Abstract
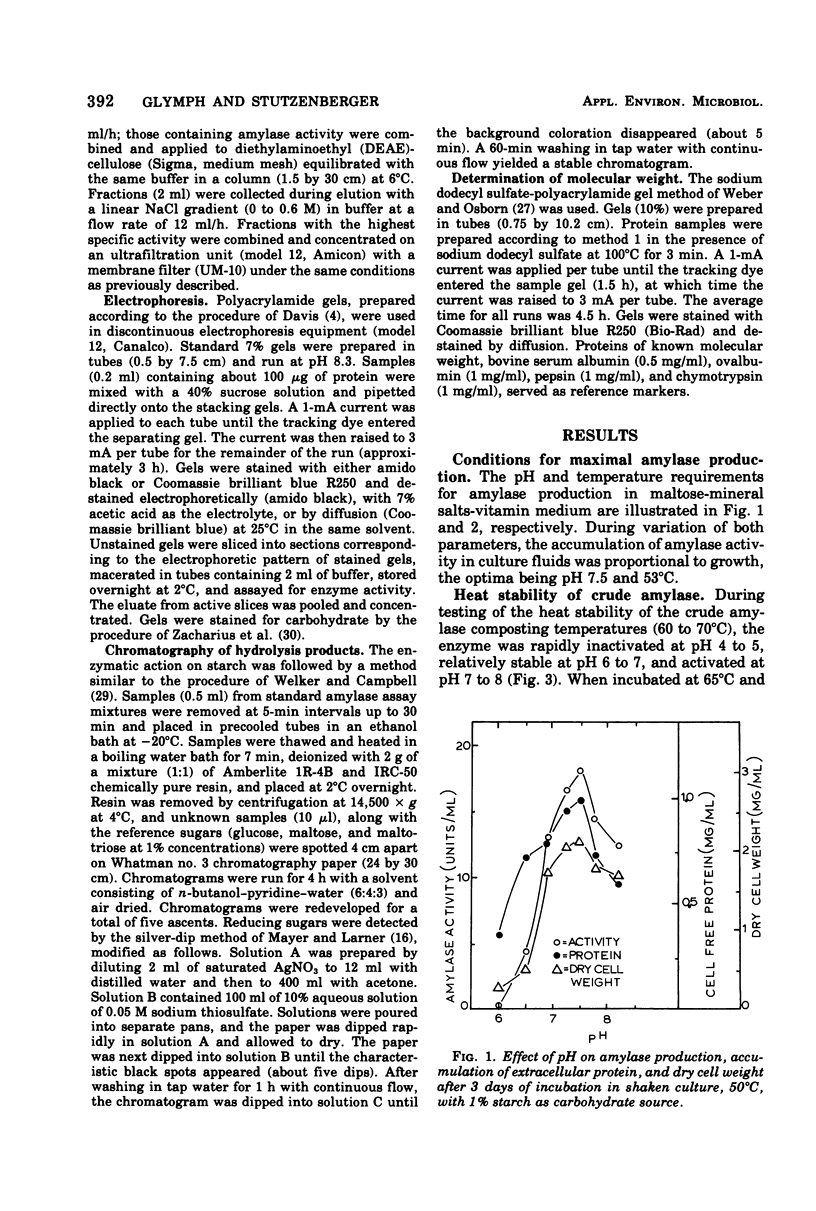
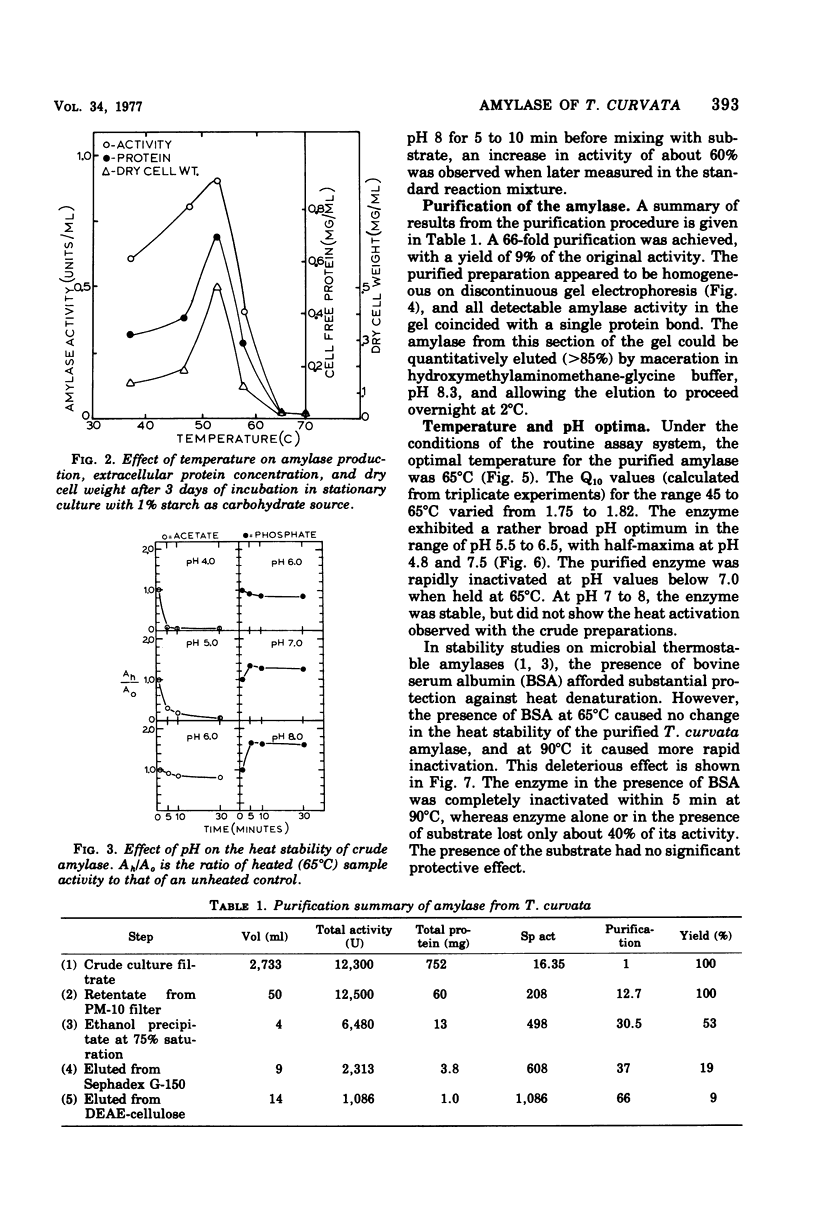
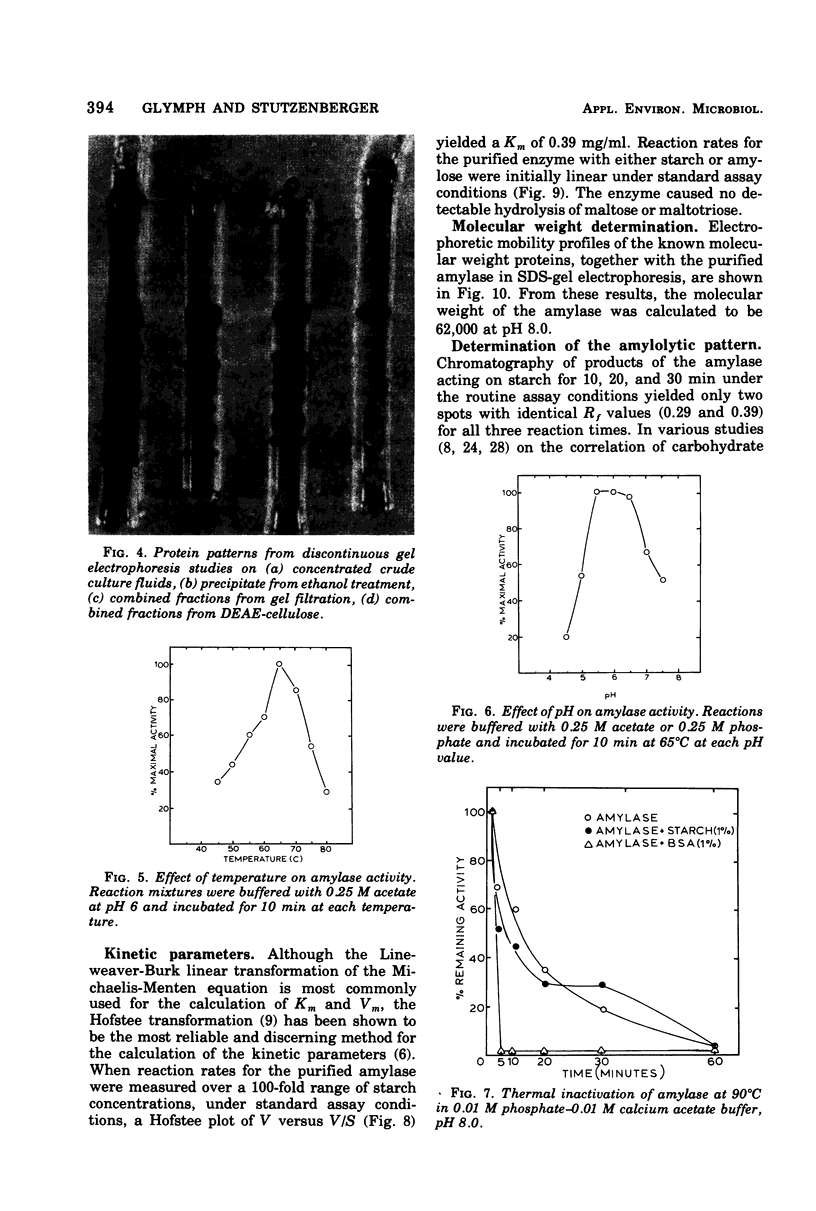
Thermomonospora curvata produces an extracellular alpha-amylase. Maximal amylase production by cultures in a starch-mineral salts medium occurred at pH 7.5 and 53 degrees C. The crude enzyme was unstable to heating (65 degrees C) at pH 4 to 6, and was activated when heated at pH 8. The enzyme was purified 66-fold with a 9% yield and appeared homogeneous on discontinuous gel electrophoresis. The pH and temperature optima for activity of the purified enzyme were 5.5 to 6.0 and 65 degrees C. The molecular weight was calculated to be 62,000. The Km for starch was 0.39 mg/ml. The amylolytic pattern consisted of a mixture of maltotetraose and maltopentaose.
Full text
PDF


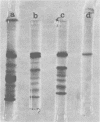
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allam A. M., Hussein A. M., Ragab A. M. Amylase of the thermophilic actinomycete Thermomonospora vulgaris. Z Allg Mikrobiol. 1975;15(6):393–398. doi: 10.1002/jobm.3630150602. [DOI] [PubMed] [Google Scholar]
- Buonocore V., Caporale C., De Rosa M., Gambacorta A. Stable, inducible thermoacidophilic alpha-amylase from Bacillus acidocaldarius. J Bacteriol. 1976 Nov;128(2):515–521. doi: 10.1128/jb.128.2.515-521.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- DePinto J. A., Campbell L. L. Purification and properties of the amylase of Bacillus macerans. Biochemistry. 1968 Jan;7(1):114–120. doi: 10.1021/bi00841a015. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- Kaufmann A., Fegan J., Doleac P., Gainer C., Wittich D., Glann A. Identification and characterization of a cellulolytic isolate. J Gen Microbiol. 1976 Jun;94(2):405–408. doi: 10.1099/00221287-94-2-405. [DOI] [PubMed] [Google Scholar]
- Kuo M. J., Hartman P. A. Isolation of amylolytic strains of Thermoactinomyces vulgaris and production of thermophilic actinomycete amylases. J Bacteriol. 1966 Sep;92(3):723–726. doi: 10.1128/jb.92.3.723-726.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. J., Hartman P. A. Purification and partial characterization of Thermoactinomyces vulgaris amylases. Can J Microbiol. 1967 Sep;13(9):1157–1163. doi: 10.1139/m67-160. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANNING G. B., CAMPBELL L. L. Thermostable alpha-amylase of Bacillus stearothermophilus. I. Crystallization and some general properties. J Biol Chem. 1961 Nov;236:2952–2957. [PubMed] [Google Scholar]
- Pfueller S. L., Elliott W. H. The extracellular alpha-amylase of bacillus stearothermophilus. J Biol Chem. 1969 Jan 10;244(1):48–54. [PubMed] [Google Scholar]
- Stutzenberger F. J. Cellulase production by Thermomonospora curvata isolated from municipal solid waste compost. Appl Microbiol. 1971 Aug;22(2):147–152. doi: 10.1128/am.22.2.147-152.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J. Cellulolytic activity of Thermomonospora curvata: nutritional requirments for cellulase production. Appl Microbiol. 1972 Jul;24(1):77–82. doi: 10.1128/am.24.1.77-82.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J. Cellulolytic activity of Thermomonospora curvata: optimal assay conditions, partial purification, and product of the cellulase. Appl Microbiol. 1972 Jul;24(1):83–90. doi: 10.1128/am.24.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J., Kaufman A. J., Lossin R. D. Cellulolytic activity in municipal solid waste composting. Can J Microbiol. 1970 Jul;16(7):553–560. doi: 10.1139/m70-093. [DOI] [PubMed] [Google Scholar]
- TURVEY J. R., WHELAN W. J. Preparation and characterization of the isomaltodextrins. Biochem J. 1957 Sep;67(1):49–52. doi: 10.1042/bj0670049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton M. E., Fogarty W. M. Production and Purification of Thermostable Amylase and Protease of Thermomonospora viridis. Appl Environ Microbiol. 1977 Jan;33(1):59–64. doi: 10.1128/aem.33.1.59-64.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN B., MEYER K., SAMPSON P., LINKER A. Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J Biol Chem. 1954 May;208(1):417–429. [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]