Abstract
A highly aligned pattern of carbon nanofibers (CNF) on polycarbonate urethane (PCU) for tissue engineering applications was created by placing a CNF–ethanol solution in 30μm width copper grid grooves on top of PCU. In vitro results provided the first evidence that fibroblasts and vascular smooth muscle cells selectively adhered to the PCU regions. However, endothelial cells did not display a preference for adhesion to the CNF compared with PCU regions. Previous studies have shown selective adhesion of osteoblasts (bone-forming cells) on CNF compared with PCU regions. Thus, the present results suggest that CNF aligned on PCU may be useful substrates for the control of spatial cell attachment, criteria useful for the design of a wide range of tissue engineering materials, from orthopedic to vascular.
Keywords: carbon nanofibers, polycarbonate urethane, osteoblasts, fibroblasts, endothelial cells, vascular smooth muscle cells, alignment
Introduction
In the last decade, carbon nanotubes–nanofibers (CNT–CNF) have aroused much interest in the bioengineering community because of their unique mechanical, electrical, and surface properties. For example, CNF have exceptional mechanical properties, including a high strength to weight ratio and a high Young’s Modulus (Price et al 2003). This, coupled with tailorable surface energy and biologically inspired nanoscale dimensions, makes CNF intriguing materials for various implant applications (Price et al 2003; McKenzie et al 2004; Khang et al 2006). Indeed, research groups have shown that CNF outperform traditional implant materials for bone and neural applications (Price et al 2003; McKenzie et al 2004; Khang et al 2006). In particular, adhesion and deposition of calcium-containing mineral by bone cells were significantly greater when cultured on CNF compared with the currently used orthopedic implant materials of commercially pure Ti, Ti6Al4V, and CoCrMo. Directed axonal extension from neurons along CNF has also been observed (McKenzie et al 2006).
Moreover, previous work has shown that aligned patterns of CNF in polymers facilitate the adhesion of osteoblasts (bone-forming cells) on the CNF region (Khang et al 2006). In fact, when cultured for up to 21 days, osteoblasts produced linear patterns of calcium-containing mineral directly on top of linear patterns of CNF placed on polymers (Khang et al 2006). Since the CNF regions are the electrically active regions on such substrates, applied voltages have also been used to further the functions of these cells (Mattson et al 2000; Supronowicz et al 2002). For example, osteoblast deposition of calcium-containing mineral was up to 3 times greater when simultaneously cultured on CNT-based materials and subjected to an alternating current of 10 μA at a frequency of 10 Hz with a duty cycle of 50% for 6 hours daily up to 21 days (Supronowicz et al 2000).
However, to design the next generation of implants, selective and spatial cell adhesion must be achieved (Vance et al 2004). Specifically, for orthopedic applications, while the functions (such as adhesion, proliferation, and deposition of calcium-containing mineral) of osteoblasts are beneficial, the functions of other cell types (like fibroblasts which contribute to fibrous soft tissue formation) are not (Vance et al 2004). In addition, for vascular applications, while the adhesion of vascular smooth muscle cells and endothelial cells to an implant surface are important, their spatial location must be different since vascular smooth muscle cells and endothelial cells are present in the inner and outer regions of vascular tissue, respectively (Darling and Linton 1972; Clayson et al 1976; DHHS 1981; Lavender et al 2005).
It was for these reasons that the objective of the present in vitro study was to examine the adhesion of a wide range of cells to linear CNF patterns formulated on one particular polymer, polycarbonate urethane (PCU), in order to ascertain their ability to serve as tissue engineering constructs. Polycarbonate urethane has been widely used in numerous tissue engineering applications as it is FDA approved for implantation (Thapa et al 2003; Miller et al 2004).
Materials and methods
Substrates
CNFs were produced by catalytic and chemical vapor deposition techniques and were obtained from Applied Sciences, Inc. (Cedarville, OH, USA). In this work, high surface energy, small diameter (60 nm) CNF were used. PCU was used as a model polymer in this study and was obtained from Thermedics, Inc. (Cleveland, OH, USA). PCU was chosen since it is biocompatible, has a good oxidative stability, and is FDA approved (Polymer Technology Group 2005). Copper grids with linear grooves of 30μm were obtained from Electron Microscopy Sciences (Hatfield, PA, USA). To obtain the alignment of CNF on PCU, first PCU was melted by sonicating it in pellet form with chloroform for about 60 minutes. Then, PCU was applied as the base layer on a coverslip (Fisher Scientific, Pittsburgh, PA) and allowed to dry for 2 hours in a vacuum oven at 60°C. Copper grids were then placed on top of the dried PCU by using the principle of surface tension between PCU and CNF. A suspension of CNF was then made by sonicating CNF with 70% ethanol for about 60 minutes; 25 μL of this suspension was placed in the copper grids. The entire sample was allowed to dry again for 1 hour. After the prescribed timed period, the copper grids were removed and a highly aligned pattern of CNF was expected on the PCU layer (Khang et al 2006). The substrates were then treated with 100% ethanol for 1 day under UV light for sterility purposes.
Substrate characterization
Scanning electron microscopy (SEM, JEOL JSM-840) was used to characterize the alignment of CNF on PCU according to standard techniques (Khang et al 2006).
Cell adhesion assays
To ascertain how a wide range of cells would adhere on the substrates of interest, fibroblasts, vascular smooth muscle cells, and endothelial cells were used. All of these cells would be found at a typical implant insertion site, specifically for the case of orthopedic and vascular implants. Fibroblasts (embryo 3T3; ATCC CRL 1658) and rat aortic smooth muscle cells (RASMC; Vec Technologies, Rensselaer, NY, USA) were cultured in Dulbecco’s Modified Eagle Medium (Gibco) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin–streptomycin (Hyclone) under standard cell culture conditions (a humidified, 95/5% air/CO2, 37°C environment). Rat aortic endothelial cells (RAEC; Vec Technologies) were cultured in MCDB media (Vec Technologies).
2500 cells/cm2 were separately seeded on the CNF–PCU samples placed in a 6-well plate and were cultured for 4 hours. At that time, the respective media was aspirated from the wells and the substrates were rinsed with phosphate buffered saline twice to remove non-adherent cells. Adherent cells were then fixed with formaldehyde (Fisher Scientific) for 10 minutes. The cell nuclei were stained with Hoechst stain (Sigma) and visualized under a fluorescence microscope. Substrates were also visualized under light microscopy to determine PCU compared with CNF regions. Fluorescent and light images (Leica) of each substrate were then merged using Image Merger (BMT Micro, Inc., Wilmington NC, USA) software. Digital images (up to 400 μm2 field of view) were taken using Image Pro Software. Experiments were repeated in triplicate, three times each. To ascertain the total number of cells that adhered onto the substrates, cells were also counted on these images and reported as cells/cm2.
Results and discussion
Substrate characterization
As expected, results of this study confirmed the alignment of CNF on PCU through the techniques described here (Figure 1).
Figure 1.
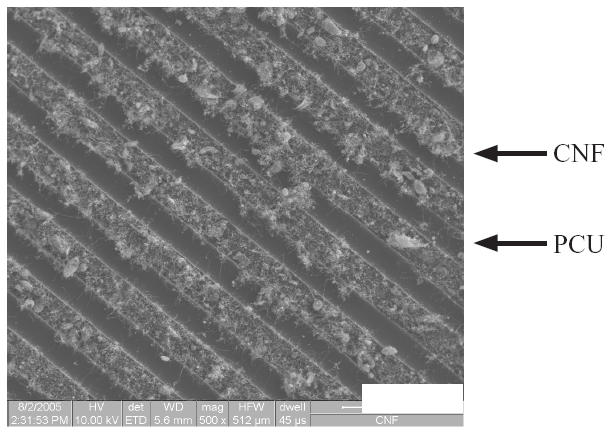
SEM image of high surface energy/area, small diameter (60 nm) CNF on PCU. PCU appears dark and CNF appear light. Bar (lower right) is 100 μm.
Abbreviations Figures 1–7: CNF, carbon nanofiber; PCU, polycarbonate urethane.
Fibroblast adhesion
Results of fibroblast adhesion revealed that about 85% selectively adhered to the PCU region and about 15% selectively adhered to the CNF region (Figures 2 and 3). This is in contrast to previous studies which found close to 90% of osteoblasts selectively adhering to the CNF (not PCU) regions (Figure 4) (Khang et al 2006). Such results highlight the ability of cells to recognize and adhere differently to PCU compared with CNF regions. Practically, such results may also give researchers the ability to spatially control osteoblast and fibroblast functions to maximize calcium-containing mineral and collagen extracellular matrix formation. This is because long bones of the body naturally possess a unique anisotropic blend of calcium-containing mineral on linear patterns of collagen (Kaplan et al 1994). Future research will be needed to determine the CNF compared with PCU width spacing that can maximize bone production for such orthopedic applications.
Figure 2.
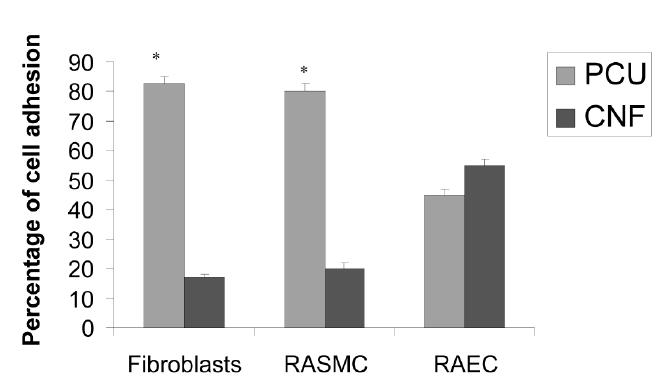
Spatial control of cell adhesion on CNF compared with PCU regions. Data = mean +/− SEM; n = 3; * p <0.01 (compared with CNF regions).
Abbreviations: RAEC, rat aortic endothelial cells; RASMC, rat aortic smooth muscle cells.
Figure 3.
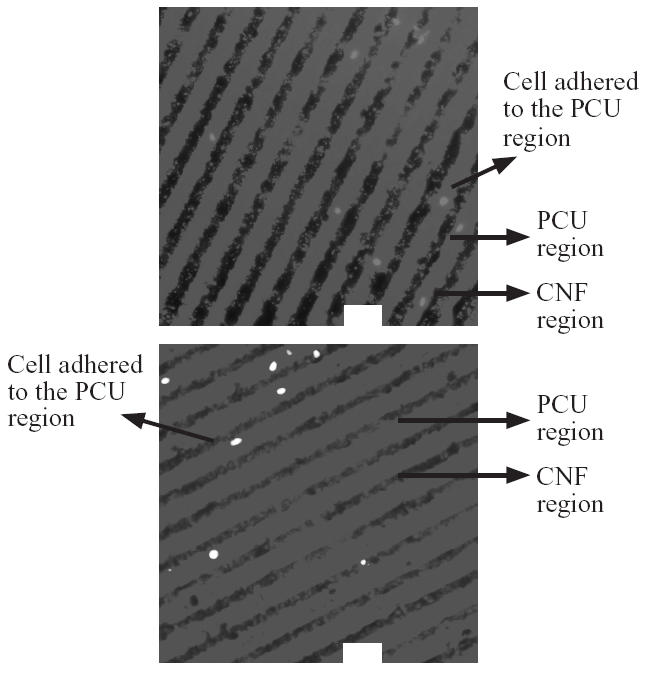
Selective fibroblast adhesion to PCU compared with CNF regions. Bars = 60 μm.
Figure 4.
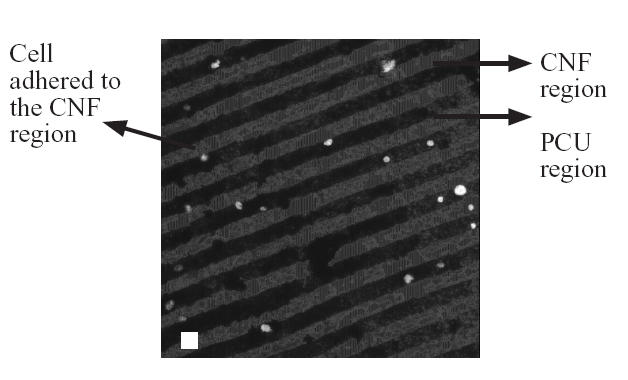
Selective osteoblast adhesion to CNF compared with PCU regions. Bar = 10 μm.
Smooth muscle cell and endothelial cell adhesion
For vascular smooth muscle cells, about 80% selectively adhered to the PCU region and about 20% selectively adhered to the CNF region (Figures 2 and 5). In contrast to fibroblast and vascular smooth muscle cell adhesion, no statistical preference was observed for endothelial cell adhesion (Figures 2 and 6). Specifically, about 45% of the endothelial cells adhered to the PCU regions while about 55% adhered to the CNF regions. These findings are of interest to both orthopedic and vascular tissue engineering applications. For orthopedic applications, angiogenesis (mediated by endothelial cells) is needed for maintaining bone health (Kaplan et al 1994). Thus, these materials could provide for new bone growth on CNF regions and angiogenesis on PCU regions (since CNF regions were almost exclusively favored by osteoblasts, leaving some favorability for PCU regions by endothelial cells). In addition, for vascular applications, these patterned materials may provide for select regions favorable for vascular smooth muscle adhesion–growth (almost exclusively on PCU regions) with different regions favorable for endothelial cell adhesion–growth (CNF regions favored somewhat by endothelial cells but not at all by smooth muscle cells). Future studies are needed to determine competitive cell adhesion on the CNF–PCU substrates to test the hypotheses stated above.
Figure 5.
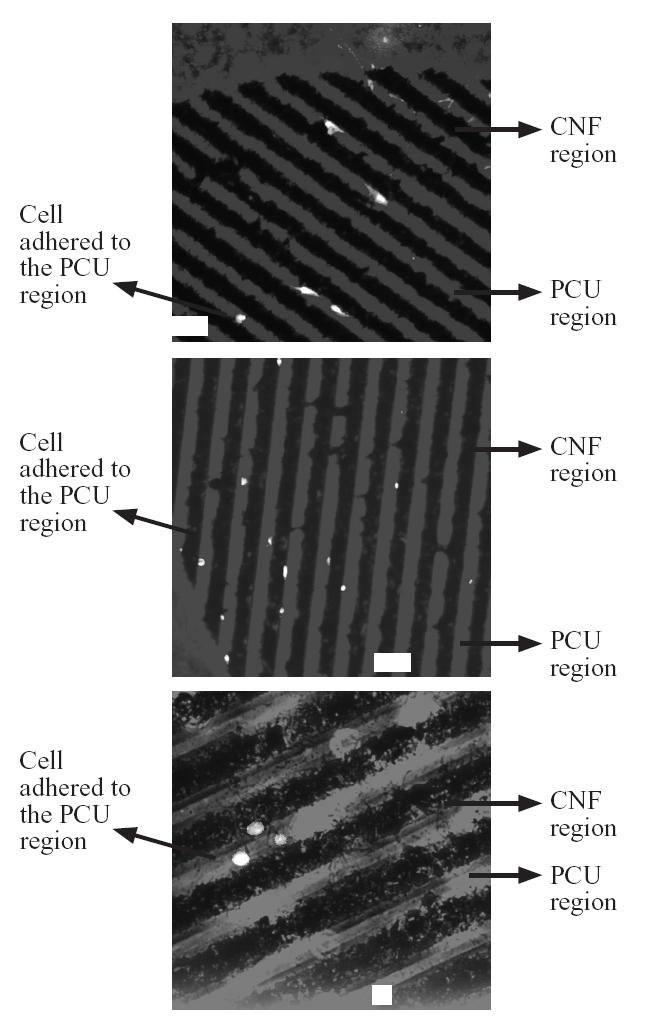
Selective vascular smooth muscle cell (RASMC) adhesion to PCU compared with CNF regions. Bars = 60 μm for the top two images and 10 μm for the bottom image.
Figure 6.
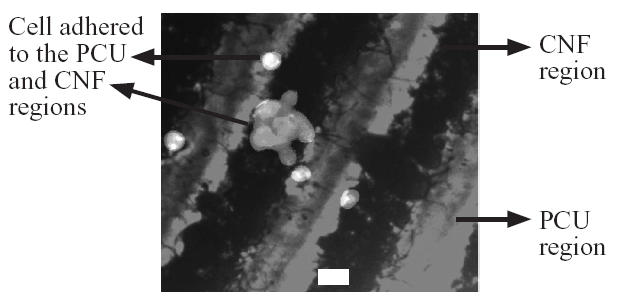
Non-selective endothelial cell (RAEC) adhesion to PCU compared with CNF regions. Bar = 10 μm.
Total cell numbers
When considering the total number of cells that adhered to the patterned surfaces, a significant portion of the original cell seeding density attached (Figure 7). Specifically, of the original cell seeding density (2500 cells/cm2), approximately 60%, 84%, and 90% of the fibroblasts, vascular smooth muscle cells, and endothelial cells adhered to the entire patterned surfaces, respectively. Previous studies (Khang et al 2006) demonstrated that 85% of the original seeding density of osteoblasts adhered on these same substrates. Importantly, when considering total cell numbers, vascular cells and osteoblasts (Khang et al 2006) adhered in significantly greater numbers than fibroblasts (Figure 7). Although the total number of adherent cells is critical in tissue engineering applications, it is important to note that a small seeding density was utilized in this study for experimental purposes. In addition, it is the selective attachment of these cells to different regions of the PCU–CNF patterned substrates that may be of unique advantage in tissue engineering applications.
Figure 7.
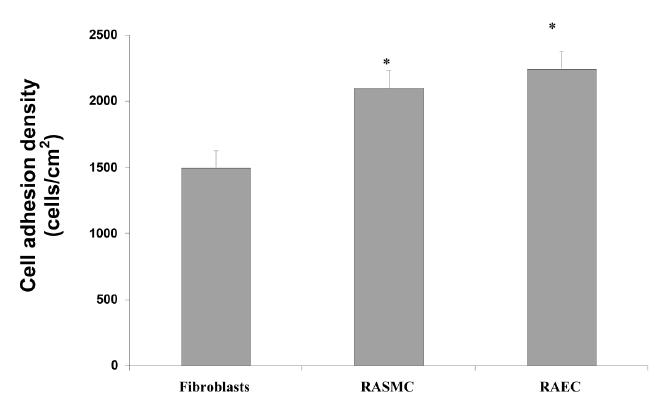
Significant cell adhesion on PCU–CNF patterned materials. Cell numbers represent the total on PCU–CNF patterned substrates. Of the original cell seeding density, approximately 60%, 84%, and 90% of the fibroblasts, RASMC (rat aortic smooth muscle cells), and RAEC (rat aortic endothelial cells) adhered, respectively. Previous studies (Khang et al 2006) demonstrated that 85% of the original seeding density of osteoblasts adhered on these same patterned substrates. Data = mean +/− SEM; n = 3; * p <0.01 (compared with fibroblasts).
Possible reasons for selective cell adhesion
Based on the above data, it is intriguing to ponder why these cells adhered differently to CNF versus PCU regions. Previous studies have provided some insights into this as they have highlighted altered initial protein interactions from serum on PCU and CNF regions (Khang et al 2006). Specifically, previous studies have shown greater vitronectin and fibronectin adsorption to CNF compared with PCU regions (Khang et al 2006). On some surfaces, greater vitronectin and fibronectin adsorption has been correlated with increased osteoblast but decreased fibroblast adhesion (Webster et al 2000); the same events of altered initial protein adsorption from serum that controls subsequent cell adhesion may be happening here. It is believed that proteins are interacting differently with CNF compared with PCU regions since these materials possess altered surface energetics (with the particular CNF used in this study having higher surface area and roughness compared with PCU).
Conclusions
Collectively, these studies provide for patterned substrates that are not chemically functionalized (with entities like amino acids or other bioactive groups that may become compromised once implanted (Mattson et al 2000)) to spatially direct cell adhesion. Specifically, osteoblast adhesion was directed to CNF patterns on PCU while fibroblast and vascular smooth muscle adhesion was directed towards PCU regions. Endothelial cell adhesion showed no preference. Coupled with the novel attributes of high mechanical strength and conductivity, CNF patterns on PCU may be novel substrates for numerous tissue engineering applications.
Acknowledgments
The authors would like to thank the SURF (Summer Undergraduate Research Fellowship) program by Purdue University for financial support and Ms Deborah Sherman for help with scanning electron microscopy.
References
- [DHHS] Department of Health and Human Services. Washington DC: DHHS; 1981. Atherosclerosis: Report of the working group on atherosclerosis of the National Heart, Lung and Blood Institute; p. 2. [Google Scholar]
- Clayson KR, Edwards WH, Allen TR, et al. Arm veins for peripheral arterial reconstructions. Arch Surg. 1976;111:1276–80. doi: 10.1001/archsurg.1976.01360290110017. [DOI] [PubMed] [Google Scholar]
- Darling RC, Linton RR. Durability of femoropopliteal reconstructions endarterectomy versus vein bypass grafts. Am J Surg. 1972;123:472–9. doi: 10.1016/0002-9610(72)90202-4. [DOI] [PubMed] [Google Scholar]
- Kaplan FS, et al. In: Bone biology. Kaplan FS, et al., editors. Columbus OH: Orthopedic basic science American Academy of Orthopedic Surgeons; 1994. p. 127. [Google Scholar]
- Khang D, Sato M, Price RL, et al. Selective adhesion and mineral deposition by osteoblasts on carbon nanofiber patterns. International Journal of Nanomedicine. 2006;1:65–72. doi: 10.2147/nano.2006.1.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender MD, Pang Z, Wallace CH, et al. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials. 2005;26:4642–53. doi: 10.1016/j.biomaterials.2004.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neuroscience. 2000;14:175–82. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- McKenzie JL, Waid MC, Shi R, et al. Decreased functions of astrocytes on carbon nanofiber materials. Biomaterials. 2004;25:1309–17. doi: 10.1016/j.biomaterials.2003.08.006. [DOI] [PubMed] [Google Scholar]
- McKenzie JL, Waid MC, Shi R, et al. Directed axonal extension from neurons on carbon nanofiber-based materials. Biomaterials. 2006 submitted. [Google Scholar]
- Miller DC, Thapa A, Haberstroh KM, et al. Endothelial and vascular smooth muscle cell function on poly (lactic-co-glycolic acid) with nano-structured surfaces. Biomaterials. 2004;25:53–61. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- Polymer Technology Group. Bionate® Polycarbonate Urethane. 2005 Accessed 23 June 2006. URL: http://www.polymertech.com/materials/bionate.html.
- Price RL, Waid MC, Haberstroh KM, et al. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003;24:1877–87. doi: 10.1016/s0142-9612(02)00609-9. [DOI] [PubMed] [Google Scholar]
- Supronowicz PR, Ajayan PM, Ullmann KR, et al. Novel current-conducting composite substrates for exposing osteoblasts to alternating current stimulation. J Biomed Mater Res. 2002;59:499–506. doi: 10.1002/jbm.10015. [DOI] [PubMed] [Google Scholar]
- Thapa A, Miller DC, Webster TJ, et al. Nano-structured polymers enhance bladder smooth muscle cell function. Biomaterials. 2003;24:2915–26. doi: 10.1016/s0142-9612(03)00123-6. [DOI] [PubMed] [Google Scholar]
- Vance RJ, Miller DC, Thapa A, et al. Decreased fibroblast cell density on chemically degraded poly-lactic-co-glycolic acid, polyurethane, and polycaprolactone. Biomaterials. 2004;25:2095–103. doi: 10.1016/j.biomaterials.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Webster TJ, Ergun C, Doremus RH, et al. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51:475–80. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]


