Abstract
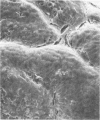
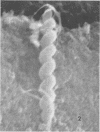
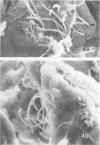
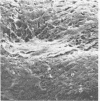
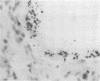
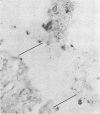
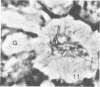
Nine male beagle dogs, housed in either a conventional or locked environment for 2.5 years, were killed, and the bacterial flora present in various regions of each gastrointestinal tract was assessed by culture techniques, light microscopy, and scanning electron microscopy. All dogs possessed a complex microflora in their colons; in almost every dog anaerobes predominated. The highest number of bacteria cultured was 1010/g (dry weight) of tissue and contents; highest counts obtained with a Petroff-Hauser counting chamber were 1010/ml (wet weight). Although there was a consistency in the detectable genera, there were also noticeable differences in the flora of dogs housed under different environmental conditions. These differences included qualitative and quantitative changes in the flora as well as alterations in the distribution and localization of microorganisms along the gastrointestinal tract and in the crypts of Lieberkuhn. No bacterial layers were detected on the surfaces of stomach or proximal bowel in any of the dogs. Dogs housed in a conventional, open, environment had bacteria that occurred in layers on their ceca and colons and in their crypts of Lieberkuhn; however, dogs housed under “locked” environmental conditions did not possess them or had them less frequently. Dogs removed from the locked environment and kept (30 days) in conventional housing conditions were the only ones with detectable segmented filamentous microbes in their ilea. This study shows that the microbial flora does not simplify when dogs are housed in a locked environment. Indeed, it may increase in complexity and cause alterations in the bacterial flora that is associated closely with gastrointestinal epithelial cells and crypts of Lieberkuhn.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Balish E., Brown J. F., Wilkins T. D. Transparent plastic incubator for the anaerobic glove box. Appl Environ Microbiol. 1977 Mar;33(3):525–527. doi: 10.1128/aem.33.3.525-527.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Shih C. N., Yale C. E., Mandel A. D. Effect of a prolonged stay in a locked environment on the microbial flora in dogs. Aerosp Med. 1974 Nov;45(11):1248–1254. [PubMed] [Google Scholar]
- Davis C. P., McAllister J. S., Savage D. C. Microbial colonization of the intestinal epithelium in suckling mice. Infect Immun. 1973 Apr;7(4):666–672. doi: 10.1128/iai.7.4.666-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Mulcahy D., Takeuchi A., Savage D. C. Location and description of spiral-shaped microorganisms in the normal rat cecum. Infect Immun. 1972 Aug;6(2):184–192. doi: 10.1128/iai.6.2.184-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P. Preservation of gastrointestinal bacteria and their microenvironmental associations in rats by freezing. Appl Environ Microbiol. 1976 Feb;31(2):304–312. doi: 10.1128/aem.31.2.304-312.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Savage D. C. Effect of penicillin on the succession, attachment, and morphology of segmented, filamentous microbes in the murine small bowel. Infect Immun. 1976 Jan;13(1):180–188. doi: 10.1128/iai.13.1.180-188.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Savage D. C. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974 Oct;10(4):948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Chase D. G. Morphological alterations in the microvillous border of villous epithelial cells produced by intestinal microorganisms. Am J Clin Nutr. 1974 Nov;27(11):1277–1286. doi: 10.1093/ajcn/27.11.1277. [DOI] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach W. D., Lee A., Stubbs R. P. Localization of bacteria in the gastrointestinal tract: a possible explanation of intestinal spirochaetosis. Infect Immun. 1973 Jun;7(6):961–972. doi: 10.1128/iai.7.6.961-972.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey T. D. Potential microbic shock in manned aerospace systems. Aerosp Med. 1966 Dec;37(12):1223–1228. [PubMed] [Google Scholar]
- Puleo J. R., Oxborrow G. S., Fields N. D., Herring C. M., Smith L. S. Microbiological profiles of four Apollo spacecraft. Appl Microbiol. 1973 Dec;26(6):838–845. doi: 10.1128/am.26.6.838-845.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. W. OBSERVATIONS ON THE FLORA OF THE ALIMENTARY TRACT OF ANIMALS AND FACTORS AFFECTING ITS COMPOSITION. J Pathol Bacteriol. 1965 Jan;89:95–122. [PubMed] [Google Scholar]
- Savage D. C., Blumershine R. V. Surface-surface associations in microbial communities populating epithelial habitats in the murine gastrointestinal ecosystem: scanning electron microscopy. Infect Immun. 1974 Jul;10(1):240–250. doi: 10.1128/iai.10.1.240-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J Exp Med. 1968 Jul 1;128(1):97–110. doi: 10.1084/jem.128.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suegara N., Morotomi M., Watanabe T., Kawal Y., Mutai M. Behavior of microflora in the rat stomach: adhesion of lactobacilli to the keratinized epithelial cells of the rat stomach in vitro. Infect Immun. 1975 Jul;12(1):173–179. doi: 10.1128/iai.12.1.173-179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Savage D. C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974 Mar;9(3):591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R., Henney M. R., Ellis W. L. Changes in the fungal autoflora of Apollo astronauts. Appl Microbiol. 1973 Nov;26(5):804–813. doi: 10.1128/am.26.5.804-813.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]