Abstract
Apoptosis, or programmed cell death, is a vital cellular process responsible for causing cells to self-terminate at the end of their useful life. Abrogation of this process is commonly linked to cancer, and rapid detection of apoptosis in vitro is vital to the discovery of new anti-cancer drugs. In this paper, we describe the application of the electrical phenomenon dielectrophoresis for detecting apoptosis at very early stages after drug induction, on the basis of changes in electrophysiological properties. Our studies have revealed that K562 (human myelogenous leukemia) cells show a persistent elevation in the cytoplasmic conductivity occurring as early as 30 minutes following exposure to staurosporine. This method therefore allows a far more rapid detection method than existing biochemical marker methods.
Keywords: biophysics, cell manipulation, electrodes, staurosporine
Introduction
Apoptosis, or programmed cell death, is an essential physiological process; dysfunction of this process has been linked to pathogenesis of cancer and other diseases (Thompson 1995). The study of apoptotic processes has helped to focus the development of new therapies for the treatment of cancer (Hughes and Mehmet 2003). A rapid method to detect the onset of apoptosis is a useful adjunct to the monitoring of surrogate tissue in order to evaluate the effects of new cancer therapies in clinical trial settings. In the study of apoptosis, focus has largely been placed on methods that address the different stages of apoptosis following treatment of cells with apoptosis-inducing agents. For example, the Annexin V assay is associated with the monitoring of changes in the proteins of the plasma membrane. This method, as with many other assays associated with the detection of apoptosis, is flow cytometry-based; early-phase apoptotic cells have exposed phosphatidyl serine (PS) residues to which FITC-conjugate Annexin V binds. Another technique uses the membrane potential sensitive probe TMRE (tetra-methyl rhodamine) to detect changes in the mitochondrial membrane potential.
There is a tremendous variability in the kinetics of apoptosis induction, some cells being fully apoptotic after only 2–4 hours of treatment with staurosporine, such as Jurkat T-lymphocytes, while some epithelial cells require nearly 24 hours of treatment with this inducer. Hence, the delay before any biochemical change can be detected after adding an inducer to the cells is significant for the assessment of the drug treatment. However, relatively little is known about the events that occur within the cell prior to detection of apoptosis using standard biochemical techniques. One event known to occur early in the apoptotic process is that changes occur in the ion concentration inside the cell (Bortner et al 1997). If such changes affect the cell electrical conductivity, apoptosis can be studied using methods capable of determining the dielectric properties of cells, which would offer a particularly and important assessment method.
Dielectrophoresis (DEP), described by Pohl (1978), is one of the techniques collectively termed AC-electrokinetics, and involves the movement of particles in non-uniform time-variant fields. DEP has numerous potential applications in the context of cell biology, as observing the cellular response to various stimuli, measured as a function of frequency of the applied electric field, allows the non-invasive determination of the dielectric properties of both the membrane and the cytoplasm of cells. DEP can provide quantitative analysis of electrophysiological properties of cells, and in our earlier studies it has been used to investigate chemotherapy resistance in cancer cells (Labeed et al 2003). Moreover, DEP has been employed in a number of other experimental settings using cancer cell line models (Hu et al 1990; Gascoyne et al 1992, 1993; Becker et al 1994; Gascoyne et al 1997; Wang et al 1997, 1999; Cristofanilli et al 2002; Labeed et al 2006). Recently, it has also been employed to accurately study the dielectric changes of human leukemic cells at different stages of apoptosis, as well as necrosis (Wang et al 2002)
In this study, we used a human chronic myelogenous leukemic cell line K562, staurosporine for apoptosis induction, and the two techniques (Annexin V-FITC assay and DEP) in order to assess whether apoptotic induction could be detected 30 minutes and 1 hour following treatment. The DEP data revealed that there is a significant change in the ionic content of the cytoplasm in K562 cells 30 minutes after treatment with staurosporine. Additionally, a second population of necrotic cells was observed that increased in number over the hour, from 25% to 33%. The latter effect was not detectable using the Annexin V technique, since no PI expression was measured during the same period. This indicates that DEP can be used as a powerful and informative tool to detect very early-stage apoptosis. Furthermore, the technique is low-cost, and has potential applications for parallelization in high-throughput systems (Hübner et al 2005).
Materials and methods
Cell culture and induction of apoptosis
Staurosporine (Alexis Corporation, Nottingham, UK) was dissolved in dimethyl sulfoxide (DMSO), and stored frozen as a 1mM stock solution and thawed prior to use. Human chronic myelogenous leukemia (K562) was grown in modified RPMI-1640 medium supplemented with 10% heat inactivated fetal calf serum (FCS; Invitrogen, Paisley, UK), 2m M L-glutamine, and 100 units/mL penicillin–streptomycin (both from Sigma-Aldrich, Poole, UK). The cells were grown under standard cell culture conditions, with 5% CO2/95% air at 37°C. A density of 6×105 cells/mL were treated with an apoptosis-inducing agent (staurosporine) at 1 μM final concentration, and incubated for 30 minutes, and 1 hour time points.
DEP experiments
K562 cells were centrifuged at room temperature at 190×g for 5 minutes. The pellets were washed and resuspended in isotonic medium consisting of 8.5% (w/v) sucrose plus 0.3% (w/v) dextrose buffer (Gascoyne et al 1997). The medium conductivity was adjusted to 2.5m S/m using PBS and the final conductivity, before use, was verified with a conductivity meter (RS components Ltd, London, UK). The final cell population was counted using a hemocytometer and adjusted to approximately 3×105 cells/mL (±15%) for DEP measurements. The DEP system used in this study was similar to that reported by Labeed et al (2003). The biophysical properties of the cells, after each staurosporine incubation period, were determined by fitting the measurement spectra to the single shell model (Irimajiri et al 1979). The best fit model was found by matching the curve to the measured data, and then altering the dielectric parameters of the membrane and the cytoplasm until a best match was found. For each staurosporine incubation period, the experiment was repeated at least 5 times, using different cell population at a time in order to reduce the effect of variation in cell number. The results, for each time point, were summed prior to modeling. In order to determine cell size for DEP calculations, cell diameters were measured using Photolite software to analyze microscope images of cells.
Annexin V assay
In normal viable cells, the phosphatidylserine (PS) molecules are located on the cytoplasmic surface of the cell membrane. When apoptosis occurs, rapid alterations in the organization of the phospholipids take place leading to exposure of PS on the cell surface (Fadok et al 1992; Martin et al 1995). For these experiments, the rapid Annexin V-FITC detection kit (CN Biosciences, Beeston, UK) was used. The assay principle uses FITC-conjugate Annexin V to detect PS exposure by flow cytometry. Simultaneously, propidium iodide (PI) was used to distinguish viable, necrotic or apoptotic cells in the late terminal stages, as PI is internalized only in cells whose membranes have become permeable.
A population of around 0.5×106 cells in culture media was used for each datum and the method was carried out according to manufacturer’s instructions. The analysis was performed using a flow cytometer (Coulter Epics XL) emitting an excitation laser light at 488nm. The detection was made using an FITC detector at 518nm and a PI detector at 620nm. Adjustments were made to minimise overlap between these two measurements.
Results and discussion
We have used DEP and Annexin V assay to investigate whether it is possible to detect cellular changes as early as 30 minutes and 1 hour post apoptosis induction by staurosporine. The Annexin V assay uses FITC-conjugate Annexin V to detect PS exposure by flow cytometry, while simultaneously, PI dye is used to distinguish viable, necrotic or apoptotic cells in the late terminal stages, as PI is internalized only in cells whose membranes have become permeable. The results of the Annexin/PI study are shown in Figure 1. At both incubation points, early apoptotic cells were not detected, and staursporine-treated cells did not differ significantly from untreated controls. Standard deviation for all data points was less than 1%.
Figure 1.
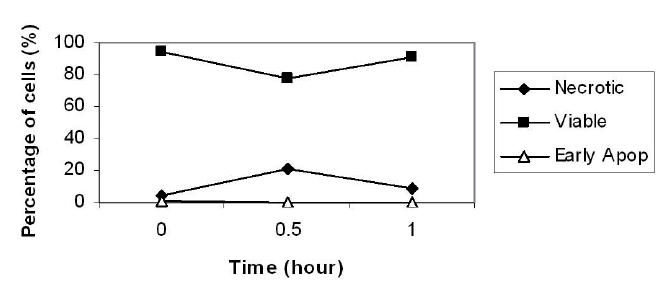
The percentage of viable, early apoptotic, and necrotic populations after different incubation periods with staurosporine, as measured using flow cytometry and Annexin V/PI. Time 0 depicts control untreated cells.
The cell collection versus frequency for the DEP of untreated K562 control cells, and after 30 and 60 minutes of treatment with staurosporine, are shown in Figure 2a, b, and c, respectively. In all three cases, cell collection was observed at frequencies of more than 10 kHz, and declined at more than 2–3 MHz. After 30 and 60 minutes of exposure, the decline in the rate of collection at the high frequency range (above 2 or 3 MHz) indicated two distinct plateaus. It has been reported that multiple plateaus indicate the presence of more than one sub-population with different properties (Broche et al 2005). In this study, the best fits for the post-treatment data were obtained after superimposing two single shell models, as suggested by Broche et al (2005). The superimposed fit can be seen in Figures 2b and c (for 30 and 60 minutes post exposure to staurosporine, respectively). The estimated dielectric parameters derived from the data obtained for each incubation period are summarized in Table 1. As can be seen, the control untreated cells exhibited a cytoplasmic conductivity (σcyto, reflecting the ionic concentration) of 0.23 S/m, and a specific membrane capacitance (Cspec, reflecting membrane morphology) of 9.7 mF/m2. After 30 minutes of staurosporine treatment, two plateaus could be observed from the DEP data suggesting the presence of more than one population in the sample. The majority of the sample (approximately 75%), referred to here as population 1, exhibited a significantly higher σcyto (0.4 S/m), and a slightly higher Cspec (10.4 mF/m2) relative control cells. The remaining proportion of the sample (approximately 25%, referred to as population 2) displayed a significantly lower σcyto (0.05 S/m). After 60 minutes of staurosporine treatment, the occurrence of two plateaus was persistent, where the majority of cells (approximately 67%) showed a persistent rise in σcyto (0.40 S/m), and a gradual increase in Cspec (12.4 mF/m2). The second population, constituting approximately 33%, exhibited a low σcyto (0.02 S/m). The surface conductance parameter Ks (reflecting cell surface charge) did not change significantly throughout these incubation periods. These results show a persistent increase in the ionic concentration of the cytoplasm in the majority of cells following staurosporine treatment relative to control untreated cells.
Figure 2.
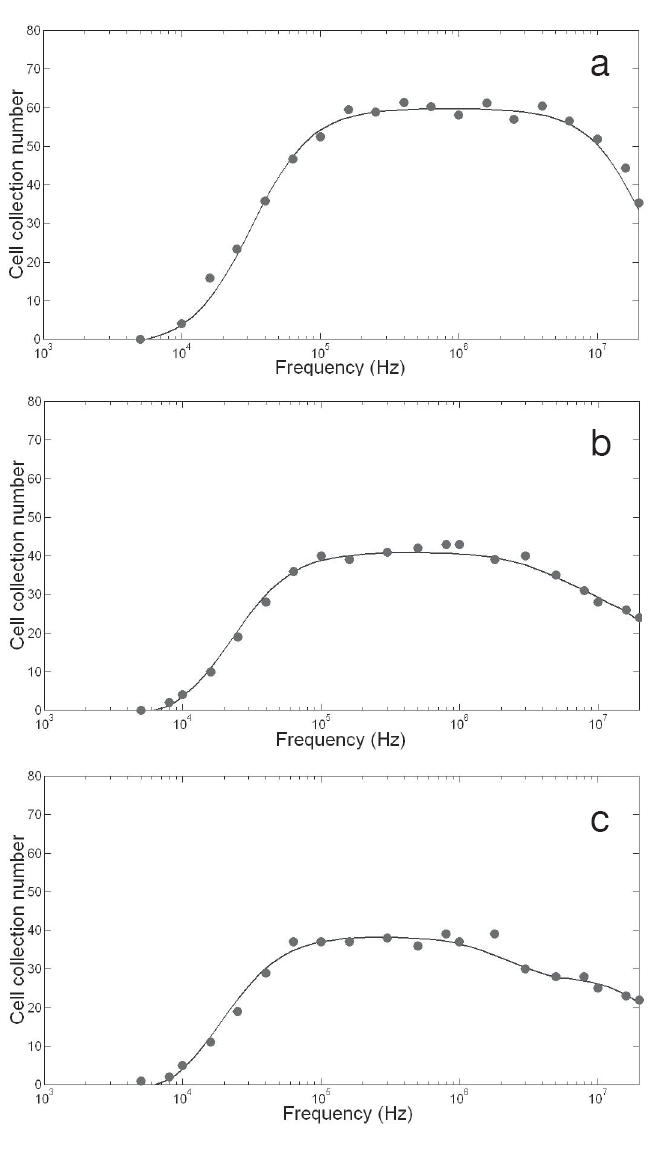
Summed dielectrophoretic collection spectra showing the number of cells collected for different energizing frequencies, for 5 experiments per condition. (a) control population; (b) cells 30 minutes after exposure to staurosporine; (c) cells 1 hour after exposure to staurosporine.
Table 1.
Electrophysiological data of K562 cells before and after apoptosis treatment with staurosporine, derived from dielectrophoresis data
| Time (minutes) after treatment with staurosporine | Proportion (%) | Cytoplasm conductivity σ (S/m) | Membrane capacitance Cspec (mF/m2) | |
|---|---|---|---|---|
| 0 (control) | 100 | 0.23 (0.22–0.24) | 9.7 (8.9–10.6) | |
| 30 | Population 1 | 75 | 0.40 (0.36–0.45) | 10.4 (9.7–11.5) |
| Population 2 | 25 | 0.05 (0.04–0.06) | 10.4 (9.7–11.5) | |
| 60 | Population 1 | 67 | 0.40 (0.30–0.50) | 12.4 (11.5–13.7) |
| Population 2 | 33 | 0.02 (0.01–0.03) | 12.4 (11.5–13.7) | |
One possible mechanism is that throughout the apoptotic process the cells become smaller, causing the cytoplasm to become more concentrated with remaining ions. Cell radii were measured before and after treatment; before treatment, the cells had a mean radius of 9.2 μm, which dropped to 8.2 μm after exposure. Although this change in radius is small, the attendant 30% reduction in volume is proportional to the observed change in conductivity, indicating that the volume may have a significant influence on the conductivity of the cytoplasm. This may be due to the remaining charged molecules (including macromolecules such as DNA and proteins) becoming more concentrated as the water is effluxed to restore the osmotic balance, increasing the conductivity of the interior. At both time points, population 2 exhibits a significantly diminished cytoplasmic conductivity. Typically, a very low value of σcyto (approaching that of the suspending medium) is indicative of necrosis, as the membrane permeabilizes and the cell interior equilibrates with the medium. The fact that σcyto in population 2, although much lower than that of population 1, is still an order of magnitude higher than the suspending medium may indicate that we are observing the onset of cell necrosis, where membrane permeabilization is relatively small. This would also explain why the PI assay fails to show a significant necrotic population, as the assay relies on significant membrane permeability in order to incorporate sufficient dye in the cytoplasm of necrotic cells.
In this paper we have shown using DEP, that the majority of K562 cells show a persistent elevation in the cytoplasmic conductivity (ionic content), occurring as early as 30 minutes following exposure to staurosporine. A possible explanation for this rise in cytoplasmic conductivity may involve many ionic or ion channel hypotheses that have been described in the literature, some of which are contradictory; however, the mechanism we suggest here is that this change is shrinkage dependent, and involves an increased ion influx. The DEP results indicate that there is an increasing proportion of necrotic cells at the two time points after staurosporine exposure, indicating that the cell population responds to apoptosis-inducing drugs either by joining a slow apoptotic cascade, or by near-immediate necrosis. Furthermore, when the DEP results were compared with that of the Annexin V assay, DEP was found to be a powerful and a more informative tool, as it was capable of detecting cellular changes associated with very early-stage apoptosis, and as early as 30 minutes post staurosporine exposure. The technique is also low-cost, and has potential applications for parallelization in high-throughput systems (Hübner et al 2005).
Acknowledgments
The authors would like to thank the Nuffield bursary scheme for funding SC, and Mrs Christine Shotton for her help with the flow cytometry experiments.
References
- Becker FF, Wang X-B, Huang Y, et al. The removal of human leukaemia cells from blood using interdigitated microelectrodes. J Phys D Appl Phys. 1994;27:2659–62. [Google Scholar]
- Bortner CD, Hughes FM, Jr, Cidlowski JA. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J Biol Chem. 1997;272:32436–442. doi: 10.1074/jbc.M303516200. [DOI] [PubMed] [Google Scholar]
- Broche LM, Labeed FH, Hughes MP. Extraction of dielectric properties of multiple populations from dielectrophoretic collection spectrum data. Phys Med Biol. 2005;50:2267–74. doi: 10.1088/0031-9155/50/10/006. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, de Gasperis G, Zhang LS, et al. Automated electrorotation to reveal dielectric variations related to HER-2/neu overexpression in MCF-7 sublines. Clin Cancer Res. 2002;8:615–19. [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- Gascoyne PRC, Huang Y, Pethig R, et al. Dielectrophoretic separation of mammalian cells studied by computerized image analysis. Meas Sci Technol. 1992;3:439–45. [Google Scholar]
- Gascoyne PRC, Pethig R, Burt J, et al. Memebrane-changes accompanying the induced-differentiation of friend murine erythroleukemia-cells studied by dielectrophoresis. Biochim Biophys Acta. 1993;1149:119–26. doi: 10.1016/0005-2736(93)90032-u. [DOI] [PubMed] [Google Scholar]
- Gascoyne PRC, Wang X-B, Huang Y, et al. Dielectrophoretic separation of cancer cells from blood. IEEE Trans Ind Appl. 1997;33:670–8. doi: 10.1109/28.585856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Arnold WM, Zimmermann U. Alterations in the electrical properties of lymphocyte T and lymphocyte B membranes induced by mitogenic stimulation-activation monitored by electrorotation of single cells. Biochim Biophys Acta. 1990;1021:191–200. doi: 10.1016/0005-2736(90)90033-k. [DOI] [PubMed] [Google Scholar]
- Hübner Y, Hoettges KF, Ogin SL, et al. Parallel measurements of drug actions on erythrocytes by dielectrophoresis, using a three-dimensional electrode design. IEE Proc Nanobiosci. 2005;154:150–4. doi: 10.1049/ip-nbt:20050011. [DOI] [PubMed] [Google Scholar]
- Hughes D, Mehmet H. Cell proliferation and apoptosis. Advanced methods. Oxford: Bios Scientific Publishers Limited; 2003. [Google Scholar]
- Irimajiri A, Hanai T, Inouye VA. Dielectric theory of “multi-stratified shell” model with its application to lymphoma cell. J Theor Biol. 1979;78:251–69. doi: 10.1016/0022-5193(79)90268-6. [DOI] [PubMed] [Google Scholar]
- Labeed FH, Coley HM, Thomas H, et al. Assessment of multidrug resistance reversal using dielectrophoresis and flow cytometry. Biophys J. 2003;85:2028–34. doi: 10.1016/S0006-3495(03)74630-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeed FH, Coley HM, Hughes MP. Differences in the biophysical properties of membrane and cytoplasm of apoptotic cells revealed using dielectrophoresis. Biochim Biophys Acta. 2006;1760:922–9. doi: 10.1016/j.bbagen.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CPM, McGahon AJ, et al. Early redistribution of plasma-membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus-inhibition by overexpressiom of BCL-2 and ABL. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl HA. Dielectrophoresis. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Wang X-B, Huang Y, Wang XJ, et al. Dielectrophoretic manipulation of cells with spiral electrodes. Biophys J. 1997;72:1887–99. doi: 10.1016/S0006-3495(97)78834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-B, Yang J, Gascoyne PRC. Role of peroxide in AC electrical field exposure effects on Friend murine erythroleukemia cells during dielectrophoretic manipulations. Biochim Biophys Acta. 1999;1426:53–8. doi: 10.1016/s0304-4165(98)00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Becker FF, Gascoyne PRC. Membrane dielectric changes indicate induced apoptosis in HL-60 cells more sensitively than surface phosphatidylserine expression or DNA fragmentation. Biochim Biophys Acta. 2002;1564:412–20. doi: 10.1016/s0005-2736(02)00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


