstract
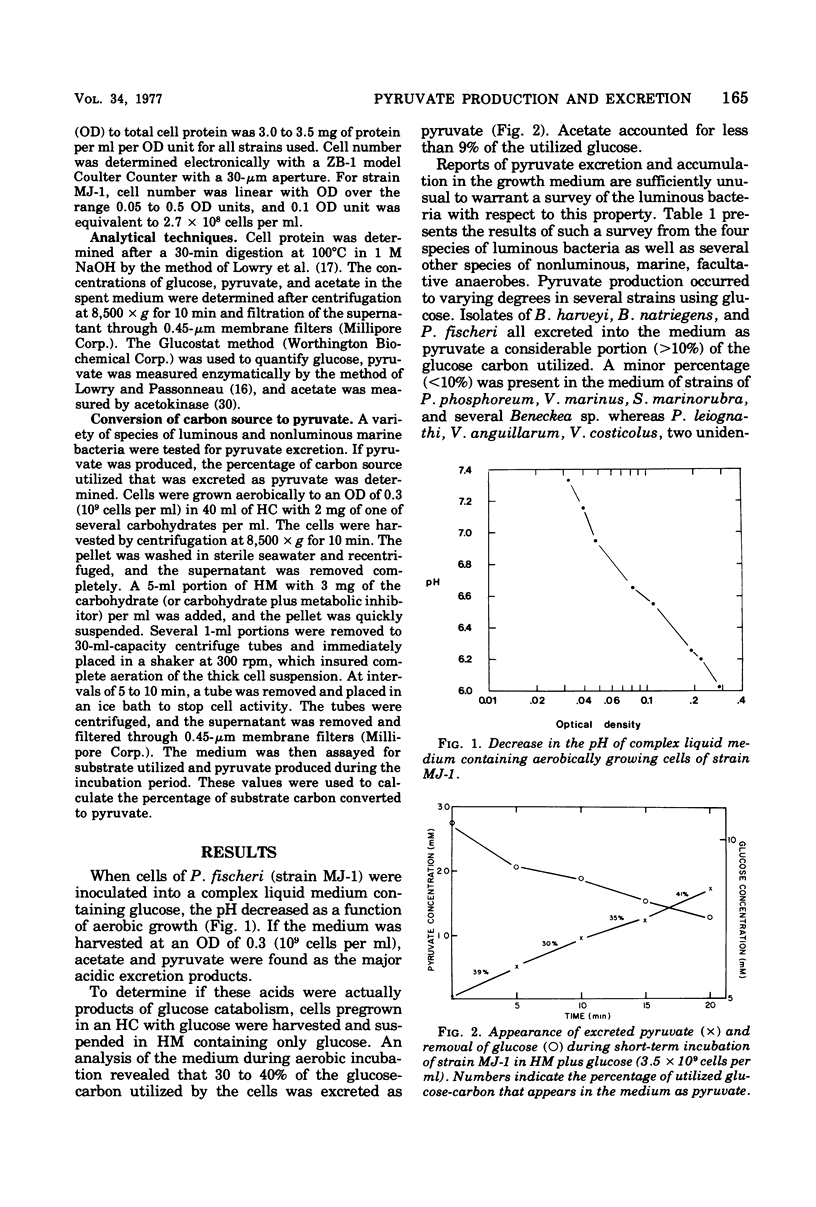
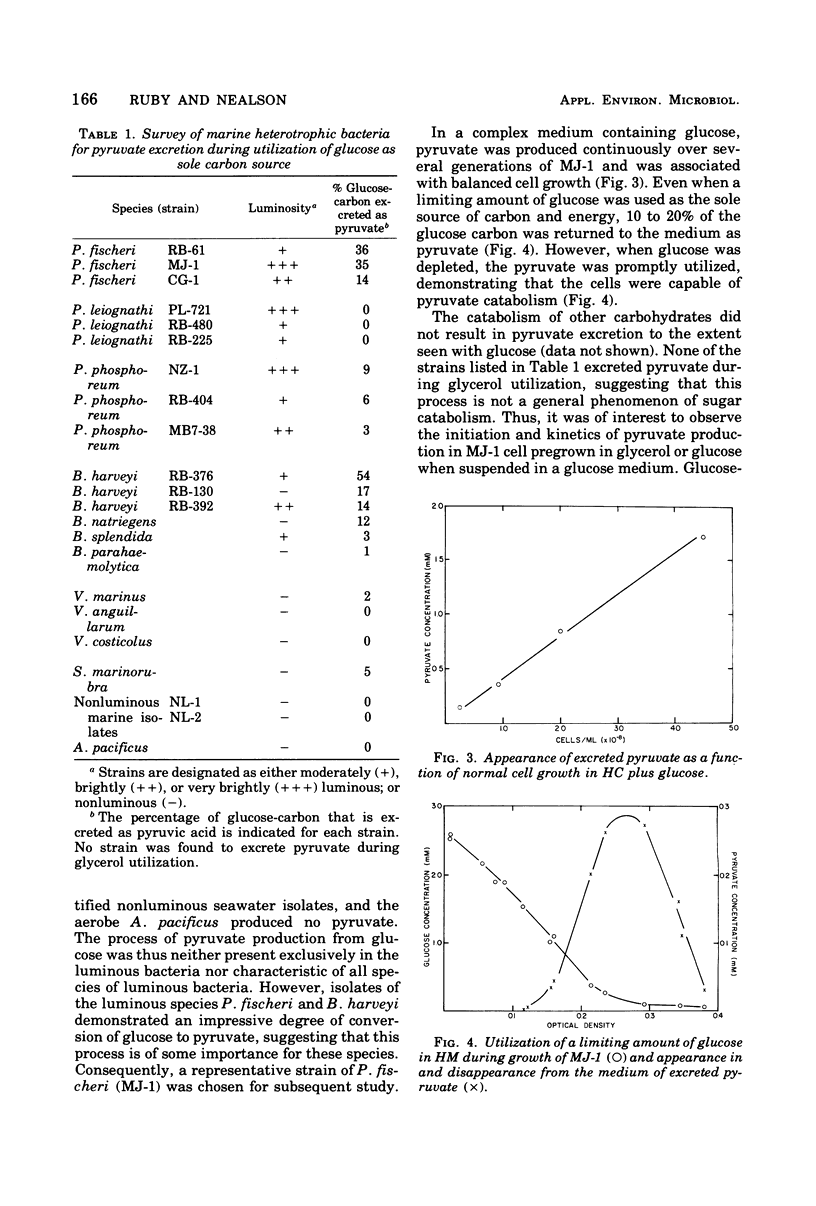
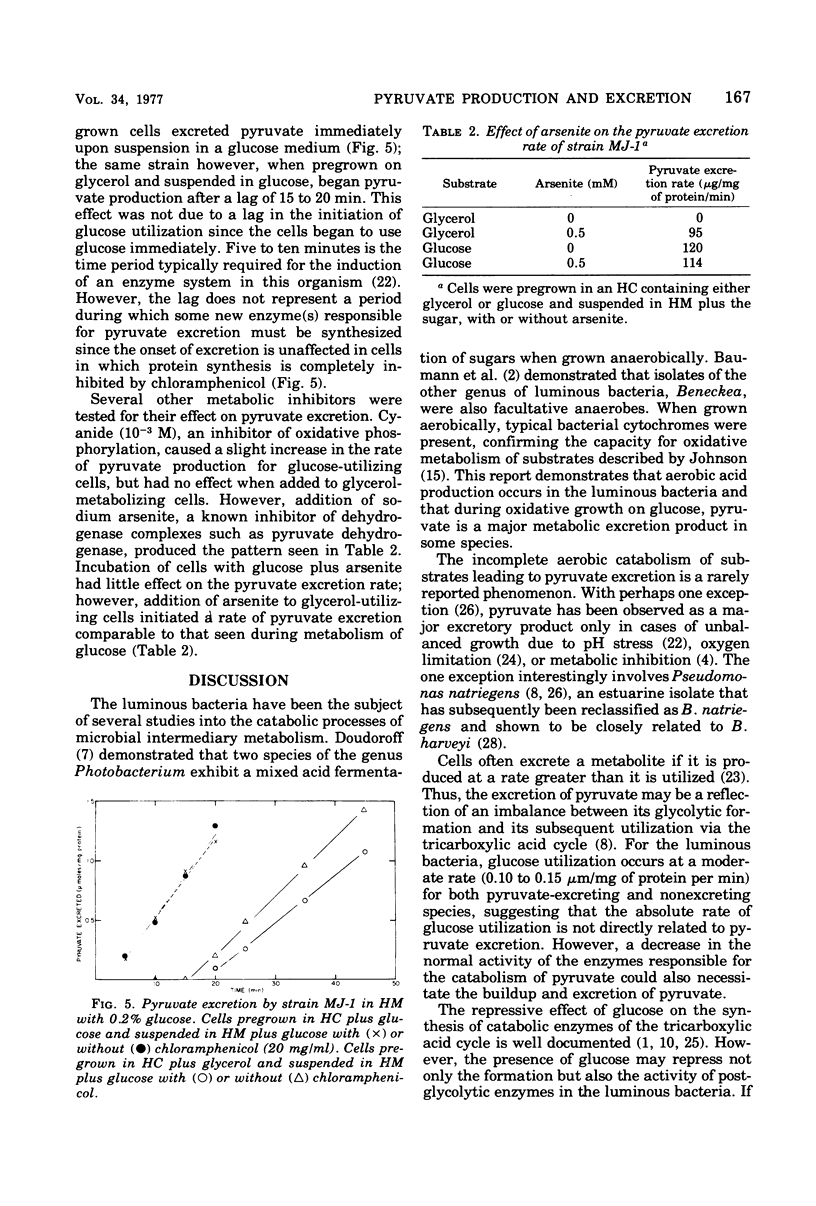
During aerobic growth on glucose, several species of luminous marine bacteria exhibited an imcomplete oxidative catabolism of substrate. Pyruvate, one of the products of glucose metabolism, was excreted into the medium during exponential growth and accounted for up to 50% of the substrate carbon metabolized. When glucose was depleted from the medium, the excreted pyruvate was promptly utilized, demonstrating that the cells are capable of pyruvate catabolism. Pyruvate excretion is not a general phenomenon of carbohydrate metabolism since it does not occur during the utilization of glycerol or maltose. When cells pregrown on glycerol were exposed to glucose, they began to excrete pyruvate, even if protein synthesis was blocked with chloramphenicol. Glucose thus appears to have an effect on the activity of preexisting catabolic enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Baumann P., Baumann L., Mandel M. Taxonomy of marine bacteria: the genus Beneckea. J Bacteriol. 1971 Jul;107(1):268–294. doi: 10.1128/jb.107.1.268-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTAWAY F. W., THOMPSON C. C. The action of inhibitors on dermatophytes. Biochem J. 1956 Aug;63(4):648–656. doi: 10.1042/bj0630648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILWORTH M. J. Oxygen inhibition in Azotobacter vinelandii pyruvate oxidation. Biochim Biophys Acta. 1962 Jan 1;56:127–138. doi: 10.1016/0006-3002(62)90533-4. [DOI] [PubMed] [Google Scholar]
- Doudoroff M. Studies on the Luminous Bacteria: II. Some Observations on the Anaerobic Metabolism of Facultatively Anaerobic Species. J Bacteriol. 1942 Oct;44(4):461–467. doi: 10.1128/jb.44.4.461-467.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G., CHO H. W. MAJOR PRODUCTS OF GLUCOSE DISSIMILATION BY PSEUDOMONAS NATRIEGENS. J Bacteriol. 1965 May;89:1209–1211. doi: 10.1128/jb.89.5.1209-1211.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNSALUS I. C. The chemistry and function of the pyruvate oxidation factor (lipoic acid). J Cell Physiol Suppl. 1953 Mar;41(Suppl 1):113–136. doi: 10.1002/jcp.1030410409. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Muscatine L., Boyle J. E., Smith D. C. Symbiosis of the acoel flatworm Convoluta roscoffensis with the alga Platymonas convolutae. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):221–234. doi: 10.1098/rspb.1974.0071. [DOI] [PubMed] [Google Scholar]
- NAKATA H. M. EFFECT OF PH ON INTERMEDIATES PRODUCED DURING GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Sep;86:577–581. doi: 10.1128/jb.86.3.577-581.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W. Induction of citrate lyase in Enterobacter cloacae grown under aerated conditions and its effect on citrate metabolism. J Bacteriol. 1975 Dec;124(3):1084–1088. doi: 10.1128/jb.124.3.1084-1088.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M. Regulation of the dicarboxylic acid part of the citric acid cycle in Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):224–234. doi: 10.1128/jb.122.1.224-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J., EAGON R. G., WILLIAMS A. K. Some observations on the physiology of Pseudomonas natriegens nov. spec. Antonie Van Leeuwenhoek. 1961;27:121–128. doi: 10.1007/BF02538432. [DOI] [PubMed] [Google Scholar]
- Reichelt J. L., Baumann P., Baumann L. Study of genetic relationships among marine species of the genera Beneckea and Photobacterium by means of in vitro DNA/DNA hybridization. Arch Microbiol. 1976 Oct 11;110(1):101–120. doi: 10.1007/BF00416975. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976 Dec;151(3):574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]


