Abstract
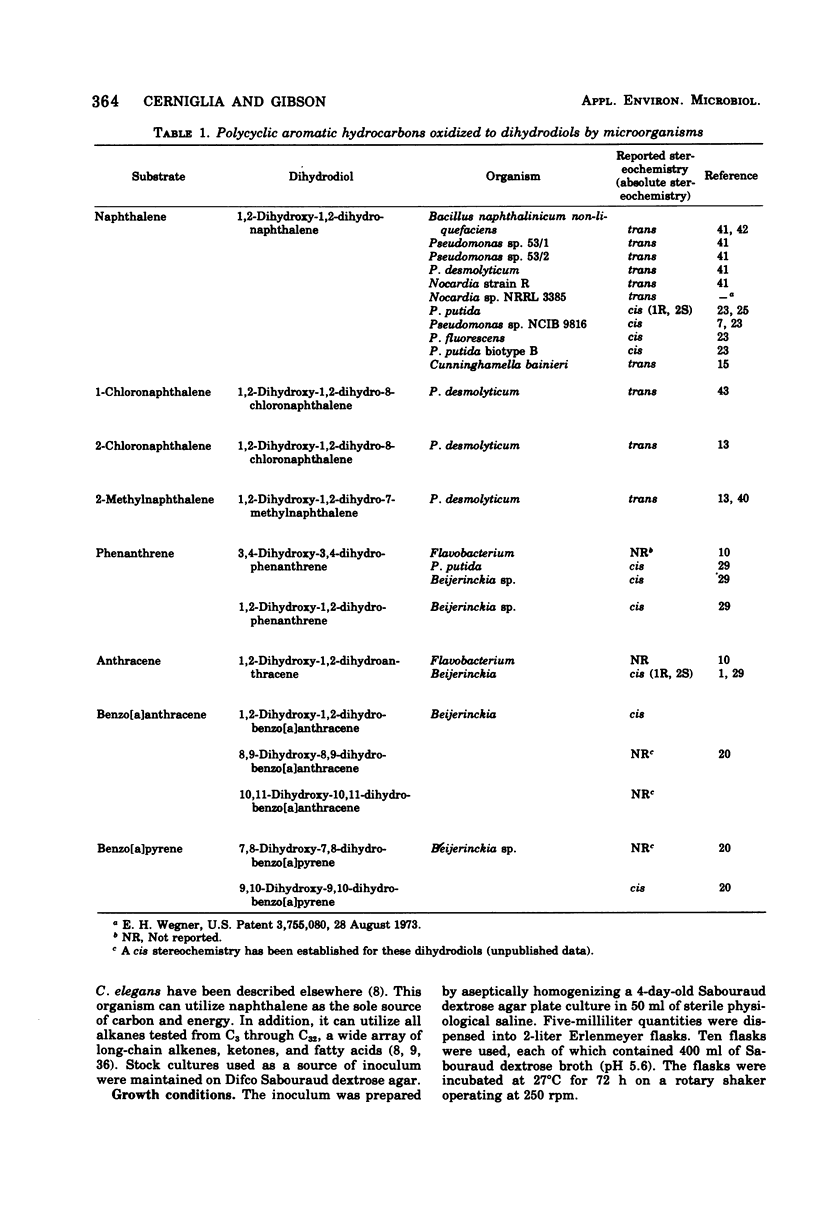
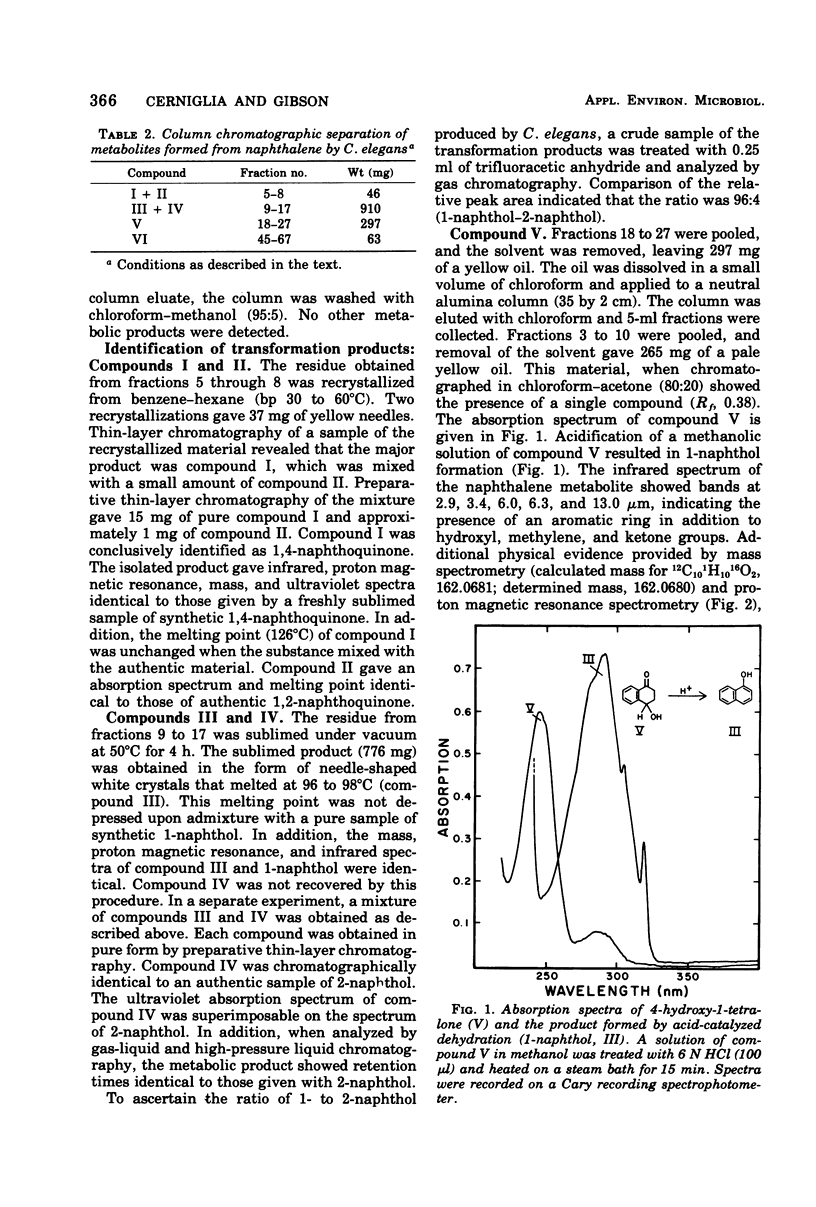
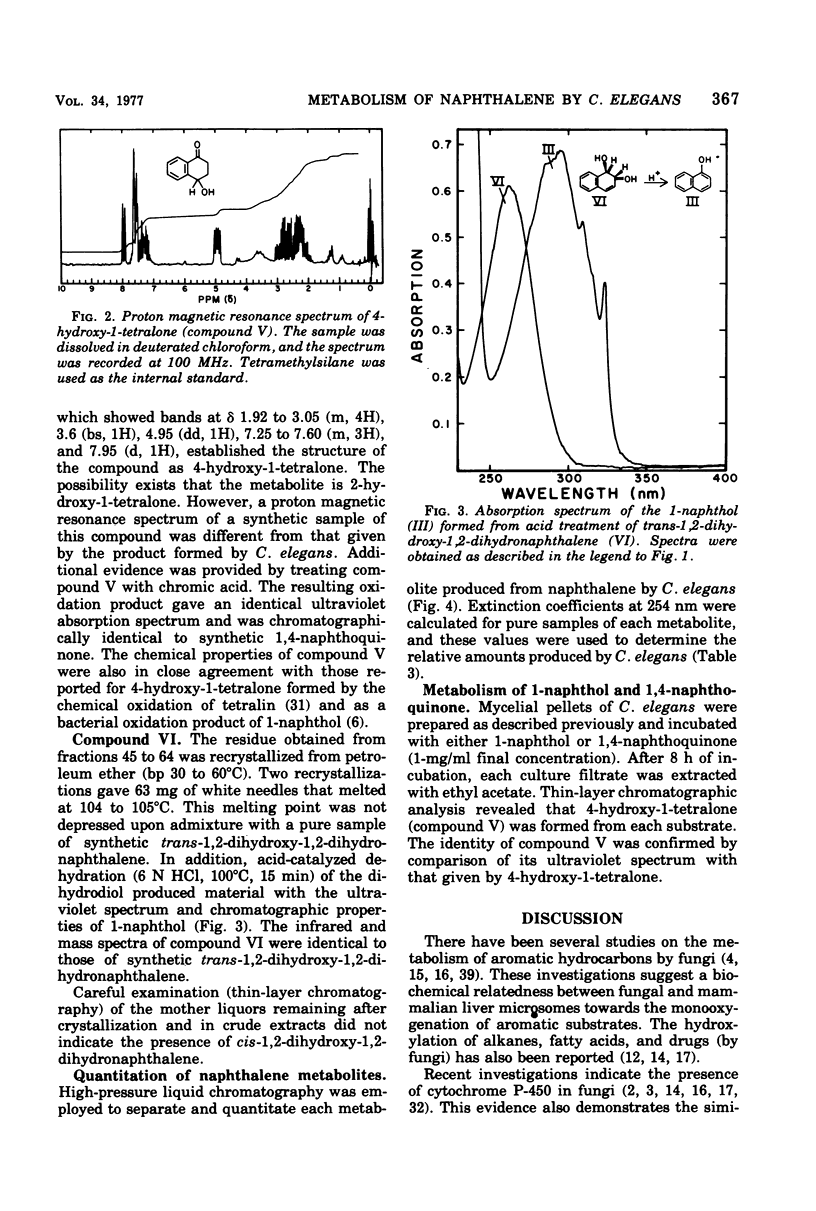
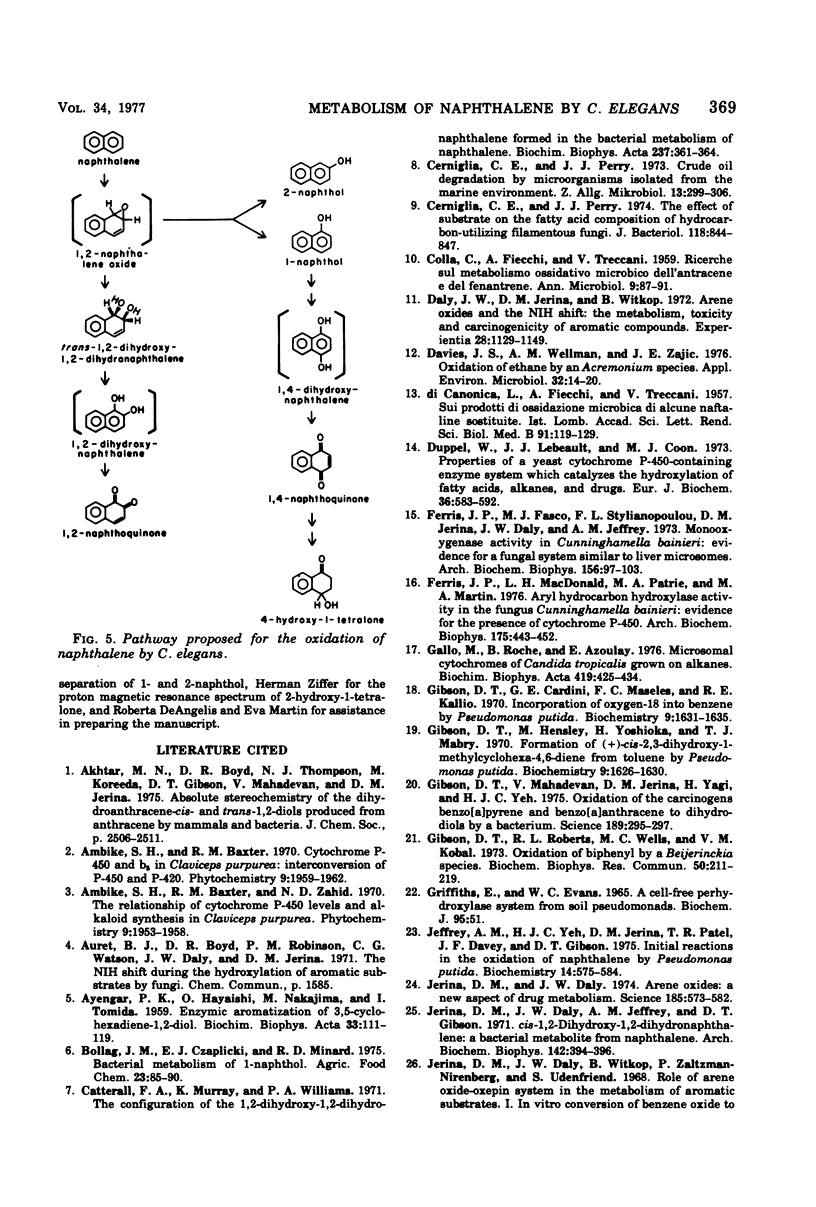
Cunninghamella elegans grown on Sabouraud dextrose broth in the presence of naphthalene produced six metabolites. Each product was isolated and identified by conventional chemical techniques. The major metabolites were 1-naphthol (67.9%) and 4-hydroxy-1-tetralone (16.7%). Minor products isolated were 1,4-naphthoquinone (2.8%), 1,2-naphthoquinone (0.2%), 2-naphthol (6.3%), and trans-1,2-dihydroxy-1,2-dihydronaphthalene (5.3%). C. elegans oxidized both 1-naphthol and 1,4-naphthoquinone to 4-hydroxy-1-tetralone. The results suggest that C. elegans oxidizes naphthalene by a sequence of reactions similar to those reported for the mammalian metabolism of this hydrocarbon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYENGAR P. K., HAYAISHI O., NAKAJIMA M., TOMIDA I. Enzymic aromatization of 3,5-cyclohexadiene-1,2-diol. Biochim Biophys Acta. 1959 May;33(1):111–119. doi: 10.1016/0006-3002(59)90504-9. [DOI] [PubMed] [Google Scholar]
- Akhtar M. N., Boyd D. R., Thompson N. J., Koreeda M., Gibson D. T., Mahadevan V., Jerina D. M. Absolute sterochemistry of the dihydroanthracene-cis- and -trans-1,2-diols produced from anthracene by mammals and bacteria. J Chem Soc Perkin 1. 1975;(23):2506–2511. [PubMed] [Google Scholar]
- Bollag J. M., Czaplicki E. J., Minard R. D. Bacterial metabolism of 1-naphthol. J Agric Food Chem. 1975 Jan-Feb;23(1):85–90. doi: 10.1021/jf60197a020. [DOI] [PubMed] [Google Scholar]
- Catterall F. A., Murray K., Williams P. A. The configuration of the 1,2-dihydroxy-1,2-dihydronaphthalene formed in the bacterial metabolism of naphthalene. Biochim Biophys Acta. 1971 May 18;237(2):361–364. doi: 10.1016/0304-4165(71)90331-x. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Perry J. J. Crude oil degradation by microorganisms isolated from the marine environment. Z Allg Mikrobiol. 1973;13(4):299–306. doi: 10.1002/jobm.3630130403. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Perry J. J. Effect of substrate on the fatty acid composition of hydrocarbon-utilizing filamentous fungi. J Bacteriol. 1974 Jun;118(3):844–847. doi: 10.1128/jb.118.3.844-847.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Jerina D. M., Witkop B. Arene oxides and the NIH shift: the metabolism, toxicity and carcinogenicity of aromatic compounds. Experientia. 1972 Oct 15;28(10):1129–1149. doi: 10.1007/BF01946135. [DOI] [PubMed] [Google Scholar]
- Davies J. S., Wellman A. M., Zajic J. E. Oxidation of ethane by an Acremonium species. Appl Environ Microbiol. 1976 Jul;32(1):14–20. doi: 10.1128/aem.32.1.14-20.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duppel W., Lebeault J. M., Coon M. J. Properties of a yeast cytochrome P-450-containing enzyme system which catalyzes the hydroxylation of fatty acids, alkanes, and drugs. Eur J Biochem. 1973 Jul 16;36(2):583–592. doi: 10.1111/j.1432-1033.1973.tb02948.x. [DOI] [PubMed] [Google Scholar]
- Ferris J. P., Fasco M. J., Stylianopoulou F. L., Jerina D. M., Daly J. W., Jeffrey A. M. Monooxygenase activity in Cunninghamella bainieri: evidence for a fungal system similar to liver microsomes. Arch Biochem Biophys. 1973 May;156(1):97–103. doi: 10.1016/0003-9861(73)90345-7. [DOI] [PubMed] [Google Scholar]
- Ferris J. P., MacDonald L. H., Patrie M. A., Martin M. A. Aryl hydrocarbon hydroxylase activity in the fungus Cunninghamella bainieri: evidence for the presence of cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):443–452. doi: 10.1016/0003-9861(76)90532-4. [DOI] [PubMed] [Google Scholar]
- Gallo M., Roche B., Azoulay E. Microsomal cytochromes of Candida tropicalis grown on alkanes. Biochim Biophys Acta. 1976 Feb 6;419(3):425–434. doi: 10.1016/0005-2736(76)90256-x. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Jerina D. M., Yogi H., Yeh H. J. Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science. 1975 Jul 25;189(4199):295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Yeh H. J., Jerina D. M., Patel T. R., Davey J. F., Gibson D. T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975 Feb 11;14(3):575–584. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970 Jan 6;9(1):147–156. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. The role of arene oxide-oxepin systems in the metabolism of aromatic substrates. 3. Formation of 1,2-naphthalene oxide from naphthalene by liver microsomes. J Am Chem Soc. 1968 Nov 6;90(23):6525–6527. doi: 10.1021/ja01025a058. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Selander H., Yagi H., Wells M. C., Davey J. F., Mahadevan V., Gibson D. T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976 Sep 15;98(19):5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- MARR E. K., STONE R. W. Bacterial oxidation of benzene. J Bacteriol. 1961 Mar;81:425–430. doi: 10.1128/jb.81.3.425-430.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Vogel G., Krippahl G., Lynen F. Patulin biosynthesis: the role of mixed-function oxidases in the hydroxylation of m-cresol. Eur J Biochem. 1974 Nov 15;49(2):443–455. doi: 10.1111/j.1432-1033.1974.tb03849.x. [DOI] [PubMed] [Google Scholar]
- Oesch F., Daly J. Conversion of naphthalene to trans-naphthalene dihydrodiol: evidence for the presence of a coupled aryl monooxygenase-epoxide hydrase system in hepatic microsomes. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1713–1720. doi: 10.1016/0006-291x(72)90807-8. [DOI] [PubMed] [Google Scholar]
- Oesch F., Jerina D. M., Daly J. W., Lu A. Y., Kuntzman R., Conney A. H. A reconstituted microsomal enzyme system that converts naphthalene to trans-1,2-dihydroxy-1,2-dihydronaphthalene via naphthalene-1,2-oxide: presence of epoxide hydrase in cytochrome P-450 and P-448 fractions. Arch Biochem Biophys. 1972 Nov;153(1):62–67. doi: 10.1016/0003-9861(72)90420-1. [DOI] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. Epoxy derivatives of aromatic polycyclic hydrocarbons. The preparation of benz( )anthracene 8,9-oxide and 10,11-dihydrobenz( )anthracene 8,9-oxide and their metabolism by rat liver preparations. Biochem J. 1971 Nov;125(1):159–168. doi: 10.1042/bj1250159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Rosazza J. P. Microbial models of mammalian metabolism. Aromatic hydroxylation. Arch Biochem Biophys. 1974 Apr 2;161(2):551–558. doi: 10.1016/0003-9861(74)90338-5. [DOI] [PubMed] [Google Scholar]
- TRECCANI V., WALKER N., WILTSHIRE G. H. The metabolism of naphthalene by soil bacteria. J Gen Microbiol. 1954 Dec;11(3):341–348. doi: 10.1099/00221287-11-3-341. [DOI] [PubMed] [Google Scholar]
- WALKER N., WILTSHIRE G. H. The breakdown of naphthalene by a soil bacterium. J Gen Microbiol. 1953 Apr;8(2):273–276. doi: 10.1099/00221287-8-2-273. [DOI] [PubMed] [Google Scholar]
- WALKER N., WILTSHIRE G. H. The decomposition of 1-chloro- and 1-bromonaphthalene by soil bacteria. J Gen Microbiol. 1955 Jun;12(3):478–483. doi: 10.1099/00221287-12-3-478. [DOI] [PubMed] [Google Scholar]


