Abstract
Macromolecular gadolinium (Gd)(III) complexes have a prolonged blood circulation time and can preferentially accumulate in solid tumors, depending on the tumor blood vessel hyperpermeability, resulting in superior contrast enhancement in magnetic resonance (MR) cardiovascular imaging and cancer imaging as shown in animal models. Unfortunately, safety concerns related to these agents’ slow elimination from the body impede their clinical development. Polydisulfide Gd(III) complexes have been designed and developed as biodegradable macromolecular magnetic resonance imaging (MRI) contrast agents to facilitate the clearance of Gd(III) complexes from the body after MRI examinations. These novel agents can act as macromolecular contrast agents for in vivo imaging and excrete rapidly as low-molecular-weight agents. The rationale and recent development of the novel biodegradable contrast agents are reviewed here. Polydisulfide Gd(III) complexes have relatively long blood circulation time and gradually degrade into small Gd(III) complexes, which are rapidly excreted via renal filtration. These agents result in effective and prolonged in vivo contrast enhancement in the blood pool and tumor tissue in animal models, yet demonstrate minimal Gd(III) tissue retention as the clinically used low-molecular-weight agents. Structural modification of the agents can readily alter the contrast-enhancement kinetics. Polydisulfide Gd(III) complexes are promising for further clinical development as safe, effective, biodegradable macromolecular MRI contrast agents for cardiovascular and cancer imaging, and for evaluation of therapeutic response.
Keywords: biodegradable, contrast agents, gadolinium, MRI, polydisulfide
Introduction
Magnetic resonance imaging (MRI) is a clinical diagnostic modality based on differences in the longitudinal and transverse relaxation rates (1/T1 or 1/T2) of water protons in different tissues (Liang and Lauterbur 2000). MRI signal intensity reflects the value of the relaxation rate of tissues. Contrast agents have been developed and used to enhance MR image contrast and to improve diagnostic accuracy. Paramagnetic metal chelates, eg, Gd(III), Fe(III), and Mn(II) complexes, can alter the relaxation rate of the surrounding water protons to allow for more effective MRI contrast enhancement (Caravan et al 1999). Gadolinium (Gd)(III) ions have seven unpaired electrons and a large paramagnetic moment. Gd(III) chelates with high stability, eg, Gd-DTPA, Gd-DOTA, or their derivatives, have been mainly developed as MRI contrast agents, which increase both 1/T1 and 1/T2 relaxivities (Weinmann et al 1984; Magerstadt et al 1986; Hayne et al 1989). Currently, Gd-containing contrast agents are used in approximately 30% of MRI examinations.
The Gd chelates approved for human use are nonspecific and have unfavorable pharmacokinetics. These low-molecular-weight agents rapidly extravasate from the vasculature and nondiscriminatively distribute in the extracellular space. The agents have a very short blood half-life and a short time window for contrast-enhanced imaging. In many situations it is difficult to estimate the timing for optimal imaging, impeding accurate diagnostic imaging. Macromolecular Gd(III) complexes have been developed to improve contrast-enhanced MR imaging. Macromolecular contrast agents are prepared by the conjugation of Gd(III) DTPA, DOTA complexes, or their derivatives to synthetic polymers (Schuhmann-Giampieri et al 1991; Bogdanov et al 1993; Wiener et al 1994; Kobayashi and Brechbiel 2004; Langereis 2004) or natural macromolecules (Lauffer and Brady 1985; Wang et al 1990; Sirlin et al 2004), or by copolymerization of the low-molecular-weight contrast agents (Kellar et al 1997; Ladd et al 1999; Duarte et al 2001). These agents have a long blood circulation time and can preferentially accumulate in solid tumor tissue depending on the hyperpermeability of the tumor vasculature (Gerlowski and Jain 1986; Hobbs et al 1998). As a result, they provide superior contrast enhancement in the blood pool and in solid tumor tissue in animal models (Gossmann et al 1999; Turetschek et al 2001; de Lussanet et al 2005).
A number of macromolecular Gd(III) complexes have been investigated as blood-pool contrast agents (Schuhmann-Giampieri et al 1991; Bogdanov et al 1993; Shibata et al 1995; Van Beers et al 1995; Roberts et al 1997; Kobayashi et al 2001b; Li et al 2001; Lee 2003; Sirlin et al 2004). These agents show molecular-weight-dependent or size-dependent plasma half-lives (Vexler et al 1994; Kobayashi et al 2001a,b), and agents with higher molecular weights and longer blood half-life produced the better contrast enhanced vascular imaging. Higher-molecular-weight chelates were prepared to increase their intravascular retention time for longer and clearer visualization of the neovascularity of neoplastic tissue (Weissleder et al 2001). Macromolecular agents with relatively high molecular weight (> 20 kDa, linear size) have a potential to detect and characterize individual tumors more specifically and accurately due to their ability to measure tumor microvascular permeability, microvascular density, and vascular recruitment. For example, albumin-(Gd-DTPA) (92 kDa) does not accumulate in benign tumors, but can gradually diffuse into the interstitial spaces of malignant tumors, which may be useful to accurately differentiate between these tumors (Gossmann et al 1999; van Dijke 1996).
Although macromolecular Gd(III) complexes have clear advantages as blood-pool contrast agents, their slow body clearance is also a disadvantage with respect to toxicity. Free Gd(III) ions are highly toxic, with an LD50 as low as 0.5 mmol/kg in rat models (Weinmann et al 1984). The dissociation of Gd(III) complexes and release of Gd(III) ions can be lethally toxic (Cacheris et al 1990). The longer body retention time increases the probability of metabolism of the macromolecular complexes and release of Gd(III) ions. The clearance rate decreases with the increase of molecular weight of the contrast agents (Kobayashi 2003). It has been reported that a Gd-DTPA polypropyleneimine dendrimer (generation 2) conjugate (7 kDa) resulted in the retention of 45% of injected dose in rats 14 days after injection (Wang 2003). The conjugation of Gd-DO3A to carboxylmethyl hydroxylethyl starch resulted in a macromolecular agent (72 kDa) that had about 47% of injected dose detected in rat body 7 days after the injection (Helbich et al 2000). Because of this safety concern, clinical development of macromolecular contrast agents has been limited.
Biodegradable polymers including poly(L-lysine), dextrans, and proteins have been used in the synthesis of macromolecular contrast agents. However, in vivo degradation of these materials takes place primarily in intracellular enzymatic compartments. MRI contrast agents are mostly extracellular agents and have low cellular uptake. In addition, the extensive chemical conjugation on the polymers can significantly reduce their biodegradability (Crepon et al 1991). Consequently, contrast agents prepared from these polymers have longer body retention times and result in significant accumulation of Gd ions in tissues (Bogdanov et al 1993; Franano et al 1995; Helbich et al 2000). Innovative approaches are needed for the design and development of safe, effective macromolecular contrast agents for clinical application.
We have recently designed and developed novel extracellular biodegradable macromolecular Gd(III) complexes based on polydisulfides to facilitate Gd(III) elimination and to minimize its tissue retention (Lu et al 2004). These complexes act as macromolecular agents for MR contrast enhancement and rapidly excrete as low-molecular-weight Gd(III) chelates after the macromolecules are broken down by the endogenous thiols via the disulfide-thiol exchange reaction. Here, we summarize the rationale of the biodegradable macromolecular contrast agents, their physicochemical and biological properties, and in vivo contrast enhancement in animal models.
Rationale of polydisulfide macromolecular MRI contrast agents
The ideal MRI contrast agent would be an agent able to accumulate in the tissue of interest long enough for imaging with conventional or dynamic MRI, then rapidly clear from the body. Currently available MRI contrast agents are primarily low-molecular-weight Gd(III) chelates, which have transient tissue retention and clear rapidly from the body. Macromolecular Gd(III) complexes exhibit long tissue retention and excrete slowly. We hypothesize that the incorporation of biodegradable structures into the backbone of macromolecules containing Gd(III) chelates can result in MRI contrast agents that will provide sufficient blood pool and tissue retention for effective contrast enhanced MR imaging. The structures will then react with biomolecules present in plasma or tissues to break down the macromolecules into smaller Gd(III) complexes that can be readily excreted (Lu et al 2004).
The disulfide-thiol exchange reaction plays a crucial role in biological systems. Free thiols including cysteine, glutathione (GSH), cysteinylglycine (Cys-Gly), and homocysteine are important endogenous biomolecules, with a total concentration of 15 μM in human blood plasma and about 10 mM in the cytoplasm (Andersson et al 1995; Deneke 2000). We have incorporated disulfide bonds into the backbone of macromolecular Gd(III) complexes to prepare biodegradable macromolecular MRI contrast agents. The disulfide bonds in the polymer chains can be readily cleaved by the thiols via disulfide-thiol exchange reaction to break down the macromolecules into smaller Gd(III) complexes, which can be excreted through renal filtration. Since Gd(III) chelates are mostly extracellular agents and the plasma thiol concentration is low, the degradation of the macromolecular agents is a slow process, which allows the agents to reside for an acceptable period in the circulation for effective contrast-enhanced MR imaging.
The concept of extracellular biodegradable macromolecular MRI contrast agents was validated with the first polydisulfide agent, Gd-DTPA cystamine copolymers (GDCC) (Lu et al 2004). GDCC was gradually degraded into smaller Gd(III) complexes by the cleavage of the disulfide bonds in the polymer backbone via the disulfide-thiol exchange reaction in the presence of 15 μM cysteine (Figure 1). The structures of degradation products were verified by MALDI-TOF mass spectrometry. GDCC resulted in more significant and prolonged contrast enhancement in the cardiovascular systems in rat than a clinical agent, Gd-(DTPA-BMA), and excreted rapidly via renal filtration. Metabolic degradation products were identified by mass spectrometry in the rat urine samples.
Figure 1.
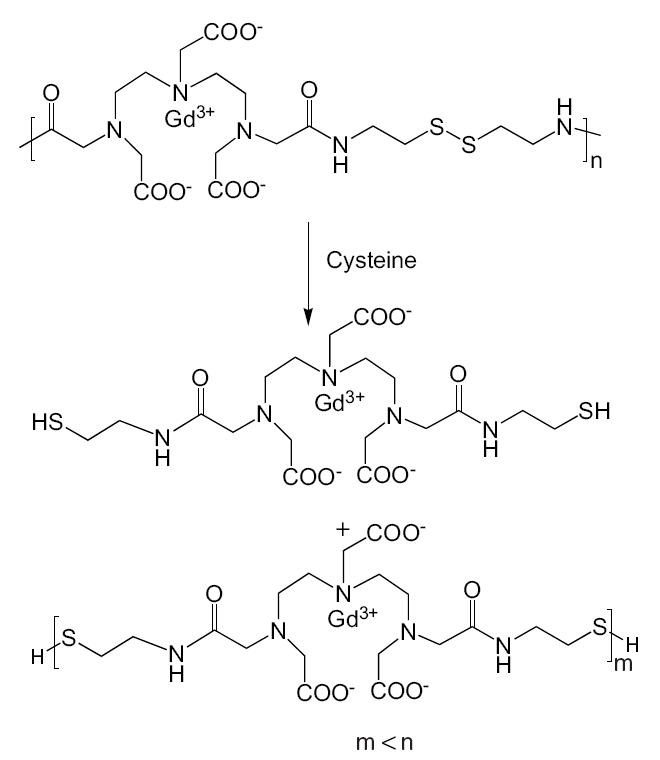
Degradation of Gd-DTPA cystamine copolymers (GDCC) in the presence of cysteine.
The structure of the polydisulfides can be readily modified to alter the physicochemical properties, pharmacokinetics, and in vivo contrast enhancement of the biodegradable MRI contrast agents. Functional groups have been introduced around the disulfide bonds by replacing cystamine with cystine. The chemistry of cystine is more versatile and the carboxylic groups in cystine can be further modified to design and prepare biodegradable macromolecular contrast agents with different properties (Mohs et al 2004; Zong et al 2005). Different substituents have been attached to cystine (Figure 2), and the modified agents demonstrated different degradability, pharmacokinetics, and in vivo contrast enhancement. The chemistry, physicochemical and pharmaceutical properties, and in vivo contrast enhancement of the polydisulfide agents are discussed in the following sections.
Figure 2.
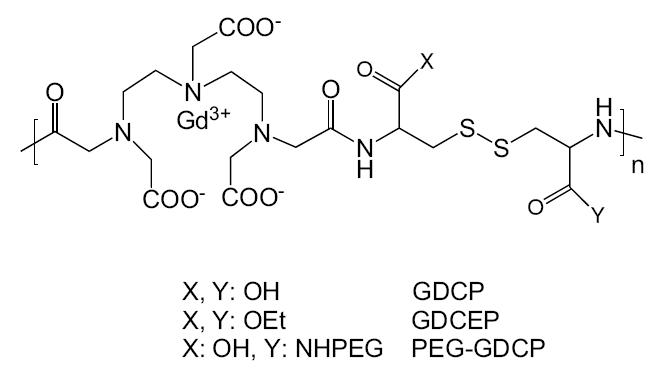
Structure of Gd-DTPA cystine copolymers (GDCP) and modified GDCP.
Abbreviations: OEt, ethoxy group; PEG, poly(ethylene glycol); GDCEP, Gd-DTPA cystine diethyl ester copolymer.
Synthesis of polydisulfide Gd(III) complexes
The synthetic procedure of polydisulfide Gd(III) complexes is described in Figure 3 with GDCC as an example. DTPA dianhydride is first copolymerized with a disulfide-containing diamine, eg, cystamine or cystine, by condensation copolymerization under basic conditions to give a polymeric ligand. Different solvents and bases can be used according to the solubility of disulfide monomers. Dimethyl sulfoxide was used as the solvent and triethylamine as the base for the monomers soluble in organic solvent. For cystine, which is not soluble in organic solvent, the copolymerization was performed in aqueous solution of sodium carbonate or sodium hydroxide. Polydisulfide Gd(III) complexes are then prepared by the complexation of the polymeric ligands with GdCl3 or Gd(OAc)3 at pH 5–6. The pH of the reaction mixture with GdCl3 decreases significantly during the complexation because of the release of HCl, and NaOH is added to maintain the pH at 5–6. There is no significant change of pH in the reaction with Gd(OAc)3.
Figure 3.
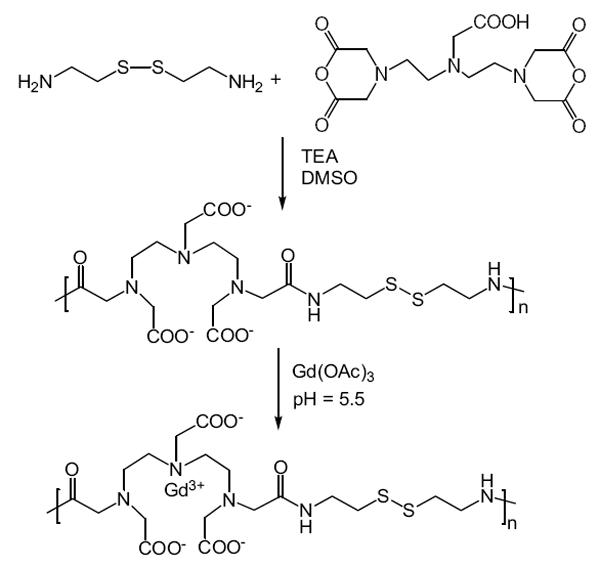
Synthesis of the polydisulfide agent Gd-DTPA cystamine copolymers (GDCC).
Abbreviations: DMSO, dimethyl sulfoxide; TEA, triethylanine.
The molecular weight of polydisulfides from the condensation copolymerization can be as high as 100 000 Da. There is a significant decrease of apparent molecular weight of copolymers after complexation with Gd(III) because of the change of hydrodynamic volume of the copolymers after complexation. The molecular weights of some paramagnetic copolymers are listed in Table 1. The polydisulfides are negatively charged and have a large hydrodynamic volume. Once the polydisulfide ligands chelate with Gd3+, the polydisulfide Gd(III) complexes are neutral or less charged and have reduced hydrodynamic volume.
Table 1.
Physicochemical parameters of some polydisulfide Gd(III) complexes
| Polymeric contrast agent | Molecular weight (kDa) | Relaxivity mM−1sec−1 | PEGylation | |||
|---|---|---|---|---|---|---|
| Mw | Mn | Mw/Mn | PEG size (Da) | PEG/Gd | ||
| GDCC | 17.7 | 15.0 | 1.2 | 4.4 (1.5T) | ||
| 35.0 | 27.0 | 1.3 | 6.3 (1.5T) | |||
| GDCP | 10.1 | 10.0 | 1.0 | 5.5 (3.0T) | ||
| GDCEP | 10.2 | 9.4 | 1.1 | 5.9 (3.0T) | ||
| PEGa-GDCP | 24.5 | 21.0 | 1.2 | 16.3 (3.0T) | 2000 | 0.33 |
| PEGb-GDCP | 22.9 | 19.9 | 1.2 | 12.7 (3.0T) | 2000 | 0.76 |
| PEG2000-GDCP | 37.7 | 28.5 | 1.3 | 8.7 (3.0T) | 2000 | 1.2 |
| PEG1000-GDCP | 37.8 | 31.1 | 1.2 | 7.8 (3.0T) | 1000 | 1.3 |
| PEG550-GDCP | 33.7 | 30.8 | 1.1 | 7.8 (3.0T) | 550 | 1.3 |
Abbreviations: PEG, polyethylene glycol; GDCC, Gd-DTPA cystamine copolymer; GDCP, Gd-DTPA cystine copolymer; GDCEP, Gd-DTPA cystine diethyl ester copolymer; Mw, averaged molecular weight; Mn, averaged molecular number.
The structure of polydisulfide Gd(III) complexes have been modified by grafting PEG of different lengths to the carboxylic groups in Gd-DTPA cystine copolymers (GDCP) (Mohs et al 2004, 2005). Monomethoxy-PEG amine (MPEG-NH2) was attached to GDCP in the presence of a coupling agent, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, in aqueous solution (Figure 4). The grafting degree can be readily manipulated by altering the ratio of mPEG-NH2 to GDCP. The modification increases apparent molecular weights of the copolymers at a low grafting degree depending on the size of PEG. Further increase of grafting degree with PEG of the same size does not further increase apparent molecular weight of the copolymers.
Figure 4.
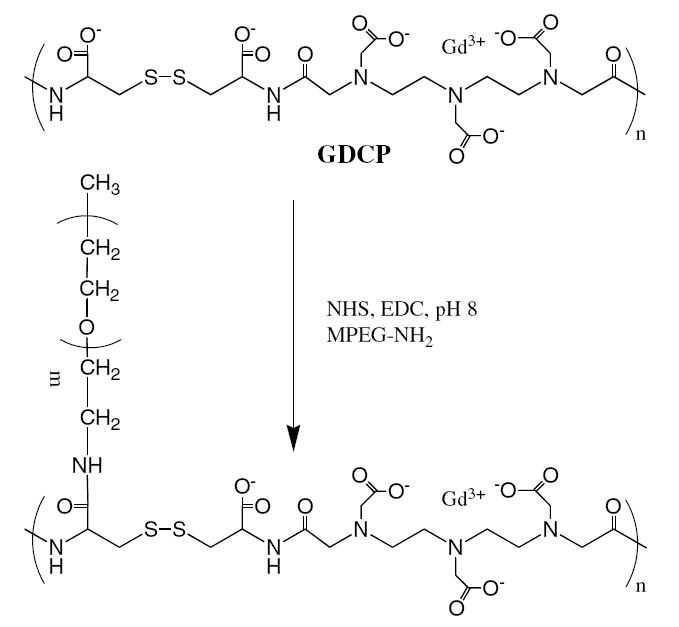
Synthesis of PEGylated Gd-DTPA cystine copolymer (GDCP).
Abbreviations: MPEG-NH2, monomethoxy-PEG amine; NHS, N-hydroxyl-succinimide; EDC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride.
The coordination feature of Gd(III) in the polydisulfide complexes is the same as that in Gd-(DTPA-BMA), a clinical MRI contrast agent. The Gd(III) is coordinated to 3 tertiary amine nitrogen atoms, 3 carboxylic groups, and 2 carbonyl groups of the amides of the DTPA bisamide. Gd-(DTPA-BMA) is a nonionic agent and has low acute toxicity in mice (LD50 = 14.8 mmol/kg) because of high stability and low transmetallation with Ca2+ (Cacheris et al 1990).
Relaxivity of polydisulfide Gd(III) complexes
The paramagnetic chelates decrease both T1 and T2 relaxation times, or increase the relaxation rates of surrounding water protons, resulting in increased signal intensity in T1-weighted imaging and decreased signal intensity in T2-weighted imaging. The chelates have a more significant effect on the relaxation rate of water protons in the coordination sphere of Gd(III) ions, less effect on water molecules immediately surrounding the chelate, and little direct effect on the bulk water molecules. Therefore, an effective Gd(III) chelate contrast agent should have at least one coordination site for water molecules. The complexed water molecule rapidly exchanges with bulk water, resulting in efficient relaxation of bulk water. The net change of the relaxation rate of water protons depends on the concentration of Gd(III) chelates and water exchange rate at the coordination site. Relaxivity is the measurement of the efficiency of a contrast agent to change the relaxation rate and can be determined from the equation (1/Ti)obs = (1/Ti)d + ri[Gd] (i = 1, 2), where (1/Ti)obs refers to the measured relaxation rate, (1/Ti)d the relaxation rate without a contrast agent, and ri the T1 or T2 relaxivity.
Gd(III) chelates are primarily used in T1-weighted, contrast-enhanced MRI. High r1 of Gd(III) chelates will result in strong relaxation or contrast enhancement. Polydisulfide Gd(III) complexes have higher T1 relaxivity than Gd-(DTPA-BMA) and the relaxivity varies based on the structure of the complexes (Table 1). GDCC, GDCP, and Gd-DTPA cystine diethyl ester copolymer (GDCEP) have similar relaxivity in the range of 4.5–6.5 mM−1sec−1. It appears that the degree of PEGylation in poly(ethylene glycol) (PEG)-modified GDCP has a more significant impact on its relaxivity than the PEG chain length. PEG-GDCP copolymers with different PEG lengths have similar relaxivity at the same grafting degree (Mohs et al 2005). For PEG-GDCP copolymers with the same PEG length (2000 Da), the increase of grafting degree significantly decreases relaxivity. The T1 relaxivity of PEG2000-GDCP with grafting ratios of 0.33, 0.76, and 1.2 mM−1s−1 decreases from 16.3 to 12.7, and 8.73 mM−1s−1, respectively. A high density of PEG on the backbones of the copolymers may interfere with the interaction of water molecules to the Gd(III) complexes because PEG can associate with water molecules via hydrogen bonding (Mohs et al 2004).
Degradability of polydisulfide Gd(III) complexes
Polydisulfide Gd(III) complexes with different structures demonstrate different degradation characteristics in the presence of cysteine. There was no degradation of GDCP in the incubation with 15 μM cysteine for 6 hours at pH 7.4 and 37 °C. GDCC gradually degraded under the same conditions and its molecular weight decreased approximately 28%, 33%, and 50% at 5, 15, and 60 minutes in the reaction (Lu et al 2004). The degradation of GDCEP was relatively slow in the first 60 minutes of incubation compared with GDCC, and its molecular weight decreased approximately 6%, 11%, and 24% at 5, 15, and 60 minutes. Both GDCC and GDCEP degraded into smaller oligomers and complexes at 6 hours (Zong et al 2005). Figure 5 shows size exclusion chromatograms of GDCEP in the incubation with 15 μM cysteine at pH 7.4 and 37 °C. All three macromolecular agents degraded more rapidly at higher cysteine concentration (150 μM) and completely degraded into the smallest repeat units in the incubation with an excess of cysteine, which was verified by mass spectrometry. GDCP and GDCEP are more sterically hindered around the disulfide bonds than GDCC, resulting in slower degradation. GDCP also has negative charges around the disulfide bonds at neutral pH, which further impede the access of the negatively charged cysteine to the disulfide bonds (Hupe and Wu 1980). Therefore, little reduction of the disulfide bonds in GDCP occurs at low cysteine concentrations.
Figure 5.
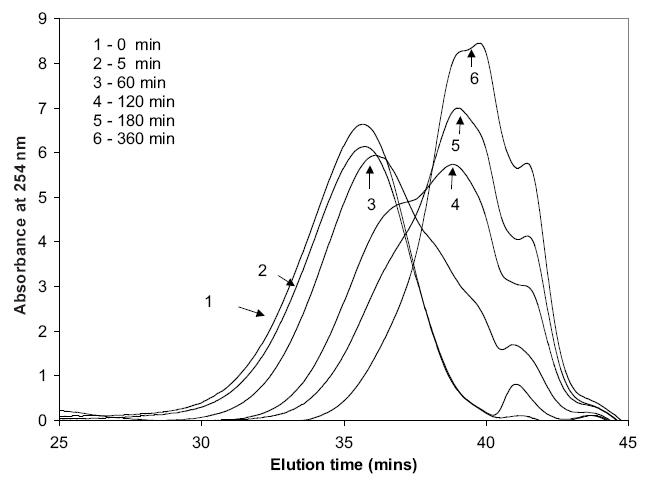
The molecular weight distribution of Gd-DTPA cystine diethyl ester copolymers (GDCEP) in the incubation with 15 μM L-cysteine at 37 °C.
NOTE: Units on y-axis are arbitrary.
The modification of GDCP also resulted in macromolecular agents with different degradability based on the length of PEG chains (Mohs et al 2004, 2005). The PEG-modified GDCP was stable at low cysteine concentration (circa 10 μM), similar to GDCP, and degraded with increased cysteine concentration. It appears that GDCP modified with short PEG chains degraded more rapidly than that with long chains. The structural modification resulted in macromolecular agents with different degradability towards the disulfide-thiol exchange reaction, which may have different pharmacokinetic properties and in vivo contrast enhancement.
Pharmacokinetics and excretion of polydisulfide Gd(III) complexes
Polydisulfide Gd(III) complexes should be able to circulate in the vasculature for an acceptable time window for effective contrast-enhanced MR imaging and then clear from the body with minimal long-term tissue retention of toxic Gd(III) ions. Pharmacokinetic data are the quantitative measurement of the circulation, excretion, and retention of the biodegradable macromolecular contrast agents in the body. A pharmacokinetic study showed that the low-molecular-weight agent Gd-(DTPA-BMA) rapidly extravasated from the vasculature, while GDCC had a higher plasma concentration in the first few minutes (approximately 5–15 minutes) post injection at the same dose (0.1 mmol-Gd/kg) in rats (Figure 6). The size of the biodegradable macromolecular agent affected the initial plasma concentration of the agent, but not the clearance from the vasculature. GDCC with high molecular weight (Mw = 60 000 Da) had a higher plasma concentration in the initial period (circa 5 minutes) after the injection than GDCC with low molecular weight (Mw = 18 000 Da). The plasma concentration of GDCC with both molecular weights reached the same level at 20 minutes post injection. Because the breakdown of the macromolecules is gradual, GDCC of the high molecular weight may produce relatively large molecules and remain in the blood at high concentration in the early stages of degradation. The large molecules eventually degrade into smaller complexes that are readily cleared via renal filtration.
Figure 6.
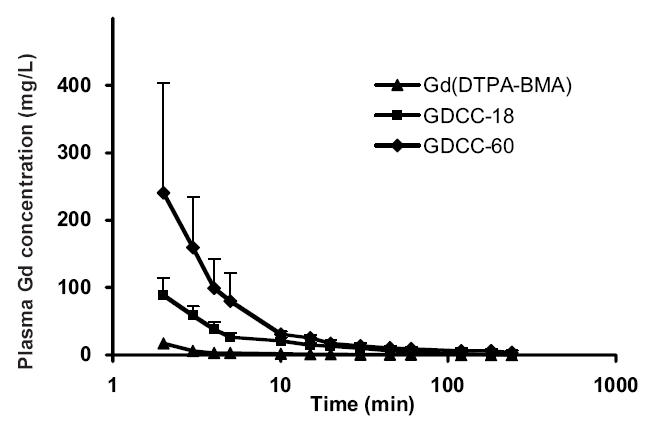
Plasma pharmacokinetics of Gd(III) complexes in rats after intravenous injection of Gd-(DTPA-BMA) (▲), GDCC-18 (18 kDa, ■), and GDCC-60 (60 kDa, ◆) at a dose of 0.1 mmol-Gd/kg
Abbreviations: GDCC, GD-DTPA cystamine copolymer.
Most of the degradation products were cleared in the urine via renal glomerular filtration and more than 50% of injected GDCC was excreted in the first 4 hours post-injection, similar to Gd-(DTPA-BMA). Consequently, rapid degradation and excretion of GDCC resulted in minimal retention of Gd(III) in the major organs and tissues 10 days post-injection, which was at the same level as Gd-(DTPA-BMA) (Wang et al 2005). The retention was much lower than that of other macromolecular Gd(III) complexes. For example, carboxymethyl hydroxyethyl starch-(Gd-DO3A) still had approximately 47% of injected dose in the body of experimental animals seven days after injection (Helbich et al 2000).
The degradation products of GDCC in the urine samples were identified as monomeric and oligomeric Gd(III) complexes by mass spectrometry. It appears that the in vivo degradation of the polydisulfide Gd(III) complexes was more complicated than the simple disulfide-thiol exchange reaction. Most of the Gd(III) complexes identified in the mass spectra were further metabolized after the cleavage of the disulfide bonds. The degradation products have a mass higher than the molecular weight of Gd-DTPA (Mw = 547.6 Da), the basic chelating unit of the agent, suggesting that the agent was excreted as stable Gd(III) chelates after degradation. These results have demonstrated that the polydisulfide agents have a relatively long blood circulation as macromolecules in the blood pool at the early stage after injection and then degrade into smaller Gd(III) complexes that excrete rapidly from the body.
In vivo MRI contrast enhancement
The polydisulfide Gd(III) complexes present superior contrast enhancement in the cardiovascular systems and tumor tissue compared with the low molecular-weight agent, Gd-(DTPA-BMA), as shown in animal models. Figure 7 shows the 3D maximum-intensity-projection (MIP), contrast-enhanced MR images of rats with Gd-(DTPA-BMA) and GDCC with molecular weights of 18 000 and 60 000 Da (0.1 mmol-Gd/kg). GDCC of both molecular weights resulted in strong contrast enhancement in rat heart and vasculature at 2 minutes post injection and the signal intensity gradually decreased. Gd-(DTPA-BMA) was rapidly extravasated from the vasculature and the contrast enhancement was much less at 2 minutes post-injection than with GDCC. GDCC with higher molecular weight resulted in more significant contrast enhancement in the small blood vessels than GDCC with lower molecular weight. Strong contrast enhancement was observed in rat kidneys and then the urinary bladder with GDCC, indicating that the biodegradable macromolecular agent was eliminated from renal filtration and accumulated in the urinary bladder. The contrast enhancement in the cardiovascular system returned to background levels at 15 minutes post-injection while the signal intensity in the bladder significantly increased.
Figure 7.
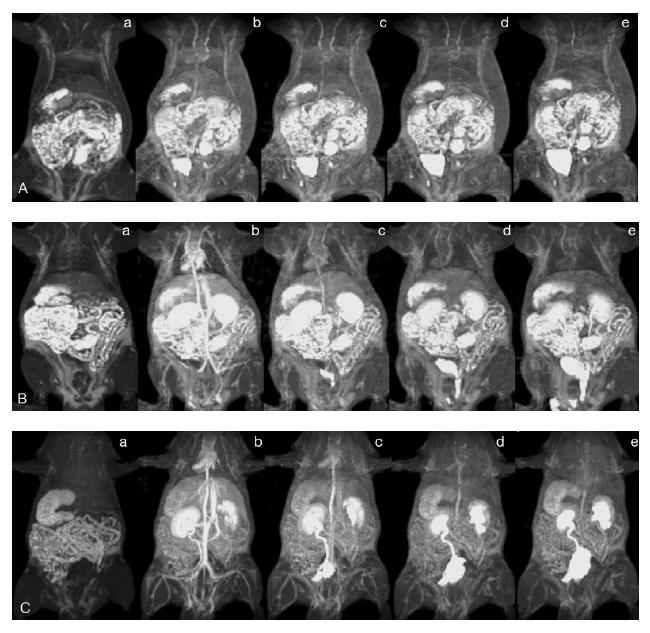
Three-dimensional maximum intensity projection (MIP) MR images of rats before (a) and 2 (b), 5 (c), 10 (d), and 15 (e) minutes after intravenous injection of Gd-(DTPA-BMA) (A), Gd-DTPA cystamine copolymer GDCC-18 (B), and GDCC-60 (C) at a dose of 0.1 mmol-Gd/kg.
Structural modification of polydisulfide agents altered in vivo contrast enhancement. PEGylation of GDCP resulted in more significant and prolonged contrast enhancement in the blood pool than GDCP. Both grafting ratio and PEG size have a significant impact on contrast enhancement profile. PEG2000-GDCP at a grafting ratio of 0.76 produced stronger and longer contrast enhancement in the blood pool than PEG2000-GDCP of a lower grafting ratio (0.33), even if the latter had higher relaxivity (Mohs et al 2004). Both agents were more effective in contrast-enhanced blood-pool imaging than GDCP (Mw = 11 400 Da) (Figure 8). With similar grafting ratios, PEG-GDCP with different PEG sizes had similar contrast enhancement in the blood pool during the initial period (2 minutes) post-injection, but the duration of the contrast enhancement decreased with decreasing size of PEG (Figure 9). PEG2000-GDCP resulted in more sustained enhancement than PEG1000-GDCP, while PEG550-GDCP had the least duration of enhancement.
Figure 8.
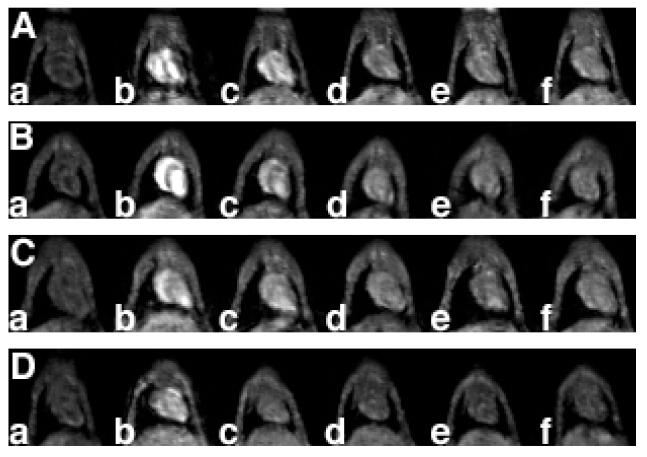
Coronal MR images of mouse hearts before (a) and 1 (b), 5 (c), 15 (d), 30 (e), and 60 minutes (f) post intravenous injection of PEGa-Gd-DTPA cystine copolymer (GDCP) (A), PEGb-GDCP (B), GDCP (C), and DTPA-BMA (D). Polymeric agents were given at a dose of 0.03 mmol/kg and DTPA-BMA was given at the standard clinical dose of 0.1 mmol-Gd/kg.
Figure 9.
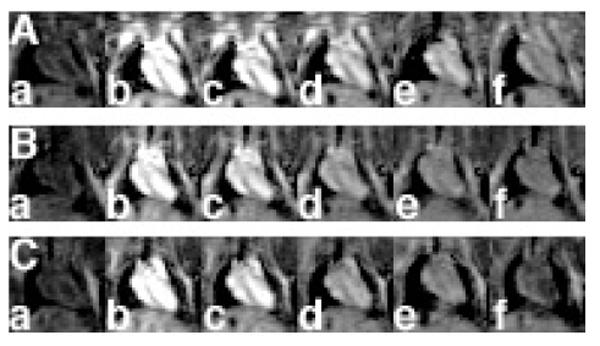
Coronal MR images of mouse hearts before (a) and 2 (b), 5 (c), 15 (d), 30 (e), and 60 minutes (f) post intravenous injection of PEG2000-Gd-DTPA cystine copolymer (GDCP) (A), PEG1000-GDCP (B), and PEG500-GDCP (C) at a dose of 0.05 mmol-Gd/kg.
In tumor MRI, the polydisulfide agents resulted in significant contrast enhancement in the tumor periphery. Figure 10 shows the coronal MR images of tumor tissue contrast enhanced by GDCC, GDCP, GDCEP, and Gd-(DTPA-BMA) in mice bearing MDA-MB-231 breast carcinoma xenografts. The biodegradable macromolecular agents produced more significant contrast enhancement in the tumor periphery than Gd-(DTPA-BMA). The modified agents GDCP and GDCEP resulted in more effective contrast enhancement than GDCC. Contrast enhancement in the tumor interstitium was less significant, possibly due to high interstitial fluid pressure, collapsed blood vessels forced by rapidly growing tumor tissue, and the formation of necrotic tissue in solid tumor (Padera et al 2004).
Figure 10.
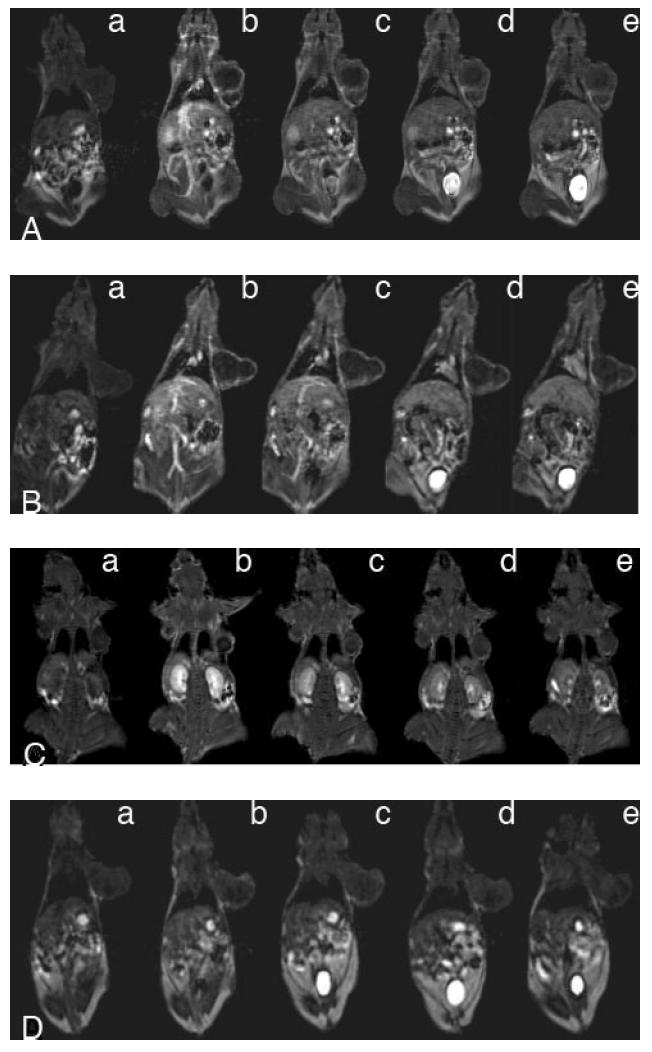
Coronal MR images of tumor-bearing mice before (a) and 5 (b), 15 (c), 30 (d), and 60 (e) minutes after intravenous injection of Gd-DTPA cystine copolymer (A), Gd-DTPA cystine diethyl ester copolymer (B), Gd-DTPA cystamine copolymer (C), and Gd-(DTPA-BMA) (D) at a dose of 0.1 mmol-Gd/kg.
The polydisulfide agents provided clear visualization of the periphery and size of solid tumor, which could be useful for the evaluation of tumor response to treatment. GDCEP has been studied in the noninvasive evaluation of the therapeutic response of solid tumor to an anti-VEGF siRNA in mice bearing MDA MB-231 breast carcinoma xenografts (Wang et al 2004). Figure 11 shows the coronal MR images of tumor-bearing mice contrast enhanced with GDCEP (0.1 mmol-Gd/kg) after the mice were treated for a period of 21 days with saline, a cationic polymer, anti-VEGF siRNA, and siRNA-polymer complex. Significant contrast enhancement was observed in the tumor rim of the mice, which clearly defined tumor size in the mice in different treatment groups. Mice treated with the siRNA and siRNA-polymer complexes had much smaller tumor sizes than mice treated with saline and cationic polymers as shown by contrast-enhanced MRI.
Figure 11.
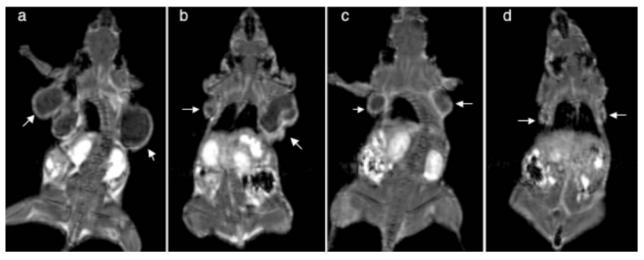
Contrast-enhanced coronal MR images of tumor-bearing mice with Gd-DTPA cystine diethyl ester copolymer (0.1 mmol-Gd/mmol) after treatment with saline (a), a cationic polyethyleneimine copolymer (b), an anti-VEGF siRNA (c), and a polymer–siRNA complex (d) for 21 days. Arrows point to the tumors.
Conclusion
Polydisulfide Gd(III) chelates have been designed and synthesized as extracellular biodegradable macromolecular MRI contrast agents. The disulfide bonds in the polymer chains can be readily cleaved by endogenous thiols into small and excretable Gd(III) complexes. The degradability of the polydisulfides can be altered by structural modification of the agents to achieve different in vivo properties. A pharmacokinetic and biodistribution study demonstrated that GDCC has a relatively longer plasma retention time for effective contrast-enhanced MRI and is rapidly excreted as low molecular-weight Gd(III) complexes, resulting in minimal long-term Gd(III) tissue accumulation comparable to Gd(DTPA-BMA). The structural modification of polydifulfide Gd(III) complexes can result in biodegradable macromolecular contrast agents with various enhancement profiles in the blood pool. The agents are effective for cardiovascular and cancer MR imaging. The polydisulfide Gd(III) complexes have a great potential to be developed as safe, effective, biodegradable macromolecular MRI contrast agents for clinical applications.
Acknowledgments
This work was supported in part by the NIH grants R01 EB00489 and R33 CA095873.
Abbreviations
- DTPA
diethylenetriaminepentaacetic acid
- DTPA-BMA
diethylenetriaminepentaacetate bismethyl amide
- DOTA
1,4,7,10-tetraazadodecanetetraacetic acid
- anti-VEGF
anti-vascular endothelial growth factor
- siRNA
small interfering RNA
References
- Andersson A, Lindgren A, Hultberg B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status off homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin Chem. 1995;41:3616. [PubMed] [Google Scholar]
- Bogdanov AA, Jr, Weissleder R, Frank HW, et al. A new macromolecule as a contrast agent for MR angiography:preparation, properties, and animal studies. Radiology. 1993;187:701–6. doi: 10.1148/radiology.187.3.8497616. [DOI] [PubMed] [Google Scholar]
- Caravan P, Ellison JJ, McMurry TJ, et al. Gadolinium (III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging. 1990;8:467–81. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- Crepon B, Jozefonvicz J, Chytry V, et al. Enzymatic degradation and immunogenic properties of derivatized dextrans. Biomaterials. 1991;12:550–4. doi: 10.1016/0142-9612(91)90049-g. [DOI] [PubMed] [Google Scholar]
- Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151–80. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- Duarte MG, Gil MH, Peters JA, et al. Synthesis, characterization, and relaxivity of two linear Gd(DTPA)-polymer conjugates. Bioconjug Chem. 2001;12:170–7. doi: 10.1021/bc000065r. [DOI] [PubMed] [Google Scholar]
- Franano FN, Edwards WB, Welch MJ, et al. Biodistribution and metabolism of targeted and nontargeted protein-chelate-gadolinium complexes:evidence for gadolinium dissociation in vitro and in vivo. Magn Reson Imaging. 1995;13:201–14. doi: 10.1016/0730-725x(94)00100-h. [DOI] [PubMed] [Google Scholar]
- Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- Gossmann A, Okuhata Y, Shames DM, et al. Prostate cancer tumor grade differentiation with dynamic contrast enhanced MR imaging in the rats:comparison of macromolecular and small-molecular contrast media-preliminary experience. Radiology. 1999;213:265–72. doi: 10.1148/radiology.213.1.r99oc43265. [DOI] [PubMed] [Google Scholar]
- Hayne L, Maravilla K, Cohen W, et al. Gd-DTPA-BMA, a new nonionic MR contrast agent: preliminary clinical results and comparison with Magnevist. Radiology. 1989;173:537–40. [Google Scholar]
- Helbich TH, Gossman A, Mareski PA, et al. A new polysaccharide macromolecular contrast agent for MR imaging: biodistribution and imaging characteristics. J Magn Reson Imaging. 2000;11:694–701. doi: 10.1002/1522-2586(200006)11:6<694::aid-jmri17>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe DJ, Wu D. Effect of charged substituents on rates of the thiol-disulfide interchange reaction. J Org Chem. 1980;45:3100–3. [Google Scholar]
- Kellar KE, Henrichs PM, Hollister RS, et al. High relaxivity linear Gd(DTPA)-polymer conjugates:the role of hydrophobic interactions. Magn Reson Med. 1997;38:712–16. doi: 10.1002/mrm.1910380506. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sato N, Kawamoto S, et al. Comparison of the macromolecular MR contrast agents with ethylenediamine-core versus ammonia-core generation-6 polyamidoamine dendrimer. Bioconjug Chem. 2001a;12:100–7. doi: 10.1021/bc000075s. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sato N, Hiraga A, et al. 3D-micro-MR angiography of mice using macromolecular MR contrast agents with poly-amidoamine dendrimer core with reference to their pharmacokinetic properties. Magn Reson Med. 2001b;45:454–60. doi: 10.1002/1522-2594(200103)45:3<454::aid-mrm1060>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawamoto S, Jo SK, et al. Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjug Chem. 2003;14:388–94. doi: 10.1021/bc025633c. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Brechbiel MW. Dendrimer-based nanosized MRI contrast agents. Curr Pharm Biotechnol. 2004;5:539–49. doi: 10.2174/1389201043376571. [DOI] [PubMed] [Google Scholar]
- Ladd DL, Hollister R, Peng X, et al. Polymeric gadolinium chelate magnetic resonance imaging contrast agents: design, synthesis, and properties. Bioconjug Chem. 1999;10:361–70. doi: 10.1021/bc980086+. [DOI] [PubMed] [Google Scholar]
- Langereis S, de Lussanet QG, Van Genderen MHP, et al. Multivalent contrast agents based on gadolinium-diethylenetriaminepentaacetic acid-terminated poly(propylene imine) dendrimers for magnetic resonance imaging. Macromolecules. 2004;37:3084–91. [Google Scholar]
- Lauffer R, Brady JT. Preparation and water relaxation properties of proteins labeled with paramagnetic metal chelates. Magn Reson Imaging. 1985;3:11–16. doi: 10.1016/0730-725x(85)90004-9. [DOI] [PubMed] [Google Scholar]
- Lee JW, Moon WK, Weinmann HJ, et al. Contrast-enhanced MR imaging of postoperative scars and VX2 carcinoma in rabbits: comparison of macromolecular contrast agent and gadopentetate dimeglumine. Radiology. 2003;229:132–9. doi: 10.1148/radiol.2291020218. [DOI] [PubMed] [Google Scholar]
- Li D, Zheng J, Weinmann HJ. Contrast-enhanced MR imaging of coronary arteries: comparison of intra- and extravascular contrast agents in swine. Radiology. 2001;218:670–8. doi: 10.1148/radiology.218.3.r01mr03670. [DOI] [PubMed] [Google Scholar]
- Liang ZP, Lauterbur PC. Principles of magnetic resonance imaging. New York: IEEE Pr; 2000. [Google Scholar]
- Lu ZR, Parker DL, K. Goodrich C, et al. Extracellular biodegradable macromolecular gadolinium(III) complexes for magnetic resonance imaging. Magn Reson Med. 2004;51:27–34. doi: 10.1002/mrm.10656. [DOI] [PubMed] [Google Scholar]
- de Lussanet QG, Langereis S, Beets-Tan RG, et al. Dynamic contrast-enhanced MR imaging kinetic parameters and molecular weight of dendritic contrast agents in tumor angiogenesis in mice. Radiology. 2005;235:65–72. doi: 10.1148/radiol.2351040411. [DOI] [PubMed] [Google Scholar]
- Magerstadt M, Gansow OA, Brechbiel MW, et al. Gd(DOTA): an alternative to Gd(DTPA) as a T1, 2 relaxation agent for NMR imaging or spectroscopy. Magn Reson Med. 1986;3:808–12. doi: 10.1002/mrm.1910030517. [DOI] [PubMed] [Google Scholar]
- Mohs AM, Wang X, Goodrich KC, et al. Poly(GdDTPA-L-cystine)-g-PEG: a biodegradable macromolecular blood pool contrast agent for MR imaging. Bioconjug Chem. 2004;15:1424–30. doi: 10.1021/bc049828r. [DOI] [PubMed] [Google Scholar]
- Mohs AM, Zong Y, Guo J, et al. PEG-g-Poly(GdDTPA-co-L-cystine): effect of PEG chain length on in vivo contrast enhancement in MRI. Biomacromolecules. 2005 doi: 10.1021/bm050194g. In press. [DOI] [PubMed] [Google Scholar]
- Padera TP, Stoll BR, Tooredman JB, et al. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- Roberts HC, Saeed M, Roberts TP, et al. Comparison of albumin-(Gd-DTPA)30 and Gd-DTPA-24-cascade-polymer for measurements of normal and microvascular permeability. J Magn Reson Imaging. 1997;7:331–8. doi: 10.1002/jmri.1880070213. [DOI] [PubMed] [Google Scholar]
- Schuhmann-Giampieri G, Schmitt-Willich H, Frenzel T, et al. In vivo and in vitro evaluation of Gd-DTPA-polylysine as a macromolecular contrast agent for magnetic resonance imaging. Invest Radiol. 1991;26:969–74. doi: 10.1097/00004424-199111000-00008. [DOI] [PubMed] [Google Scholar]
- Shibata DK, Schmiedl UP, Yuan C, et al. Two prototype blood-pool agents for contrast-enhanced magnetic resonance angiography of the portal vein in pigs. Acad Radiol. 1995;2:705–8. doi: 10.1016/s1076-6332(05)80439-8. [DOI] [PubMed] [Google Scholar]
- Sirlin CB, Vera DR, Corbeil JA, et al. Gadolinium-DTPA-dextran: a macromolecular MR blood pool contrast agent. Acad Radiol. 2004;11:1361–9. doi: 10.1016/j.acra.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Turetschek K, Huber S, Floyd F, et al. MR imaging characterization of microvessels in experimental breast tumors by using a particulate contrast agents with histopathologic correlation. Radiology. 2001;218:562–9. doi: 10.1148/radiology.218.2.r01fe37562. [DOI] [PubMed] [Google Scholar]
- Van Beers BE, Mottet I, Delos M, et al. Acute occlusive ischemia of the rat intestine:early detection by MR imaging with polylysine-Gd-DTPA enhancement. J Magn Reson Imaging. 1995;5:509–13. doi: 10.1002/jmri.1880050505. [DOI] [PubMed] [Google Scholar]
- van Dijke CF, Brasch RC, Roberts TP, et al. Mammary carcinoma model: correlation of macromolecular contrast-enhanced MR imaging characterizations of tumor microvasculature and histologic capillary density. Radiology. 1996;198:813–18. doi: 10.1148/radiology.198.3.8628876. [DOI] [PubMed] [Google Scholar]
- Vexler VS, Clement O, Schmitt-Willich H, et al. Effect of varying the molecular weight of the MR contrast agent Gd-DTPA-polylysine on blood pharmacokinetics and enhancement patterns. J Magn Reson Imaging. 1994;4:381–8. doi: 10.1002/jmri.1880040325. [DOI] [PubMed] [Google Scholar]
- Wang SC, Wikstrom MG, White DL, et al. Evaluation of Gd-DTPA-labeled dextran as an intravascular MR contrast agent: imaging characteristics in normal rat tissues. Radiology. 1990;175:483–8. doi: 10.1148/radiology.175.2.1691513. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Brechbiel M, Wiener EC. Characteristics of a new MRI contrast agent prepared from polypropyleneimine dendrimers, generation 2. Invest Radiol. 2003;38:662–8. doi: 10.1097/01.rli.0000084887.47427.75. [DOI] [PubMed] [Google Scholar]
- Wang X, Zong Y, Goodrich KC, et al. MR imaging of tumor response to the treatment with anti-VEGF siRNA. 3rd Annual Meeting of the Society for Molecular Imaging; September 9-12; Honolulu, HI, USA. 2004. [Google Scholar]
- Wang X, Feng Y, Ke T, et al. Pharmacokinetics and long-term Gd tissue accumulation of (Gd-DTPA)-cystamine copolymers, a biodegradable macromolecular MRI contrast agent. Pharm Res. 2005;22:596–601. doi: 10.1007/s11095-005-2489-7. [DOI] [PubMed] [Google Scholar]
- Weinmann HJ, Brasch RC, Press WR, Wesbey GE. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984;142:619–24. doi: 10.2214/ajr.142.3.619. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Bogdanov A, Jr, Tung CH, et al. Size optimization of synthetic graft copolymers for in vivo angiogenesis imaging. Bioconjug Chem. 2001;12:213–19. doi: 10.1021/bc000091p. [DOI] [PubMed] [Google Scholar]
- Wiener EC, Brechbiel MW, Brothers H, et al. Dendrimer-based metal chelates:a new class of magnetic resonance imaging contrast agents. Magn Reson Med. 1994;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- Zong Y, Wang Y, Goodrich KC, et al. Contrast enhanced tumor MRI with new biodegradable macromolecular Gd(III) complexes in mice. Magn Reson Med. 2005;53:835–42. doi: 10.1002/mrm.20402. [DOI] [PubMed] [Google Scholar]


