Abstract
Axonal receptors for class 3 semaphorins (Sema3s) are heterocomplexes of neuropilins (Nrps) and Plexin-As signalling coreceptors. In the developing cerebral cortex, the Ig superfamily cell adhesion molecule L1 associates with Nrp1. Intriguingly, the genetic removal of L1 blocks axon responses of cortical neurons to Sema3A in vitro despite the expression of Plexin-As in the cortex, suggesting either that L1 substitutes for Plexin-As or that L1 and Plexin-A are both required and mediate distinct roles. We report that association of Nrp1 with L1 but not Plexin-As mediates the recruitment and activation of a Sema3A-induced focal adhesion kinase–mitogen-activated protein kinase cascade. This signalling downstream of L1 is needed for the disassembly of adherent points formed in growth cones and subsequently their collapse response to Sema3A. Plexin-As and L1 are coexpressed and present in common complexes in cortical neurons and both dominant-negative forms of Plexin-A and L1 impair their response to Sema3A. Consistently, Nrp1-expressing cortical projections are defective in mice lacking Plexin-A3, Plexin-A4 or L1. This reveals that specific signalling activities downstream of L1 and Plexin-As cooperate for mediating the axon guidance effects of Sema3A.
Keywords: axon guidance, cerebral cortex, focal adhesion, receptor, semaphorins
Introduction
Class 3 semaphorins (Sema3s) are secreted guidance cues whose repulsive activity restricts the trajectory of a great number of axon subsets to prevent their aberrant growth during the development of neuronal connectivity (He et al, 2002). Axonal receptors for Sema3s comprise binding subunits, the Nrps, complexed with signalling co-receptors, the Plexin-As, which mediate the guidance activity of Sema3s by activating various intracellular pathways, including RhoGTPases, CRMPs, mitogen-activated protein kinase (MAPK), PI3K and Src kinase families (Kruger et al, 2005). Ig superfamily cell adhesion molecules (IgSFCAMs) have also been found to contribute to Sema3 responses, but their function has remained elusive so far (Castellani and Rougon, 2002; Falk et al, 2005). The IgSFCAM L1 associates with neuropilin (Nrp)1 and was proposed to be a component of the Sema3A receptor (Castellani et al, 2000; Castellani, 2002; Itoh et al, 2004). Similarly, another member of the L1-CAM subgroup, NrCAM, was found to associate with Nrp2 and to be required for growth cone responses to Sema3B and Sema3F in vitro (Falk et al, 2005). In both cases, the interaction is mediated by the ectodomain of IgSFCAMs and Nrp proteins.
During the development of cortical connectivity, Sema3A is thought to guide axons at several levels, from their initial growth out of the cortical plate of the cerebral cortex, through the corpus callosum and during their navigation to subcortical targets in the internal capsule and at the entry of the spinal cord (Bagnard et al, 1998; Polleux et al, 1998; Castellani et al, 2000; Gu et al, 2003). Interestingly, the genetic removal of L1 also disturbs these cortical pathways, causing midline crossing defects and size reduction of the corpus callosum (Dahme et al, 2007; Cohen et al, 1998; Demyanenko et al, 1999; Castellani et al, 2000). Disorganization of fibres in the internal capsule and defective pyramidal decussation have also been reported in L1−/y mice (Dahme et al, 2007; Ohyama et al, 2004). The contribution of Plexin-As to the guidance effects of Sema3A on cortical axons has not yet been investigated, but all plexin-A members are detected at the mRNA level in the developing cerebral cortex over the period of cortical neuron migration and axon extension (Murakami et al, 2001). Intriguingly, experiments conducted with explants from mice lacking L1 showed that cortical axons are unresponsive to Sema3A, indicating that Plexin-As cannot compensate for the lack of L1 in vitro (Castellani et al, 2000). These data lead to the question of the precise role of L1 in the Sema3A receptor and why Plexin-As cannot compensate their loss.
Four Plexin-A members have been identified and most of them are able to confer a morphological retraction to COS cells exposed to Sema3A when coexpressed with Nrp1, although the ligand binding affinity differs between the complexes (Togashi et al, 2006). Co-culture assays with DRG explants from mice lacking Plexin-A3 and Plexin-A4 have shown that Sema3A-responsive axons primarily utilize Plexin-A4 for their repulsive behaviour to Sema3A (Suto et al, 2005; Yaron et al, 2005). However, cooperative effects exist between different Plexin-As as some DRG axons from Plexin-A4−/− mice only exhibited reduced sensitivity to Sema3A but were fully desensitized by additional genetic removal of Plexin-A3 (Yaron et al, 2005). In the context of the cerebral cortex, L1 and Plexin-A subunits could both be required in a common receptor to perform distinct and/or redundant roles. Alternatively, L1 could mediate Sema3A signalling and thus substitute to Plexin-As in these neurons. Finally, L1 and Plexin-As could also segregate in subpopulations of cortical axons to form distinct receptor complexes.
The goal of this study was to address these possibilities to gain insights into the role of L1 in the response of cortical neurons to Sema3A. We report that interaction of L1 but not Plexin-As with Nrp1 specifically mediates the activation of a focal adhesion kinase (FAK)–mitogen-activated protein kinase (MAPK) signalling pathway controlling a crucial process of the repulsive behaviour, the disassembly of adhesion points of the growth cones. We provide evidence from biochemical experiments, culture assays and analysis of mutant mice lacking Plexin-A3 that Plexin-A4 and L1 are coexpressed and initiate distinct signalling cascades that cooperate for mediating the growth cone responses to Sema3A. These data assign a specific function to L1 in Sema3A signalling during axon guidance and elucidate the lack of functional compensation for L1 by Plexin-A proteins in L1-deficient mice.
Results
FAK is required for the growth cone collapse response to Sema3A
The first issue was to determine whether L1 shows signalling activity in the Sema3A receptor. The FAK acts downstream of integrin activation by extracellular matrix components and has a major role in the dynamics of focal adhesions (Mitra et al, 2005). Interestingly, FAK also participates in the signalling of guidance cues including Sema3B (Miao et al, 2000; Carter et al, 2002; Li et al, 2004; Liu et al, 2004; Ren et al, 2004; Falk et al, 2005) although its recruitment to Plexins has not been reported (Barberis et al, 2004). We investigated whether FAK participates in Sema3A signalling in cortical neurons and if so if it could be recruited by L1. Immunocytochemistry performed on E15 embryonic brain sections showed that L1, Nrp1 and FAK are expressed by post-mitotic neurons in the cortical plate of the developing cerebral cortex, consistent with some previous observations (Contestabile et al, 2003), and distributed over the cell body layer and the intermediate zone containing cortical efferents (Figure 1A). FAK and pFAK could also be detected in cultured cortical neurons (Figure 1A and data not shown). To address whether FAK mediates Sema3A signaling, different FAK variants were electroporated into cortical neurons. These included variants with a kinase-dead domain (FAKΔkin), a mutation on the Y397, which is the autophosphorylation site of FAK (FAKY397), deletion of the FERM domain rendering FAK constitutively active (FAKΔFERM; Cohen and Guan, 2005; Mitra et al, 2005) or wild-type FAK (FAKWT) as control. Collapse assays were performed on electroporated cortical neurons (Figure 1B, a total of 80 growth cones assessed per condition from 12 neonatal brains). Overexpression of FAKWT did not interfere with the collapse response observed for wild-type neurons. FAKΔFERM significantly increased the basal level of collapse observed under control conditions. Nevertheless, Sema3A application further increased the level of collapsed growth cones (Figure 1B). In contrast, FAKΔkin and FAKY397 forms had no detectable effects under control treatment but totally abrogated the collapse normally exerted by Sema3A (Figure 1B). Thus, FAK is required for Sema3A to collapse neuronal growth cones.
Figure 1.
FAK contributes to Sema3A-induced growth cone collapse. (A) FAK, L1 and Nrp1 are expressed by neurons residing in the cortical plate and detected in the intermediate zone where their efferents navigate to exit the cortex. Scale bar: 100 μm. Axonal localization of pFAK, L1 and Nrp1 in cultured cortical neurons is shown. Scale bar: 50 μm. (B) Microphotographs illustrating neurons labelled with antibodies to HA-tagged overexpressed FAK and magnification of the growth cone morphology under control and Sema3A-treated conditions. Overexpression of FAKY397 and FAKkinΔ but not FAKWT and FAKΔFERM prevents the growth cone collapse. Western blot shows the expression of FAK proteins from the plasmids used in electroporation. The histogram shows the percentage of growth cone collapse under control and Sema3A-treated conditions showing that the response is abolished by FAKY397 and FAKkinΔ. (C) Western blot showing FAK precipitation with Nrp1 and L1 from the purified membrane fraction of neonatal mouse cortex. Sema3A stimulation of fresh cortical tissue induces Y397 FAK phosphorylation. lpIP: lysate post immunoprecipitation; NS, nonsignificant; *P<0.001, χ2 test.
FAK recruitment to the Sema3A receptor is mediated by L1/Nrp1 complex formation and is ligand-induced
Next, we investigated the mechanisms underlying the contribution of FAK to Sema3A effects. First, to assess whether FAK is recruited to the Sema3A receptor, neonatal cortical tissue was collected and then processed for membrane purification and precipitation experiments with antibodies to Nrp1. Western blot analysis showed that FAK co-precipitates with Nrp1 and L1 from this tissue (Figure 1C). To examine FAK activation, FAK was precipitated from control and Sema3A-stimulated fresh cortical tissue. Western blot analysis showed that Sema3A induced Y397 phosphorylation of FAK (Figure 1C). Second, to search for the partners of the Sema3A receptor that mediate the recruitment of FAK, 293 cells were transfected to express various combinations of receptor subunits (Figure 2A and D). The signalling properties of the L1/Nrp1 complex were compared with that of the prototypal Sema3A receptor Plexin-A1/Nrp1 (Toyofuku et al, 2005; Togashi et al, 2006). Interestingly, FAK co-precipitated with L1 from L1/Nrp1-expressing cells but not with Plexin-A1 from Nrp1/Plexin-A1-expressing cells. Likewise, when coexpressed with Nrp1, L1/FAK association could be observed by precipitation with antibodies to FAK and L1, whereas antibody to neither FAK nor to the VSV tag of Plexin-A1 could allow the detection of FAK/Plexin-A1 interaction (Figure 2A and B). This was not due to deficiency of Plexin-A1 construct, as expression of this construct with Nrp1 in COS7 cells conferred upon them a collapse response to Sema3A (data not shown).
Figure 2.
L1/Nrp1 mediates Sema3A-induced recruitment and activation of FAK. (A, B) FAK precipitates with L1 from L1/Nrp1-expressing 293 cells but not with Plexin-A1VSV (plexVSV) from Plexin-A1/Nrp1-expressing 293 cells. Sema3A treatment (3A) stimulates L1/FAK co-precipitation. The L1/FAK complex could be detected in the control condition by precipitation with anti-L1 but not anti-FAK antibodies. (C, D) FAK does not precipitate with Nrp1, plexVSV or L1 when expressed alone whereas it precipitates with L1 from cells expressing L1 complexed with Nrp1 truncated from its cytoplasmic domain (Nrp1Δcyt). (E) Sema3A induces FAK autophosphorylation detected with anti-py397 in cells expressing L1/Nrp1 but not Nrp1/PlexVSV or PlexVSV. None of the Plexin-A2-A3-A4/Nrp1 complexes mediate FAK phosphorylation upon Sema3A stimulation. (F) Sema3A stimulates FAK/Src association in L1/Nrp1-expressing cells. (G) Precipitation of L1 in Sema3A-stimulated HEK cells expressing L1, Nrp1 and FAK forms for detection of FAK. FAKΔkin, FAKY397, FAKWT but not FAKΔFERM co-precipitated with L1. The photographs show bands at 125 kDa for FAKΔkin, FAKY397 and FAKWT and at 70 kDa for FAKΔFERM. (H) Precipitation of FAK in Sema3A-stimulated HEK cells expressing various truncated L1 forms lacking one or several functional domains of L1 and Nrp1 for the detection of L1. L11176, L11180, L1ΔRSLE but not L11146 co-precipitated with FAK. ERM: binding site to ERM proteins; AP-A: binding site for AP-2/chlatrin-dependent endocytosis; ANK: binding site to ankyrin.
These data suggest that FAK associates with L1. An alternative is that FAK is recruited by Nrp1, but only in an L1/Nrp1 complex and not when Nrp1 is complexed with Plexin-A1. This latter possibility was invalidated by the finding that an Nrp1/FAK interaction could not be detected in cells expressing only Nrp1 or Plexin-A1 (Figure 2C). Furthermore, co-precipitation of L1 with FAK from cells expressing a complex of L1 and a cytoplasmic deleted form of Nrp1 could be detected, which demonstrates that L1 is the subunit that triggers the recruitment of FAK (Figure 2C). Notably, we also observed that FAK/L1 association was almost undetectable in cells expressing L1 alone, indicating that the recruitment of FAK is conditional, based on the formation of the L1/Nrp1 complex (Figure 2D). Moreover, Sema3A treatment increased the recruitment of FAK to the L1/Nrp1 complex, as shown by precipitation with antibodies to L1 and FAK (Figure 2A and B).
L1/Nrp1 complex formation triggers Sema3A-induced FAK activation
Although the recruitment of FAK to the Sema3A receptor was found to depend on L1 but not Plexin-A1, its activation could require Plexin-A in the complex or result from signalling events downstream of Plexin-A. The phosphorylation of FAK was assessed with an antibody that selectively recognizes the pY397. Phosphorylation at Y397 could be detected in Sema3A-treated L1/Nrp1-expressing cells but not in cells expressing Plexin-A1 alone or complexed with Nrp1 (Figure 2E). The recruitment of FAK upon L1/Nrp1 complex formation was not sufficient to induce strong activation, as pY397 FAK was only weakly detected in the untreated condition. As described above, cortical neurons express several Plexin-As other than Plexin-A1 that could share with L1 the capacity of recruiting and activating FAK. We thus examined this possibility by immunoprecipitation of FAK from Sema3A-treated-transfected HEK cells and found that none of the Nrp1/Plex-A2, Nrp1-Plex-A3 and Nrp1/Plex-A4 complexes mediated FAK recruitment and activation (Figure 2E and Supplementary Figure 1). Thus, the L1/Nrp1 but not the Plexin-As/Nrp1 complex could mediate FAK phosphorylation. FAKY397 autophosphorylation creates a binding site for src (Mitra et al, 2005). Consistently, we found in L1/Nrp1-expressing cells that Sema3A stimulates FAK/src co-precipitation (Figure 2F).
To delineate the domains of FAK and L1 required for their association, HEK cells expressing L1/Nrp1 with FAKΔkin, FAKΔFERM, FAKY397 or FAKWT were stimulated with Sema3A and processed for precipitation with antibodies to L1 (Figure 2G). Western blot analysis showed that the removal of FAK kinase domain and the Y397 mutation did not affect FAK recruitment to L1, whereas deletion of the FERM domain totally abolished it (Figure 2G). Next, we tested L1 constructs having various cytoplasmic truncations removing key functional domains: L1-1146 truncated after the fourth cytoplasmic residue, L1-1176 lacking the binding domain for the cytoskeletal adaptor ankyrin and the AP-2/chlatryn-dependent internalization motif but still containing the binding site for ezrin–radixin–moeisin (ERM) proteins, L1-1180 lacking only the Ank domain and L1-RSLE lacking only the RSLE motif (Cheng et al, 2005). FAK was precipitated from Sema3A-stimulated L1/Nrp1-expressing cells for western blot detection of L1. We found that L1-1180 but not L1-1146 could recruit FAK. FAK/L1 co-precipitation was also detected with L1-1176 and L1-RSLE. Thus, FAK recruitment to L1 requires a sequence comprising 1146–1176 amino acids, including the ERM binding domain but excluding the RSLE and ankyrin binding domains (Figure 2H).
Sema3A-induced FAK-dependent erk1/2 phosphorylation downstream of L1/Nrp1 complex
FAK has complex roles during the turnover of adherent contacts. Recent work established that FAK initiates a signalling cascade leading to activation of the MAPK pathway, which controls the disassembly of adherent contacts (Webb et al, 2004). Interestingly, MAPK activation downstream of L1 also mediates some of the major functions of L1 in cell adhesion and axon growth (Kamiguchi and Lemmon, 2000). Moreover, MAPK activation is already linked to Sema3A-induced growth cone collapse, although the nature of the process affected by this signalling remains unclear (Campbell and Holt, 2003).
As expected, we could detect erk1/2 phosphorylation in neonatal cortical tissues stimulated with Sema3A (Figure 3A). We thus examined whether Sema3A could induce an MAPK signalling in L1/Nrp1-expressing cells. Stimulated cells were processed for immunoprecipitation and western blot analysis. Sema3A could trigger erk phosphorylation in cells expressing L1/Nrp1 but not Nrp1 alone (Figure 3A). To determine whether FAK signalling is required for MAPK activation, cells were first transfected with L1, Nrp1 and either egfp or dominant-negative FAKΔkin. We found that FAKΔkin but not egfp coexpression abolished Sema3A-induced erk1/2 phosphorylation (Figure 3B). Second, cells were transfected with Nrp1 and L1−1146 mutant lacking FAK recruitment and in this condition Sema3A-induced erk phosphorylation was also abrogated (Figure 3C). Next, we examined whether constitutively active FAK although uncoupled to L1 is sufficient for inducing erk phosphorylation. Cells were transfected with L1, Nrp1 and either FAKWT or FAKΔFERM. Western blot analysis showed that erk phosphorylation was still dependent on stimulation by Sema3A (Figure 3D). This ligand-dependent erk phosphorylation was not due to the recruitment of endogenous FAK to L1, as FAKΔFERM overexpression resulted in loss of association of L1 with both FAKΔFERM and endogenous FAK (Figures 2F and 3E). Thus, Sema3A-induced FAK recruitment and activation by L1 can be overcome by uncoupled active FAK, which is nevertheless insufficient for erk phosphorylation. Additional L1-dependent signalling induced by Sema3A might also occur to trigger erk phosphorylation. Consistent with this hypothesis, Sema3A-induced erk phosphorylation was lost in cells expressing Nrp1, FAKΔFERM and the L1−1146 mutant lacking almost all the cytoplasmic domain (Figure 3F).
Figure 3.
L1/Nrp1 mediates Sema3A-induced erk phosphorylation. (A) Stimulation by Sema3A of fresh cortical tissue induces erk1/2 phosphorylation, which is reproduced in L1/Nrp1- but not Nrp1-expressing HEK cells. (B) Introduction of dominant-negative FAKΔkin abrogates Sema3A-induced erk1/2 phosphorylation in L1/Nrp1-expressing cells. (C) Sema3A-induced erk phosphorylation is lost in cells expressing Nrp1 and L1−1146 mutant lacking FAK recruitment. (D) Sema3A still triggers erk phosphorylation in cells overexpressing constitutively active FAK (FAKΔFERM) even though this construct blocks the recruitment of endogenous FAK. (E) The blots show bands at 125 kDa (endogenous FAK, FAKWT, FAKΔkin) and at 70 kDa (FAKΔFERM). (F) Sema3A triggers erk phosphorylation in cells expressing L1WT/FAKWT and L1WT/FAKΔFERM but not L1−1146/FAKΔFERM. (G) Introduction of dominant-negative Plex-GPI does not abrogate Sema3A-induced erk1/2 phosphorylation in L1/Nrp1-expressing cells.
Finally, we examined whether the cascade downstream of L1 requires Plexin-As signalling because HEK cells express endogenous Plexin-As. However, erk1/2 phosphorylation was still detected in HEK cells transfected with L1/Nrp1 and dominant-negative truncated form of Plexin-A1 in which the cytoplasmic domain has been removed and replaced by a GPI anchor (Plex-A1GPI; Toyofuku et al, 2004; Figure 3G).
L1-mediated FAK and MAPK activation is required for Sema3A-induced growth cone collapse
To address whether L1-mediated MAPK pathway is required for cortical responses to Sema3A, we disturbed this activation. Two L1 serine residues, mutated in the L1-related human neurodevelopmental disease, have been found to alter the ability of L1 to trigger erk1/2 phosphorylation (Needham et al, 2001). The pathological substitution of the serines S1194 and S1224 by leucine prevented neither L1 cell surface expression nor interaction with Nrp1 (Supplementary Figure 1D). When coexpressed with Nrp1 in HEK cells, these mutated L1 constructs still mediated Sema3A-induced autophosphorylation of FAK. In contrast, erk1/2 phosphorylation was totally lost (Figure 4A). L1WT, L1−1146, L1S1194L and L1S1224L IRES EGFP vectors were overexpressed in cortical neurons in collapse assays. Overexpression of L1−1146 but not L1WT totally blocked Sema3A-induced growth cone collapse (Figure 4B, a total of 320 cones from 12 neonatal brains). Similarly, the L1 serine mutations partially (for L1S1224L) and totally (for L1S1194L) abrogated Sema3A-induced growth cone collapse (Figure 4B, a total of 383 cones from 12 neonatal brains). To confirm that L1 signalling is required for the guidance of cortical axons, we took advantage of the previously developed slice overlay assay, assessing axon trajectory of dissociated cortical neurons grown on top of cortical slices. In normal conditions, about 65% of the axons are directed towards the white matter by Sema3A repulsive activity emanating from the pial side (Polleux et al, 1998). Using this assay, we found that overexpression of FAKΔkin and L1S1194L profoundly disorganized axon trajectory, as only 21 and 27% of axons were properly guided towards the white matter side, respectively (Supplementary Figure 2).
Figure 4.
L1 mutants interfere with Sema3A-induced erk phosphorylation, release of Paxillin/L1 interaction and collapse response. (A) The L1 serine mutations 1194 and 1224 abolished the Sema3A-induced erk1/2 phosphorylation in L1/Nrp1-expressing cells. Y397 FAK phosphorylation is still detected in cells expressing complexes of Nrp1 and L1S1194L and L1S1224L. (B) Collapse assays with cortical neurons overexpressing the L1 variants L1S1224L, L1S1194L and L1−1146. Microphotographs illustrate the loss of collapse response due to these overexpressions. Histograms depict the percentage of growth cone collapse showing defective responsiveness of neurons expressing L1S1224L, L1S1194L, L1−1146 but not L1WT. *P<0.001 with χ2 test. Scale bar: 25 μm. (C) Co-precipitation of L1 with Paxillin but not talin and vinculin, indicating that L1 complexes in the cerebral cortex contain paxillin. lpIP: lysate post immunoprecipitation. Paxillin/L1 co-precipitation is decreased by Sema3A. (D) Sema3A induces Paxillin release from L1 in L1/Nrp1-expressing cells. (E) L1/Nrp1 mediates Sema3A-induced paxillin phosphorylation at Y31. (F) Sema3A-induced release of Paxillin/L1 interaction is not prevented by the application of the MEK1 inhibitor U0126.
L1-mediated FAK–MAPK signalling controls the disassembly of paxillin+ adhesion points induced by Sema3A
The nature of the process regulated by this L1-dependent FAK–MAPK activation found required for Sema3A-induced growth cone collapse remained undetermined. The FAK–MAPK cascade was shown to control focal adhesion disassembly in migrating cells (Webb et al, 2004). Moreover, other recent work described that Sema3A at a non-collapsing dose destabilizes adherent points of the growth cones, visualized by the focal adhesion marker Paxillin (Woo and Gomez, 2006). Paxillin is an adaptor protein recruited to dynamic focal contacts and has a crucial role in adhesion turnover. Interestingly for the present context, Paxillin forms a scaffold for activation of erk at focal contacts (Ishibe et al, 2003). It constitutively binds MEK. On phosphorylation by FAK in response to extracellular stimuli, Paxillin recruits inactive erk and active Raf, resulting in local erk activation. This prompted us to investigate first the links between L1 and Paxillin and second the possibility that L1-dependent FAK–MAPK activation mediates Sema3A-induced adhesion disassembly. First, fresh cortical tissues were stimulated with control and Sema3A treatment and L1 was immunoprecipitated for western blot analysis of Paxillin. Strong interaction of L1 with Paxillin but not other proteins of focal adhesions such as talin and vinculin was found in control condition, indicating that L1 might be associated with focal adhesion complexes (Figure 4C). Notably, this Paxillin-L1 association was strongly decreased by Sema3A (Figure 4C). Likewise, quantification of the bands showed that the ratio between precipitated L1 and Paxillin was reduced by 51 and 71% for each Paxillin band in the Sema3A condition compared to the control condition. Second, this Sema3A-induced regulation of Paxillin/L1 interaction could be fully recapitulated in L1/Nrp1-expressing cells (Figure 4D). Third, in these cells, Sema3A could induce paxillin phosphorylation at Y31, a residue known to be phosphorylated by FAK (Mitra et al, 2005; Figure 4E). Next, we assessed whether the S1194L L1 mutation could interfere with the dynamic of L1/paxillin interaction. Strikingly, L1S1194L still bound Paxillin but was totally unable to release it upon Sema3A stimulation (Figure 4D). Thus, the S1194L mutation blocks both Sema3A-induced Paxillin release and erk phosphorylation. When L1/Nrp1-expressing cells were stimulated in the presence of the MEK inhibitor U0126 (10 μM), erk phosphorylation was totally abolished whereas but paxillin release from L1 was not prevented (Figure 4F). Thus, Paxillin release does not occur downstream of erk phosphorylation.
Overall, these data pointed to a model in which L1 couples the Sema3A receptor to Paxillin+ focal adhesion points and enables Sema3A to modulate their dynamics. To test this model, we first examined whether modulation of adherent points induced by low dose of Sema3A (0.1 nM, found inefficient to induce collapse, data not shown) could be detected in our culture model. Phalloidin and paxillin co-labelling was examined by confocal microscopy. As expected, punctate paxillin staining was distributed over the growth cone structures and was reduced by Sema3A application (Figure 5A). When Paxillin was co-labelled with Nrp1, Nrp1 labelling was qualitatively and quantitatively unchanged by low-dose Sema3A application, whereas paxillin staining was profoundly reduced and no longer spread throughout the growth cone (Figure 5A and data not shown). Quantitative analysis of the fluorescence (see Materials and methods) confirmed that Sema3A significantly decreased Paxillin fluorescence (Figure 5B and C). FAKΔkin overexpression and application of the MAPK pharmacological inhibitor U0126 (5 μM) could block the normal decrease of Paxillin (Figure 5B and C). Application of U0126 (5 μM) in the control condition did not modify the basal Paxillin level. Next, L1WT, L1S1194L and L1−1146 were co-electroporated in cortical neurons. The introduction of L1WT did not interfere with the Sema3A-dependent removal of paxillin whereas L1S1194L strikingly abolished it (Figure 5B and C). Thus, Sema3A-induced FAK and MAPK activation controls the disassembly of adherent points, and L1 mutants lacking FAK and MAPK activation disrupt this process. Plexins mediate the suppression of integrin-dependent adhesions (Serini et al, 2003; Barberis et al, 2004; Toyofuku et al, 2005). Thus, by preventing new contact formation or maturation, Plexin signalling should also trigger paxillin decrease in Sema3A-treated neuronal growth cones. As several plexin-As are expressed in cortical neurons, we thought that overexpression of cytoplasmic deleted Plexin-A1 form should favour the formation of non-functional Plexin–Nrp1 complexes, thus interfering with the signalling by all endogenous Plexin-As. We observed that Sema3-induced paxillin removal was very marked in cortical neurons overexpressing Plex-A1WT, whereas it was effectively weakened but still significant in neurons overexpressing Plex-A1GPI, consistent with still active signalling downstream of L1/Nrp1 (Figure 5B and C).
Figure 5.
The L1-dependent FAK–MAPK pathway mediates Sema3A-induced disassembly of paxillin+ adhesion points in cortical growth cones. (A) Confocal images of phalloidin and Paxillin staining in cortical growth cones showing Paxillin decrease induced by subcollapsing dose of Sema3A. The two lower panels illustrate that Paxillin (in red) but not Nrp1 (in blue) is decreased by Sema3A. (B, C) Quantitative analysis and illustrations of paxillin fluorescence in cortical growth cones. Pharmacological inhibition of MAPK and overexpression of dominant-negative FAKΔkin and L1 variants abolish Paxillin decrease normally induced by low dose of Sema3A in non-transfected condition (NT). Paxillin decrease takes place in cells overexpressing PlexA1WT and dominant-negative Plex-A1GPI. Scale bars: 25 μm.
L1 and Plexin-As are coexpressed in cortical growth cones
To get further insights into the links between L1 and Plexin-As, we investigated their distribution to determine whether they segregate in subsets or cortical neurons or rather colocalize in common pools. Co-labelling of L1 with three Plexin-A members was performed in cultured cortical neurons. We could not analyse the expression of Plexin-A4, owing to the lack of available antibodies. Interestingly, Plexin-A1, Plexin-A2 and Plexin-A3 were individually found coexpressed with L1 in cortical growth cones and thus did not label distinct pools of cortical neurons. However, they only partially colocalized within the growth cone (Figure 6).
Figure 6.
Coexpression of Plexin-As and L1 in cortical neurons and dominant-negative effects of Plexin-A1 and L1 variants on Sema3A-induced growth cone collapse. (A) Confocal images of cortical neurons and their cortical growth cones, showing coexpression of L1 (in green) with Plexin-A1, Plexin-A2 and Plexin-A3 (in red). The distribution of L1 and Plexin-A proteins only partially colocalized both in the growth cone and the soma. Pictures illustrate cases of cortical growth cones with variable levels of Plexin-A/L1 colocalization. Scale Bar: 50 μm. (B, C) Collapse assays with cortical neurons overexpressing L1WT, L1Δcyt, Plex-A1WT, Plex-A1GPI and Plex-A1sol. (B) Microphotographs illustrate the morphological collapse induced by Sema3A on growth cones, visualized by EGFP labelling. The histogram depicts the percentage of growth cone collapse and the significant reduction of responsiveness to Sema3A of neurons overexpressing L1Δcyt but not L1WT, compared to wild-type neurons. (C) Overexpression of Plex-A1GPI but not of Plex-A1WT or Plex-A1sol disrupted Sema3A-induced growth cone collapse. *P<0.001, with χ2 test. Scale bar: 10 μm. (D, E) Precipitation of L1 (D) and Plexin-As (E) from neonatal (P0) cortical tissues showing that L1 and plexin-A proteins are present in common complexes.
Cytoplasmic deleted forms of L1 and Plexin-A1 disrupt growth cone collapse induced by Sema3A
Next, we introduced in cortical neurons L1 and Plexin-As cytoplasmic deleted forms to interfere with their signalling activities. First, wild-type and cytoplasmic deleted form of L1 were overexpressed with EGFP by electroporation of cortical neurons. We observed that overexpression of L1Δcyt but not L1WT significantly reduced Sema3A-induced growth cone collapse response (Figure 7B, a total of 254 growth cones scored from 8 neonatal brains). Second, overexpression of full-length Plexin-A1 did not abolish the collapse-inducing effect of Sema3A, whereas the Plex-A1GPI form totally blocked it (Figure 7C, a total of 240 growth cones scored from 8 neonatal brains). Overexpression of a secreted form of the Plexin-A1 ectodomain (Plex-A1sol) was in contrast inefficient (Figure 7C). Thus, both cytoplasmic truncated forms of L1 and Plexin-A interfere with Sema3A-induced collapse responses, indicating that L1 and Plexin-As might be jointly required. If so and although not directly interacting, L1 and Plexin-As might be present in vivo in common receptor complexes through association with Nrp1. Co-precipitation experiments performed on neonatal cortical tissue, using antibodies to L1 and a mixture of antibodies to Plex-A1, Plex-A2 and Plex-A3 to collectively detect these Plexin-As, confirmed this hypothesis (Figure 7D).
Figure 7.
Defects of Nrp1+ cortical projections in mice lacking Plexin-A3, Plexin-A4 or L1. (A) Horizontal sections of neonatal brains immunolabelled with antibodies to Nrp1. The upper panels show reduction of Nrp1+ axons in the intermediate zone of the cerebral cortex (white arrows) and in the internal capsule (black arrows) in mice lacking Plexin-A3, Plexin-A4 and L1, compared with wild-type mice. The intermediate panels show that the dorsal positioning of Nrp1+ axons in the corpus callosum is maintained in different null mutants, but in Plexin-A4 knockout mice, axons prematurely stop crossing the midline (asterisk). The lower panels show a magnified image of the internal capsule and delineate the striatum (black lines) and the area occupied by Nrp1+ axon bundles (white lines) in each type of mouse. (B, C) Nrp1+ corticospinal axons (black arrows) diverging from Nrp1+ corticothalamic axons (white arrows in the magnifications) are detected in wild-type and Plexin-A4 null mice but are lost in Plexin-A3 and L1 null mutants. (D) Magnification showing the reduced area (white line) occupied by cortical axons innervating the dorsal thalamus (delineated by a black line) in Plexin-A3, Plexin-A4 and L1 null mutants compared with wild-type mice. (E) The Nrp1+ corticospinal tract in the cerebral peduncle is lost in mice lacking Plexin-A3 and L1, and reduced in mice lacking Plexin-A4 (black arrows). Scale bar: 1 mm.
Mice lacking Plexin-A3, Plexin-A4 or L1 have profound defects of Nrp1+ cortical projections
If L1 and Plexin-As mediate together the guidance effects of Sema3A, the genetic removal of L1 or Plexin-As should thus impact the formation of cortical pathways. Cortical axons navigate into two main pathways, the corpus callosum and the internal capsule for reaching, respectively, cortical and subcortical targets. We thus examined these cortical pathways in mice lacking Plexin-A3, Plexin-A4 or L1. Because both Plexin-As and L1 are implicated in axonal responses to cues other than Sema3A, we focused our analysis on projections expressing Nrp1. In coronal sections of neonatal brains, Nrp1+ axons extending in the intermediate zone of the cerebral cortex and coursing in the internal capsule were strongly reduced in mice lacking Plexin-A3, Plexin-A4 and L1 compared to wild type mice (Figure 8Aa). This reduction was particularly marked for laterally oriented axon bundles and also clearly detected in horizontal brain sections (Supplementary Figure 3). Remnant medial bundles were in addition abnormally oriented in mice lacking Plexin-A3 and L1 but not obviously in mice lacking Plexin-A4. In coronal and horizontal sections of wild-type mice, Nrp1+ axons of the corpus callosum are present at dorsal and anterior parts. This spatial organization was not modified to a greater extent in the different Plexin mutant genotypes, except that in mice lacking L1, Nrp1+ axons were absent in the anterior pole of the corpus callosum (Supplementary Figure 4). In addition, in Plexin-A4−/− mice but not Plexin-A3−/Y mice, Nrp1+ callosal axons prematurely stopped crossing the midline at caudal levels and accumulated on ipsilateral sides (Figure 8Aa). The corticospinal tract was also strikingly altered in these mutant mice. Nrp1+ corticospinal axons diverging from corticothalamic axons in the reticular thalamic nucleus were clearly detected in wild-type mice. Notably, this Nrp1+ diverging corticospinal tract was totally absent in Plexin-A3−/Y and L1−/Y mice, whereas it was still detected, although reduced, in Plexin-A4−/− mice (Figure 8Ab and c). Consequently, Nrp1+ corticospinal axons were also absent in the cerebral peduncle of mice lacking Plexin-A3 and L1 (Figure 8Ae). Finally, the innervation of the thalamus was also defective. Nrp1+ cortical axons innervating the dorsal thalamus were almost absent in mice lacking Plexin-A4 and L1, and were only partially detected in mice lacking Plexin-A3 (Figure 8Ad). Thus, the genetic removal of Plexin-A3, Plexin-A4 and L1 leads to important alteration of cortical projections and L1 knockouts combined the defects of Plexin-A3 and Plexin-A4 knockouts.
Figure 8.
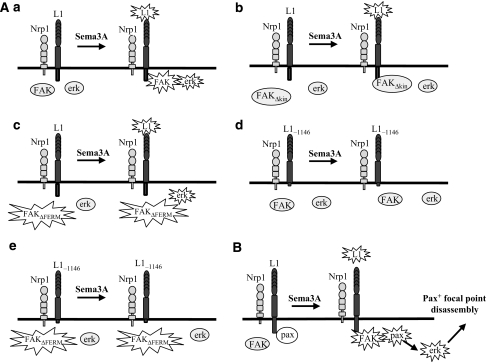
Model for Sema3A signalling downstream of L1. (A) Activation of FAK and MAPK. (a) Sema3A induces recruitment of FAK to L1, FAK and erk activation. Sema3A also triggers activation of L1, presumably involving the S1194 residue. (b) Kinase-dead FAK (FAKΔkin) binds L1 upon Sema3A stimulation but erk phosphorylation is prevented. (c) Constitutively active FAK (FAKΔFERM) prevents Sema3A-induced FAK recruitment to L1 but not erk phosphorylation, reflecting that FAK activity is required but not sufficient, and that other signalling triggered by Sema3A is also needed, for erk phosphorylation. (d, e) Consistently, L1-1146 having truncation of almost all the cytoplasmic domain is unable to mediate erk phosphorylation even in the context of activated FAK (FAKΔFERM). (B) Model for the cascade triggering adhesion disassembly. Upon Sema3A stimulation, L1 in the L1/Nrp1 complex is modified, recruits FAK and releases Paxillin. These activations trigger erk phosphorylation leading to disassembly of Paxillin+ focal points.
Discussion
Sema3A-induced FAK–MAPK signalling triggered by L1/Nrp1 interaction controls the disassembly of adhesion points of the growth cones
From the initial observation that L1 and Nrp1 ectodomains interact and that neuronal growth cones lacking L1 are insensitive to Sema3A in vitro (Castellani et al, 2000), a recurrent question has been the nature of L1-dependent processes occurring during growth cone steering. The present findings show that L1 does not simply act as a binding partner for Nrp1 but controls important events of the growth cone collapse. Our results support a model in which L1/Nrp1 interaction activates a FAK–MAPK cascade triggering the disassembly of adhesion contacts, which is required for Sema3A-induced collapse behaviour to take place (Figure 8A and B).
Several data support this model. First, the Sema3A receptor complex is associated by L1 to a structural protein of dynamic focal adhesions, Paxillin, and this interaction is released by Sema3A. This coupling may thus provide a basis for Sema3A to suppress adhesion points during growth cone steering. Second, Sema3A stimulates Y397 FAK, Y31 Paxillin and erk1/2 phosphorylation in L1/Nrp1-expressing cells, a cascade initially described to control the disassembly of adhesion sites at the leading edge of protrusions in migrating cells (Webb et al, 2004). Consistently, subcollapsing dose of Sema3A elicits the release of Paxillin+ adhesions in cortical growth cones. Pharmacological inhibition of MAPK and overexpression of inactive FAK variant, L1−1146 and L1S1194L mutants deficient for FAK and/or MAPK activation all abolish Sema3A-mediated disassembly of Paxillin+ adhesions. Finally, our data indicate that this L1-dependent removal of Paxillin+ dynamic adhesions is needed for Sema3A-induced guidance response, as overexpression of inactive FAK and L1 mutants also blocks Sema3A-induced growth cone collapse of cortical neurons and impairs the trajectory of cortical axons in slice overlay assays.
Several issues remain open concerning the mechanisms by which L1 in the Sema3A receptor couples FAK and erk activation to control adhesion disassembly. Our data indicate that FAK signalling is necessary but not sufficient for erk activation. First, overexpression of inactive FAK blocks erk phosphorylation but constitutively active FAK does not mimic Sema3A, as erk phosphorylation remains ligand-dependent. This is also reflected in collapse assays, as kinase-dead FAK abolishes Sema3A-induced growth cone collapse whereas active FAK only induces modest increase of collapse in the absence of Sema3A. Second, FAK but not erk phosphorylation can be detected in cells expressing L1S1194L instead of L1WT, suggesting that FAK is not the only trigger of erk phosphorylation. The cytoplasmic domain of L1 contains several tyrosine and serine residues, which are phosphorylated upon stimulation by extracellular cues (Kamiguchi and Lemmon, 2000). S1194 phosphorylation has not been described so far but given the pathogenic consequences of its mutation, this residue might be critical for L1 functions. Likewise, integrin-dependent stimulation of cell migration by L1 was found impaired by this mutation (Thelen et al, 2002). We do have evidence that L1 becomes serine phosphorylated upon Sema3A stimulation in an S1194-dependent manner (unpublished observations). Additional work will be needed for identifying the pathways controlling this phosphorylation. Interestingly, here we found that in addition to preventing erk phosphorylation, the S1194L mutation disrupts Sema3A-induced Paxillin release. Previous work established that upon phosphorylation by FAK, Paxillin recruits inactive erk and triggers its activation at focal contacts and that Paxillin acts upstream of erk phosphorylation in adhesion disassembly in cell protrusions (Ishibe et al, 2004; Webb et al, 2004). Thus in the present context, erk phosphorylation could be controlled by Paxillin, upon its release from L1. Alternatively, FAK activation and paxilin release could enable L1 to recruit ranBPM, a signalling molecule that interestingly can trigger erk phosphorylation and is also downstream of Plexin-A in the Sema3A response (Cheng et al, 2005; Togashi et al, 2006). Future study will also focus on the mechanisms by which adhesion disassembly is achieved. It could result from actinomyosin contraction triggered by the myosin-like chain kinase downstream of FAK–MAPK signaling, which can break adherent contacts and could also involve RHOA kinase signalling (Crowley and Horwitz, 1995; Mitra et al, 2005).
Intriguingly, FAK has been implicated as a positive and negative regulator of axon growth and branching and, in the context of axon guidance, it participates in both attractive and repulsive guidance effects (Li et al, 2004, 2006; Liu et al, 2004; Rico et al, 2004; Falk et al, 2005; Parri et al, 2007). As in cell protrusions, FAK continuously relocalizes from disassembled adhesion points to newly formed ones in advancing growth cones and, possibly, remodelling of adherent contacts might be required for coupling adhesion turnover and growth cone steering. Whatever the attractive or repulsive nature of the guidance effects, polarization of adherent points could be correlated with the direction of the growth cone steering. Alternatively and owing to its multifunctional nature, FAK could recruit different signalling effectors to control events that would be specific for attractive and repulsive effects.
Interaction of Nrp1 with L1 but not Plexin-A mediates Sema3A-induced FAK signalling
We found that FAK does not constitutively associate with Plexin-A, Nrp1 or L1 individual subunits but only with L1 in the L1/Nrp1 complex. Sema3A strengthens this recruitment and also induces the phosphorylation of FAK and its downstream target paxillin. Thus, this strongly suggests that we have not identified a general binding partner for L1 but rather a specific signalling effector of L1 function in the Sema3A receptor. Notably, we failed to detect any interaction between Plexin-As and FAK, consistent with previous work (Barberis et al, 2004). Overexpression of a truncated Plex-A1 totally disrupted Sema3A-induced growth cone collapse, probably due to the loss of Plexin signalling to actin dynamics. However, removal of adhesion sites of growth cones still occurred, presumably reflecting L1 signalling. It should be noted from this condition however that the loss of paxillin was less pronounced than when the integrity of Plexin signalling was preserved. Thus, the activation of the FAK cascade likely specifically relies on the L1 subunit in the Sema3A receptor but the final downregulation of adhesion sites might be controlled by coincident Plexin and L1 signalling. Several previous works indeed reported that Plexin signalling suppresses integrin-dependent adhesions (Barberis et al, 2004; Serini et al, 2004; Garrity, 2005; Toyofuku et al, 2005). It is thus plausible that the Sema3A receptor initiates a Plexin signalling to inhibit the formation and/or the maturation of new adhesion points, and an L1 signal to disassemble existing contacts, with both effects converging on the downregulation of adherent points.
Joint requirement for L1 and Plexin-As in the development of cortical pathways
Immunolabelling revealed that cortical growth cones coexpress L1 with at least one Plexin-A and segregation of cortical neurons in pools expressing either a Plexin-A or L1 was not found, consistent with joint requirement for at least one Plexin-A member with L1 in the same cortical neurons. Interestingly, association of L1 and Plexin-A proteins could be detected in the developing cerebral cortex and, in brain sections, L1 was also found expressed by cortical fibres whose trajectory was disorganized in mice lacking Plexin-A3 and Plexin-A4. We cannot exclude that L1 and Plexin-As segregate in subsets of cortical axons projecting to common targets, but it is however noticeable that a majority of cortical neurons that were isolated from the whole cerebral cortex were sensitive to the dominant-negative effects of both truncated L1 and Plexin-A1 overexpression. Analysis of mouse models provides substantiate this conclusion, as the consequences of genetic removal of L1 and Plexin-A3 or Plexin-A4 show striking similarities in the reduction of Nrp1-expressing fibres along cortical pathways. Likewise, the genetic removal of Plexin-A3, Plexin-A4 or L1 leads to drastic reduction of Nrp1+ axons in the intermediate zone of the cerebral cortex and the internal capsule. Moreover, both Plexin-A3 and L1 knockout mice lack Nrp1+ corticospinal axons, whereas Plexin-A4 and L1 knockout mice show common strong reduction of Nrp1+ cortical axons innervating the dorsal thalamus. These data thus strongly suggest that Plexin-A3 and Plexin-A4 act with L1 to specify the trajectory of Nrp1+ axons. It is also clear from this analysis that the defects are more marked in L1 knockouts than in Plexin-A3 and Plexin-A4 knockouts. This could be due to compensation for the loss of Plexin-A3/A4 by other Plexin-As. Alternatively, the genetic removal of L1 could alter another important signalling for the guidance of Nrp1+ axons. Conversely, some of the defects observed in the L1−/Y mice are unlikely due to loss of Sema3A responses, as abnormal hyper-fasciculation of axon bundles expressing DCC but not Nrp1 was observed in the internal capsule. Similarly, we cannot exclude that additional Plexin-A-dependent signalling also accounts for the defects described here, as Plexin-As also contribute to the response to other semaphorins. Overall, the observation that different Plexins are used by different axons to mediate the response to a given semaphorin has a precedent in the finding that Sema3F effects require Plexin-A3 in hippocampal fibres but Plexin-A4 in axons forming the anterior commissure (Cheng et al, 2001; Suto et al, 2005; Yaron et al, 2005).
Altogether, these data support the idea that L1 and Plexin-A might be joint components of common Sema3A receptors. However, given their only partial colocalization in cortical growth cones, an additional possibility is that partitioning of some Plexin-A and L1 pools in physically separated membrane domains of the growth cone could also underlie their recruitment to different receptor complexes and the initiation of distinct signalling cascades at distinct sites of the same growth cone. Future direction will focus on a detailed analysis of spatio-temporal dynamics of L1, Plexin-As and their downstream effectors during growth cone steering. Finally, independent activation of the Plexin-A/Nrp1 and L1/Nrp1 complexes to modify growth cone adhesion properties without inducing collapse could also enable Sema3A to control processes other than growth cone steering during axon navigation, such as axo–axonal and axon–substrate interactions.
Materials and methods
Immunohistochemical analysis of Plexin-A3/A4 and L1 mutants
Genotypes were determined by PCR as described by Itoh et al (2004) for L1 mice and by Yaron et al (2005) for Plexin-A3 and Plexin-A4 mice. Free-floating 60-μm-thick horizontal and coronal sections from neonatal brains were incubated overnight with antibodies to DCC (Oncogen, 1:100), to L1 (Hybridoma Bank, 1:50) or to Nrp1 (R&D, 1:50) and processed for DAB labelling as described by Falk et al (2005).
cDNA construction
Point mutations in L1 were generated by the Quickchange site-directed mutagenesis kit (Promega) according to the manufacturer's specifications and were confirmed by DNA sequencing. Mutations were produced by using pcDNA3.1-L1 as template and the following primers: for mutation S1194L, 5′-GGCAGCAGCCAGCCATTGCTCAACGGGACATC-3′ and 5′-GATGTCCCCGTTGAGCAATGGCTGGCTGGCTGCTGCC-3′; for mutation S1224L, 5′-GTTCAACGAGGATGGTTTGTTCATTGGCCAGTACAG-3′ and 5′-CTGTACTGGCAATGAACAAACCATCCTCGTTGAAC-3′. The ires EGFP L1 construct was cloned by PCR amplification for the ires EGFP (pIRES2-EGFP) with the primers 5′-ATCTCGAGCAAATGTATGGCTGATTATGATC-3′ and (5′-GAGGTCTATATAAGCAGAGCTGGTTTAGTG-3′. The amplified fragment and L1 constructions were digested by Xho1 and ligated.
Electroporation of neuronal cell cultures, collapse and slice overlay assays
P0 mouse cortices were dissected, dissociated and electroporation was performed with Nucleofector (Amaxa). Transfected cells were plated onto polylysin-coated coverslips and grown for 24 h in DMEM/F12 medium supplemented with B27, Glutamax and antibiotics. Collapse assays were performed as described by Castellani et al (2004) and slice overlay assays as described by Polleux et al (1998). Briefly, 250 thick cortical slices were incubated in laminin-coated inserts. Dissociated cortical neurons were electroporated with Nucleofector and placed on top of the slices. After 1 DIV, the cultures were fixed and processed for analysis.
Biochemistry and immunocytochemistry
The following antibodies were used: anti-phosphoserine (Sigma), anti-ERK15C165 and anti-phospho-ERK(E-4) (Santa Cruz), anti-FAK(A-17) (Tebu), anti-phospho-FAK Y397 (Cell Signaling), anti-VSV (Sigma), anti-HA clone 12CA5 (Roche), anti-paxillin (BD Transduction Laboratories) and anti-phospho-Y31 Paxillin (Biosource). The pharmacological inhibitor U0126 (Calbiochem) was added 30 min before control and Sema3A treatments. HEK 293T cells were transfected with vectors encoding L1, L120V, L1Δcyt, L1S1194L, L1S1224L, Nrp1 and Nrp1Δcyt with Exgene (Fermentas). Cells were lysed with immunoprecipitation buffer (HEPES, 25 mM; EDTA, 5 mM; MgCl2, 1 mM; PMSF, 2 mM; glycerol, 10%; and Triton X-100, 1%; pH 7). For biochemistry on fresh tissue, brains from P0 embryos were dissected out, cut into small pieces and stimulated with Sema3A and control supernatants as described by Falk et al (2005). Tissues were lysed for 1 h in a mixture containing 50 mM Tris pH 7.5, 1 mM EDTA, 1 mM EGTA, 0.5 mM Na3VO4, 0.1% 2-mercaptoethanol, 1% Triton X-100, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 10 mM sodium β-glycerol phosphate, 0.1 mM PMSF and 1 μg/ml each of aprotinin, pepstatin and leupeptin. In some experiments, purification of the membrane fraction was carried out as described by Falk et al (2005). For immunocytochemistry, cells were fixed with 4% paraformaldehyde for 15 min at room temperature (RT) and permeabilized or not with 0.02% Triton X-100 in PBS for 15 min at RT. Cells were incubated with primary antibodies for 2 h at RT followed by incubation with secondary antibodies for 1 h at RT. Immunohistochemical labelling of brain sections was performed according to Falk et al (2005). Polyclonal rabbit antibodies to Plexin-A1, Plexin-A2 and Plexin-A3 (Santa Cruz, 1:100) were used for immunodetection in neuronal cultures.
Quantitative analysis of Paxillin staining
Cultures of cortical neurons were treated with Sema3A-AP or AP supernatants for 20 min, fixed with 4% paraformaldehyde and processed for triple immunolabelling to detect Paxillin, Nrp1 and the electoporated construct by its tag. Growth cones were imaged with a microscope (Axiovert 200M, Zeiss). Images were captured under constant parameters with a Coolsnap CCD camera (Photometrics, Evry, France) under non-saturating exposure conditions and analysed with the ImageJ software (National Institutes of Health, USA). The growth cone surface was delineated with Nrp1 labelling. After background subtraction, the intensity of Nrp1 and paxillin staining was measured within the outline and the mean intensity per pixel was calculated. Intensity values were normalized to the respective control experiments.
Supplementary Material
Supplementary Figures 1–4
Supplementary Figure Legends
Acknowledgments
We are grateful to S Garrel for helpful discussion, F Rathjen for anti-L1 antibodies and A Püschel and S Strittmatter for Plexin-As constructs. This work was supported by grants to VC from the CNRS ATIP programme, Association Française Contre les Myopathies (AFM), Fondation Pour la Recherche Médicale (FRM) and ANR Neuroscience.
References
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J (1998) Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development 125: 5043–5053 [DOI] [PubMed] [Google Scholar]
- Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L (2004) Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J 18: 592–594 [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE (2003) Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 37: 939–952 [DOI] [PubMed] [Google Scholar]
- Carter N, Nakamoto T, Hirai H, Hunter T (2002) EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat Cell Biol 4: 565–573 [DOI] [PubMed] [Google Scholar]
- Castellani V (2002) The function of neuropilin/L1 complex. Adv Exp Med Biol 515: 91–102 [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G (2000) Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27: 237–249 [DOI] [PubMed] [Google Scholar]
- Castellani V, Falk J, Rougon G (2004) Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol Cell Neurosci 26: 89–100 [DOI] [PubMed] [Google Scholar]
- Castellani V, Rougon G (2002) Control of semaphorin signaling. Curr Opin Neurobiol 12: 532–541 [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tcssier-Lavigne M (2001) Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron 32: 249–263 [DOI] [PubMed] [Google Scholar]
- Cheng L, Lemmon S, Lemmon V (2005) RanBPM is an L1-interacting protein that regulates L1-mediated mitogen-activated protein kinase activation. J Neurochem 94: 1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LA, Guan JL (2005) Residues within the first subdomain of the FERM-like domain in focal adhesion kinase are important in its regulation. J Biol Chem 280: 8l97–8207 [DOI] [PubMed] [Google Scholar]
- Cohen NR, Taylor JS, Scott LB, Guillery RW, Soriano P, Furley AJ (1998) Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol 8: 26–33 [DOI] [PubMed] [Google Scholar]
- Contestabile A, Bonanomi D, Burgaya F, Girault JA, Valtorta F (2003) Localization of focal adhesion kinase isoforms in cells of the central nervous system. Int J Dev Neurosci 21: 83–93 [DOI] [PubMed] [Google Scholar]
- Crowley E, Horwitz AF (1995) Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol 131: 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N (2007) Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet 17: 346–349 [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, Maness PF (1999) Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J Neurosci 19: 4907–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, Sanes JR, Castellani V (2005) Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 48: 63–75 [DOI] [PubMed] [Google Scholar]
- Garrity PA (2005) Tinker to Evers to Chance: semaphorin signaling takes teamwork. Nat Neurosci 8: 1635–1636 [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD (2003) Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 5: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wang KC, Koprivica V, Ming G, Song HJ (2002) Knowing how to navigate: mechanisms of semaphorin signaling in the nervous system. Sci STKE RE1 [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Liu ZX, Cantley LG (2004) Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell 22: 257–267 [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Zhu X, Cantley LG (2003) Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. MolCell 12: 1275–1285 [DOI] [PubMed] [Google Scholar]
- Itoh K, Cheng L, Kamei Y, Fushiki S, Kamiguchi H, Gutwein P, Stoeck A, Arnold B, Altevogt P, Lemmon V (2004) Brain development in mice lacking L1-L1 homophilic adhesion. J Cell Biol 165: 145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V (2000) IgCAMs: bidirectional signals underlying neurite growth. Curr Opin Cell Biol 12: 598–605 [DOI] [PubMed] [Google Scholar]
- Kruger RP, Aurandt J, Guan KL (2005) Semaphorins command cells to move. Nat Rev Mol Cell Biol 6: 789–800 [DOI] [PubMed] [Google Scholar]
- Li W, Aurandt J, Jurgensen C, Rao Y, Guan KL (2006) FAK and Src kinases are required for netrin-induced tyrosine phosphorylation of UNC5. J Cell Sci 119: 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL (2004) Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci 7: 1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y (2004) Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci 7: 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B (2000) Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol 2: 62–69 [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6: 56–68 [DOI] [PubMed] [Google Scholar]
- Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H (2001) Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn 220: 246–258 [DOI] [PubMed] [Google Scholar]
- Needham LK, Thelen K, Maness PF (2001) Cytoplasmic domain mutations of the L1 cell adhesion molecule reduce L1–ankyrin interactions. J Neurosci 21: 1490–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Tan-Takeuchi K, Kutsche M, Schachner M, Uyemura K, Kawamura K (2004) Neural cell adhesion molecule L1 is required for fasciculation and routing of thalamocortical fibres and corticothalamic fibres. Neurosci Res 48: 471–475 [DOI] [PubMed] [Google Scholar]
- Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, Ramponi G, Chiarugi P (2007) EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem 282: 19619–19628 [DOI] [PubMed] [Google Scholar]
- Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A (1998) Patterning of cortical efferent projections by semaphoring–neuropilin interactions. Science 4: 1904–1906 [DOI] [PubMed] [Google Scholar]
- Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, Song HJ, Mei L, Xiong WC (2004) Focal adhesion kinase in netrin-1 signaling. Nat Neurosci 7: 1204–1212 [DOI] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF (2004) Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci 7: 1059–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Püschel AW, Bussolino F (2003) Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424: 391–397 [DOI] [PubMed] [Google Scholar]
- Suto F, lto K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H (2005) Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci 25: 3628–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen K, Kedar V, Panicker AK, Schmid RS, Midkiff BR, Maness PF (2002) The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J Neurosci 15: 4918–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Schmidt EF, Strittmatter SM (2006) RanBPM contributes to Semaphorin3A signaling through plexin-A receptors. J Neurosci 26: 4961–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H (2005) FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci 8: 1712–1719 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H (2004) Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 18: 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF (2004) FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6: 154–161 [DOI] [PubMed] [Google Scholar]
- Woo S, Gomez TM (2006) Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci 26: 1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M (2005) Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 45: 513–523 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1–4
Supplementary Figure Legends