Abstract
Pegylated liposomal doxorubicin is a formulation of doxorubicin in which the molecule itself is packaged in a liposome made of various lipids with an outer coating of polyethylene glycol. Liposomal technology is being used in increasing amounts in the therapy of a variety of cancers, including ovarian cancers. This article reviews the mechanistic actions of this formulation, the Phase II and Phase III data that helped define the role of pegylated liposomal doxorubicin in recurrent ovarian cancer, as well as a discussion of some of the side-effects and their management.
Keywords: pegylated lipopsomal doxorubicin, ovarian cancer
Introduction
According to the American Cancer Society (2006), an estimated 20 180 new cases of ovarian cancer will be diagnosed in the US in 2006. Approximately 15 310 of these women will die of this disease. The vast majority will present with advanced disease and will require chemotherapy, and the majority of these will relapse. Currently, in the US, the initial treatment consists of maximal surgical debulking followed by carboplatin and taxane chemotherapy. When the disease recurs, the patient and physician are presented with a host of chemotherapy options. One drug that is increasingly being used is pegylated liposomal doxorubicin (DOXIL ® [US], Caelyx ® [outside US]; Tibotec Therapeutics, a division of Ortho Biotech Products, L.P., Bridgewater, NJ, USA. http://www.tibotec.com).
Mechanism of action
Pegylated liposomal doxorubicin is one of a new class of drug formulations that is delivered in vesicles called liposomes. The doxorubicin molecules in pegylated liposomal doxorubicin are encapsulated in a bilayer sphere of lipids. This vesicle is then surrounded by a dense layer of polyethylene glycol (PEG), hence the name pegylated liposomal doxorubicin (Figure 1). The size of the liposomes, approximately 100 nm, prevents them from entering tissues with tight capillary junctions, such as the heart and gastrointestinal tract, as well as selectively depositing the liposome into the tumor (Waterhouse et al 2001) (Figure 2). In contrast to normal vessels, the vessels of the tumor are tortuous, dilated, have morphologically abnormal endothelial cells, and are leaky due to large spaces between pericytes (Figure 3) (Jain 2005). These physical characteristics allow more extravasation of the vesicles into the tumor, thus encouraging more deposition of the chemotherapy agent into the tumor. The PEG coating on the liposome creates a hydrophilic layer around the liposome that buffers the liposome wall from the surrounding milieu. This decreases proteins from binding to the lipid bilayer. These proteins act as opsonins, attracting foreign particles that in turn activate the mononuclear phagocytic cells. This leads to break down of the liposome and release of the drug. Therefore, the PEG coating on the liposome increases the longevity of the liposome.
Figure 1.
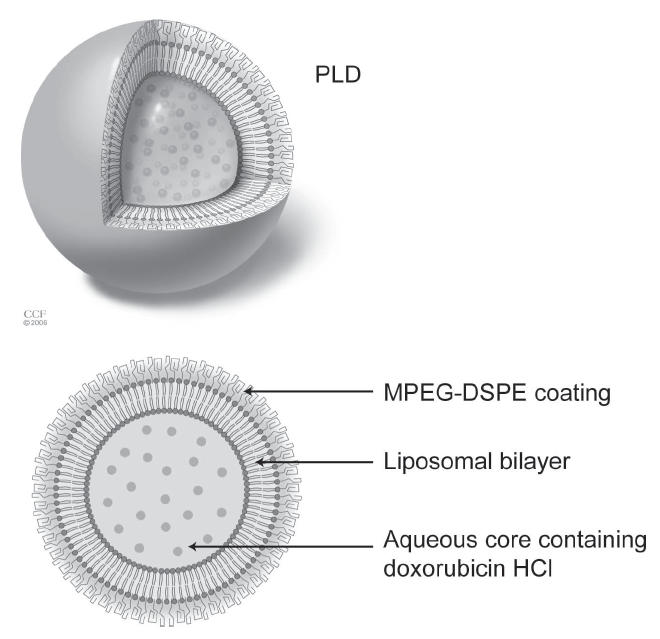
Pegylated liposomal doxorubicin (PLD) molecule. Reprinted with the permission of the Cleveland Clinic Foundation
Abbreviations: MPEG-DSPE, N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt.
Figure 2.
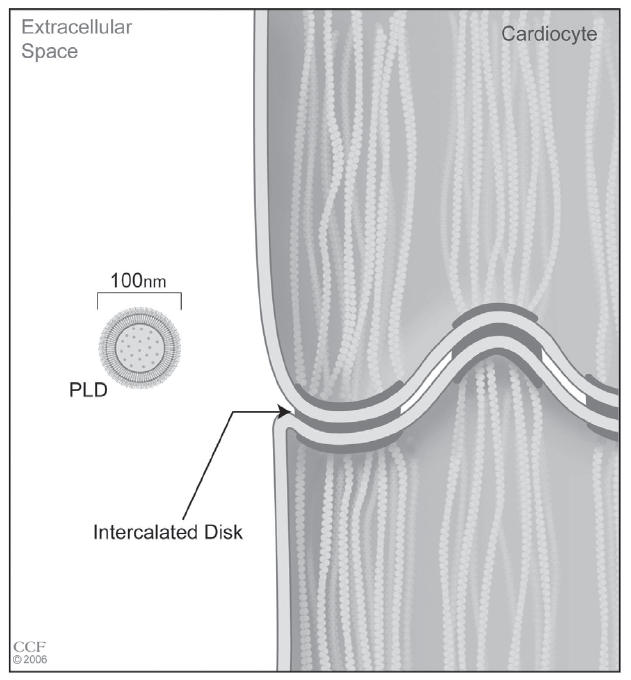
Scale drawing of the aforementioned (Figure 1) macule in cardiac tissue. Reprinted with the permission of the Cleveland Clinic Foundation.
Figure 3.
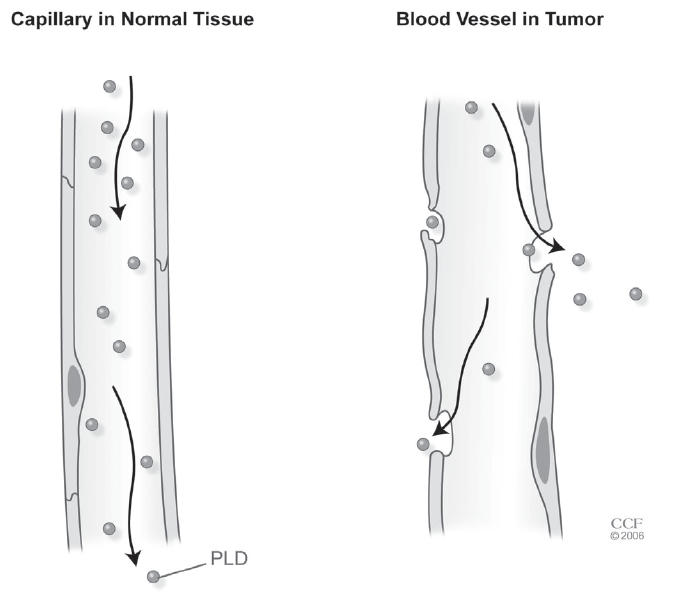
Pegylated liposomal doxorubicin (PLD) molecule in normal (left) and tumor (right) vessels. Reprinted with the permission of the Cleveland Clinic Foundation.
When the liposome does leave the intravascular compartment, in normal tissues it is cleared via the lymphatic system and returned to the circulation. In tumor tissue, however, there are no lymphatics. Therefore, when the liposome is deposited it remains for a longer time. This allows a higher dose of doxorubicin to be released in the tumor, and a lower dose in normal tissue (Gordon et al 2000). Collectively, there is preferential uptake and decreased clearance of the drug delivery system, increasing the exposure of the tumor to the drug. This was termed by Cattel et al (2003) as the enhanced permeation retention effect.
Phase II single-agent studies
Table 1 summarizes the multiple Phase II trials of pegylated liposomal doxorubicin in recurrent ovarian cancer. In 1997 Muggia et al (1997) reported 35 consecutive patients at 2 institutions who had recurred following platinum- and paclitaxel-based chemotherapy. Patients were treated with pegylated liposomal doxorubicin at a dose of 50 mg/m2 every 21 days. There were 9 (25.5%) responses, including 1 complete response. The median progression-free survival was 5.7 months and the median overall survival was 11 months. Thirteen (37%) patients had a grade 3 or 4 toxicity, but were managed by decreasing the dose to 40 mg/m2, or by lengthening the dosing interval to 4 or 5 weeks.
Table 1.
Phase II trials – single agent
| Author | Year | Drug (dose) | n = | Responses (%) | Platinum resistant | Stable disease |
|---|---|---|---|---|---|---|
| Muggia | 1997 | PLD (50 mg/m2) q 3 wks | 35 | 9 (25.5%) | 100% | NR |
| Gordon | 2000 | PLD (50 mg/m2) q 4 wks | 79 | 13 (16.9%) | 100% | 36 (57%) |
| Markman | 2000 | PLD (40 mg/m2) q 4 wks | 44 | 4 (8%) | 100% | 8 (16%) |
| Campos | 2001 | PLD (40 mg/m2) q 4 wks | 72 | 19 (27%) | 40% | NR |
| Rose | 2001 | PLD (50 mg/m2) q 4 wks | 37 | 5 (13.5%) | 100% | 18 (48.6%) |
| Rose | 2001 | PLD (40 mg/m2) q 4 wks | 39 | 3 (7.7%) | 100% | 20 (51.3% |
| Lorusso | 2004 | PLD (35 mg/m2) q 3 wks | 37 | 5 (13%) | 46% | 18 (48%) |
| Arcuri | 2004 | PLD (50 mg/m2) q 4 wks | 20 | 7 (26.6%) | 66% | NR |
| Wilailak | 2004 | PLD (40 mg/m2) q 3 wks | 14 | 3 (23%) | 100% | NR |
| Chou | 2005 | PLD (45 mg/m2) q 4 wks | 29 | 7 (23%) | 100% | NR |
Abbreviations: NR, not reported; PLD, pegylated liposomal doxorubicin.
In a subsequent Phase II study, Gordon et al (2000) evaluated 79 better-defined patients all of whom were platinum and taxane refractory. Eighty-five percent of the patients had received more than 2 prior chemotherapy regimens. These “doubly refractory” patients were treated with 50 mg/m2 of pegylated liposomal doxorubicin every 4 weeks. Fourteen partial responses and 1 complete response were reported for an overall response rate of 16.9%. The median time to response was 15 weeks. The median progression-free survival for all patients treated in this study was 19.3 weeks (range 0.7–86 weeks). In addition, 36 patients (57%) were classified as having stable disease, and achieved a median progression-free survival of 21.9 weeks. This was one of the first studies to show that disease stabilization in recurrent ovarian cancer is of clinical benefit. All patients reported at least 1 adverse event, but the majority were grade 1 or 2. Asthenia and palmar-plantar erythrodysesthesia (PPE) were seen in 41.6%. Only 1 patient experienced any cardiac complications, and there were no treatment-related deaths. This study demonstrated that pegylated liposomal doxorubicin was useful in this drug-resistant setting, and associated with no life-threatening toxicities.
In the same year, Markman et al (2000), with the intent to find a more tolerable dose of pegylated liposomal doxorubicin, published a Phase II trial of 40 mg/m2. They evaluated 44 patients with platinum- and paclitaxel-refractory ovarian, peritoneal, and fallopian tube cancer. The patients received a median of 2 (range 1–12) treatments. There were 4 patients (8%) with evidence of a partial response or benefit, defined as decreasing symptoms and a decrease in CA-125 (cancer antigen 125). Another 8 (16%) were classified as stable disease and received more than 6 doses. There were 6 cases (12%) of grade 2 PPE, 4 (8%) grade 2 mucosal toxicities, but no grade 3 toxicities. Six of the patients required dose reductions that were treatment-related. They concluded that in the setting of palliative therapy for refractory ovarian cancer, this dosing regimen presented an acceptable option for patients.
Campos et al (2001) published a retrospective study of 71 patients with recurrent ovarian cancer who received 40 mg/m2 every 28 days. A partial response was seen in 16 patients (23%) and a complete response in 3 (4%) for an overall response rate of 27%. Additionally, there were 12 (17%) patients with stable disease, defined as <50% reduction in the sum of bidimensional measurements, a <50% reduction in CA-125, and no progression for 3 weeks. Surprisingly, among 51 platinum-resistant patients, there were 14 partial and 2 complete responses for an overall response rate of 35%, which was a slightly higher response rate than in the study overall. The median progression-free survival was 5.3 months (range 2.1–12.1). Also, Rose et al (2001) retrospectively compared 40 patients treated with pegylated liposomal doxorubicin at a dose of 50 mg/m2 with 38 patients treated at a dose of 40 mg/m2. Patients were treated with the higher dose until 1999 and a lower dose regimen afterward. No difference in progression-free survival was noted. However, dose reductions were required in 27% of the group in the higher dose and in none of the patients treated at the lower dose (Rose et al 2001).
Lorusso et al (2004) treated 37 patients in a Phase II study at a dose of 35 mg/m2 every 3 weeks in an attempt to decrease the incidence of skin toxicity. Although patients were pretreated with a median of 2 other chemotherapy regimens, and 32.4% had received at least 4 different chemotherapy regimens, 20 (54%) remained platinum sensitive. All received at least 2 courses of the pegylated liposomal doxorubicin. While there were no complete responses, 5 patients had a partial response and 16 patients had stable disease. The median time to response was 12 weeks. There were 2 partial responses in the platinum-sensitive population and 3 in the platinum-resistant group. In the heavily pretreated population (>3 prior regimens) there was only 1 response among 17 patients (5.8%). In contrast, 4 out of 20 patients who had received 1 or 2 prior treatment regimens responded (20%). Although not statistically significant, this suggests that using pegylated liposomal doxorubicin earlier in the course of recurrent disease is preferable.
Arcuri (2006) reported on 30 patients who recurred following prior platinum-based chemotherapy and were treated with single-agent pegylated liposomal doxorubicin at 50 mg/m2 every 28 days. There were 6 (20%) partial responses and 2 (6.6%) complete responses for an overall response rate of 26.6%. Among 23 patients defined as platinum resistant, only 2 (8.7%) responded. Again, the median time to response was 12 weeks. At this dose, a higher frequency of toxicities was noted, with a 23.3% incidence of grade 3 or 4 neutropenia. Grade 3 or 4 PPE was reported in 10% of the patients and grade 3 or 4 mucositis also occurred in 10% of the patients.
In smaller studies of single-agent pegylated liposomal doxorubicin in recurrent ovarian cancer, Wilailak and Linasmita (2004) at a dose of 40 mg/m2 every 3 weeks and Chou et al (2005) at a dose of 45 mg/m2 every 4 weeks both reported response rates of 23%.
Randomized single-agent studies
There have been 2 randomized trials of pegylated liposomal doxorubicin in recurrent ovarian cancer (Table 2). Gordon et al (2001) reported on 474 patients randomized to either pegylated liposomal doxorubicin at 50 mg/m2 every 4 weeks or topotecan 1.5 mg/m2 daily for 5 days every 3 weeks. Response rates for pegylated liposomal doxorubicin and topotecan were 19.7% and 17%, respectively (p=0.390). At the time of the original publication only a trend in increased progression-free survival was noted in favor of the pegylated liposomal doxorubicin group (p=0.095). Although the study was not designed for subgroup analysis, among the platinum-sensitive group there was a progression-free survival (p=0.037) and overall survival advantage (108 vs 71.1 weeks, p=0.008) in favor of the pegylated liposomal doxorubicin arm. In contrast, the platinum-resistant patients showed no survival advantage. The toxicity profiles between these two chemotherapy agents were quite different. Although nearly all patients reported at least 1 adverse event, there were more grade 4 events in the topotecan group (71% vs 17%). In the pegylated liposomal doxorubicin group there was a 49% incidence of PPE (22% grade 3 and 0.8% grade 4) and a 40% incidence of stomatitis. In the topotecan group, >90% experienced hematologic toxicity and two thirds were grade 3 or 4. Also of significance was that 3.8% (9 patients) of the topotecan group developed treatment-related sepsis, and 3 of these patients died as a result of it. There were no treatment-related deaths in the pegylated liposomal doxorubicin group and these patients had fewer treatment modifications. There were a similar number of patients who withdrew from the study due to adverse events (43 in the pegylated liposomal doxorubicin group and 37 in the topotecan group). A long-term follow up on this study was subsequently reported (Gordon et al 2004). At the time of this evaluation 87% (413) had died. There was a significant survival advantage for all patients entered on this study in favor of the pegylated liposomal doxorubicin arm (p=0.050), resulting in an 18% reduction in the risk of death, a difference of more than 3 weeks. Among the patients with platinum-sensitive disease a more significant benefit was seen, resulting in a 30% reduction in death and a total survival of 107.9 vs 70.1 weeks. This is the first study to show a survival advantage between 2 non-platinum agents.
Table 2.
Randomized studies single-agent trials
| Author | Year | Drug (dose) | n = | Survival advantage resistant | Platinum advantage | Side-effect |
|---|---|---|---|---|---|---|
| Gordon | 2001 | PLD (50 mg/m2) q 4 wks, vs topotecan 1.5 mg/m2/d for 5 days q 3 weeks | 474 | Yes – PLD in both platinum sensitive and in the study overall | 50% | Yes – PLD |
| O’Byrne | 2002 | PLD (50 mg/m2) q 4 wks vs paclitaxel 175 mg/m2 q 3 wks | 214 | None | 100% | none |
Abbreviations: PLD, pegylated liposomal doxorubicin.
In a second randomized trial, O’Byrne et al (2002) studied 214 patients randomized to either pegylated liposomal doxorubicin (50 mg/m2) every 4 weeks or paclitaxel 175 mg/m2 every 3 weeks. The study was suspended due to poor accrual, as paclitaxel became incorporated into first-line therapy. A preliminary review of the data showed that there were no significant differences in response rates, progression-free survival, or overall survival. The number of adverse events was similar.
Administration and side-effects
Pegylated liposomal doxorubicin is associated with a number of adverse effects. The first adverse effect seen is an acute hypersensitivity reaction. This reaction is characterized by flushing, facial edema, headache, back pain, rigors, hypotension, chest/throat tightness and dyspnea. This hypersensitivity reaction is seen in 6.8% of patients and usually occurs during the first administration (Uziely et al 1995). This differs from the classic hypersensitity reactions, which occur only after previous exposure to the allergen. To overcome this reaction the initial administration of pegylated liposomal doxorubicin is given over 2 hours with pretreatment with diphenhydramine, dexamethasone, and famotidine. If no infusion reaction occurs, the subsequent doses can be given over 1 hour without pretreatment. If hypersensitivity is not seen initially, it will usually not be seen in subsequent administrations. If a hypersensitive reaction does occur, the reaction is managed by temporarily discontinuing the infusion until symptoms resolve. The infusion can then be restarted at 25% of the original rate. This usually alleviates any further hypersensitivity.
PPE and mucositis are the most common serious side-effects of pegylated liposomal doxorubicin. Both are dose- and schedule-dependent. PPE, also referred to as hand-foot syndrome, although seen with other chemotherapy agents, is extremely common with liposomal therapy and can be very severe. Following administration, the liposomes tend to pool in the post-capillary venules where the liposome is degraded and the drug is released. Accumulation of liposomal doxorubicin can also be seen in areas of skin trauma, which may be subclinical. Subclinical skin trauma may occur as a result of tight-fitting clothing or shoes, intercourse, repetitive contact, or even recent surgical incisions. Symptoms usually present 14–21 days after the third cycle of therapy. The syndrome is characterized by a prodrome of paraesthesia of the extremities, usually occurring 3–5 days before cutaneous symptoms become identifiable. This can progresses to painful erythema and cracking of the palmar and plantar surfaces, followed by desquamation (Gordon et al 2001). Grade 3 or 4 PPE is seen in 17.5% of patients with pegylated liposomal doxorubicin at a dose of 50 mg/m2 every 4 weeks (Nagore et al 2000). The symptoms worsen with repeated doses, especially at higher doses and shortened dosing intervals. It is theorized that the accumulation in skin, and thus this side-effect, is due to vascular permeability at contact–pressure points (Cattel et al 2003). While PPE cannot be prevented, its severity can be decreased with dose modification, either by decreasing the dose or lengthening the dosing interval. Dose modification often allows continued treatment without recurrence of PPE. In Lorusso’s (2004) study with a decreased dose of pegylated liposomal doxorubicin utilized (35 mg/m2), there was a 21.6% incidence of PPE but it was grade 3 in severity in only 1 (2.8%) and no patient had grade 4 toxicities. In a series of patients treated at 40 mg/m2 (Kim et al 2005) there was only a 1% rate of grade 3 and no grade 4 toxicities among 90 patients. There are preliminary data indicating that some drugs, including B6, lysine, topical dimethyl sulfoxid, and topical or systemic steroids may decrease the rate and/or severity of PPE; however, this has not been extensively studied. Drake et al (2004) reported a prospective study of 23 patients treated at a dose of 50 mg/m2. Nine patients (39%) developed grade 2–4 PPE. These symptoms were significantly decreased in 6 patients treated with oral dexamethasone 8 mg bid beginning 24 hours before the infusion
Mucositis is often a dose-limiting toxicity and can be seen after a single injection of high-dose pegylated liposomal doxorubicin as well as after numerous cycles. Its severity can also be modulated by altering the dosing regimen. Our regimen for mucositis prophylaxis includes L-lysine 500 mg tid (3 tabs tid) with the use of either or both of the following mouth washes when patients develop oral symptoms: benadryl, maalox, xylocaine, or tetracycline 400 mg, hydrocortisone 110 mg, 30 mL nystatin, 240 mL chlor-trimeton 5 cc swish and spit qid.
In an effort to prevent PPE and mucositis a number of behavioral modifications have been utilized. Avoiding activities that increase blood flow or cause trauma to the skin or mucosal membranes, such as hot liquids, hot foods, hot baths, tight fitting clothes, bras, and shoes. Alternatively, ice packs to the hands and feet have been utilized to decrease blood flow. Unfortunately, the efficacy of these interventions has not been prospectively studied.
Myelotoxicity is generally mild, and cumulative toxicity is not observed, indicating minimal damage to the marrow. Severe myelosuppression is so rare, Campos et al (2001) were able to treat 10 patients who had limited bone marrow reserve after failing high-dose therapy with peripheral stem cell support.
In contrast to free doxorubicin, cardiotoxicity has been shown to be extremely rare. In patients in whom there was an associated change in the status of the heart, often it was either not clinically significant and picked up only on screening echocardiograms, or it presented in patients with significantly compromised cardiac status. This was confirmed in a study of 509 breast cancer patients who were randomized to pegylated liposomal doxorubicin 50 mg/m2 every 4 weeks or doxorubicin 60 mg/m2 every 3 weeks (O’Brien et al 2004). This study was designed as a non-inferiority study and showed equivalent efficacy. However, cardiotoxicity was significantly less with pegylated liposomal doxorubicin (p= <0.001, HR=3.16). Myelosuppression and alopecia were significantly improved as well.
The dosing for pegylated liposomal doxorubicin and conventional doxorubicin are different (40–50 mg/m2 every 4 weeks vs 60–90 mg/m2 every 3 weeks) (Rose 2004). Pegylated liposomal doxorubicin has a plasma concentration nearly 1000-fold different compared with the concentration time curve for free doxorubicin. Normally, less than 1% of free doxorubicin actually reaches the tumor; however, with pegylated liposomal doxorubicin there is a 10-fold increase in the concentration of doxorubicin in the tumor. The peak uptake of the drug occurs 48–72 hours after administration, highlighting the stability of the delivery vehicle. The drug itself is released from the stealth liposomes in the interstitial fluid of the tumor over a period of days to weeks, thus exposing the tumor to a continuous drug exposure over time (Cattel 2003).
Rarely, nausea, vomiting, or alopecia can be seen with pegylated liposomal doxorubicin.
Doublet combinations
In view of the activity of pegylated liposomal doxorubicin with its minimal hematologic toxicity, this agent has been studied in numerous Phase I and II chemotherapy combinations involving 2 or 3 agents.
Phase I combination studies
The Phase I doublets studied are listed in Table 3. One of the concerns about combination regimens is the potential to use subtherapeutic doses in an effort to achieve an acceptable regimen. One case in example is a study by Rose et al (2002) which was a Phase I trial of the combination of prolonged oral etoposide and pegylated liposomal doxorubicin. The study had 2 dose escalations planned: dose escalation for hematologic toxicity with oral etoposide followed by dose escalation for non-hematologic toxicity with pegylated liposomal doxorubicin. The starting dose of pegylated liposomal doxorubicin was 20 mg/m2, followed by 50 mg/m2 of etoposide daily beginning on day 2. The maximum tolerable dose (MTD) of etoposide was found to be at 12 days. However, at this dose the authors were not able to increase the pegylated liposomal doxorubicin dose due to stomatitis and PPE. Five (29%) responses were noted. Of significance, 4 of the 7 patients who progressed on this combination subsequently stabilized with single-agent pegylated liposomal doxorubicin at 40 mg/m2 every 28 days, implying that the lower dose of pegylated liposomal doxorubicin was not a therapeutic dose in ovarian cancer.
Table 3.
Phase I combinations
| Author | Year | Drug (dose) | MTD |
|---|---|---|---|
| Doublets | |||
| Ryan | 2000 | PLD | aPLD 20 mg/m2 day 1 |
| topotecan | topotecan 1.0 mg/m2 day 1–5 | ||
| Lyass | 2001 | PLD | PLD 50 mg/m2 day 1 |
| cisplatin | cisplatin 50 mg/m2 day 1 | ||
| D’Agostino | 2002 | PLD | PLD 30 mg/m2 day 1 |
| gemcitabine | gemcitabine (1000 mg/m2) q day 1 and 8 every 21 days | ||
| Rose | 2002 | PLD | PLD 20 mg/m2 day 1 |
| Oral etoposide | etoposide 50 mg/m2 daily on day 2 for 12 days | ||
| Androulakis | 2002 | Weekly PLD | Weekly PLD 10 g/m2 |
| Weekly paclitaxel | Weekly paclitaxel 80 mg/m2 | ||
| Fracasso | 2002 | PLD | PLD 20 mg/m2 day 1,15 |
| gemcitabine | gemcitabine 1000 mg/m2 day 1,15 | ||
| Goncalves | 2003 | PLD | PLD 35 mg/m2 day 1 |
| carboplatin | carboplatin AUC 5 | ||
| Recchia | 2003 | PLD | PLD 40 mg/m2 |
| oxaliplatin | oxaliplatin 130 mg/m2 | ||
| Fracasso | 2003 | PLD | PLD 20 mg/m2 |
| docetaxel | docetaxel 40 mg/m2 day 1,15 | ||
| Tambaro | 2003 | PLD | PLD 30 mg/m2 day 1 |
| vinorelbine | vinorelbine 25 mg/m2 day 1,8 | ||
| Fracasso | 2005 | PLD | PLD 25 mg/m2 day 1 |
| valspodar | valspodar 0.42 mg kg/h 72 hr CI | ||
| Mirchandani | 2005 | PLD | PLD 40 mg/m2 day 1 |
| CI topotecan | topotecan 0.4 mg/m2 day1–14 | ||
| Triplets | |||
| Gibbs | 2002 | PLD | PLD 30 mg/m2 |
| carboplatin | carboplatin AUC 6 | ||
| paclitaxel | paclitaxel 175 mg/m2 q 28 days or PLD 20 mg/m2 carboplatin AUC 5 paclitaxel 175 mg/m2 q 21 days | ||
| Rose | 2000 | PLD | PLD 30 mg/m2 q 42 days carboplatin |
| carboplatin | AUC 5 q 21 days | ||
| paclitaxel | paclitaxel 175 mg/m2 q 21 days |
Excessive toxicity seen at this dose
Abbreviations: AUC, area under the curve; CI, continuous infusion; MTD, maximally tolerated dose; PLD, pegylated liposomal doxorubicin.
As expected, in view of the activity of platinum compounds in ovarian cancer, investigators have studied the combinations of pegylated liposomal doxorubicin with platinum agents including cisplatin, carboplatin, and oxaliplatin (Lyass et al 2001; Goncalves et al 2003; Recchia et al 2003). Other investigators have studied the combinations of pegylated liposomal doxorubicin with taxanes, both paclitaxel and docetaxel (Fracasso et al 2003; Androulakis et al 2002) or other second-line agents such as topotecan, vinorelbine, and gemcitabine (Ryan et al 2000; D’Agostino et al 2003; Fracasso et al 2002, 2005; Tambaro et al 2003; Garcia et al 2005; Mirchandani et al 2005; Valerio et al 2006)
Gibbs et al (2002) and Rose et al (2000) both studied chemotherapy-naïve ovarian cancer patients with escalating pegylated liposomal doxorubicin doses with standard dose carboplatin area under concentration time curve (AUC) 5 and paclitaxel at 175 mg/m2over 3 hours. Both studies demonstrated the maximum tolerated dose schedule was carboplatin AUC 5 or 6, paclitaxel 175 mg/m2, and pegylated liposomal doxorubicin 30 mg/m2 every 28 days. Gibbs et al (2002) found that reducing the dose of paclitaxel did not allow an increase in the dose of pegylated liposomal doxorubicin. Rose et al (2000) demonstrated that administering the liposomal doxorubicin every other cycle allowed for the maximum dose of carboplatin and paclitaxel to be administered. This schedule was taken forward into the randomized trial GOG 182-ICON 5 (Copeland et al 2003).
An alternative method of adding pegylated liposomal doxorubicin to the carboplatin paclitaxel regimen is by using the drugs sequentially rather than simultaneously. Potamianou et al (2005) treated patients with 4 cycles of carboplatin (AUC 6) and paclitaxel at 175 mg/m2 followed by 4 cycles of carboplatin (AUC 6) with pegylated liposomal doxorubicin 40 mg/m2 for 4 cycles. They reported a 78% response rate (49% complete responses and 29% partial responses). Seven (17%) had stable disease. The 2-year survival was 67%.
Phase II combination studies
Phase II studies of pegylated liposomal doxorubicin in combination with various other chemotheraputic agents are summarized in Table 4. In platinum-sensitive recurrent ovarian cancer patients, doublet therapy with pegylated liposomal doxorubicin and carboplatin has been studied. The GINECO (Ferrero et al 2004) Phase II trial enrolled 105 patients who were treated with pegylated liposomal doxorubicin at 30 mg/m2 and carboplatin at an AUC of 5 on day 1, every 4 weeks. They observed a complete response rate of 38% and an overall response rate of 63%. The median progression-free survival was 9 months. Vorobiof et al (2004) reported a Phase II trial of pegylated liposomal doxorubicin (50 mg/m2) and carboplatin at an AUC of 5 every 4 weeks. The overall response rate was 62.5% with a complete response rate of 37.5%. The median time to treatment failure was >287 days (range 29–819 days).
Table 4.
Phase II doublet combinations
| Author | Year | Drug (dose) | n = | Responses (%) | Stable disease | Response in platinum resistant |
|---|---|---|---|---|---|---|
| D ’Agostino | 2003 | PLD (30 mg/m2) q day 1 gemcitabine (1000 mg/m2) q day 1 and 8 every 21 days | 70 | 23 (34.3%) | 26 (38.8%) | 25% |
| Campos | 2003 | PLD (30 mg/m2) q 3 wk, paclitaxel (70 mg/m2) q wk | 40 | 11 (29%) | NR | 17% |
| Ferrero | 2004 | PLD (30 mg/m2) q 4 wk, carboplatin (AUC 5) q 4 wk | 105 | (63%) | NR | 0% |
| vorobiof | 2004 | PLD (50 mg/m2) q 4 wk, Carboplatin (AUC 5) q 4 wk | 21 | 13 (62.5%) | 5 (23%) | 0% |
| Ferrandina | 2005 | PLD (50 mg/m2) q 3 wk, gemcitabine (1000 mg/m2) q 3 wk | 106 | 35 (34%) | 38 (34%) | 21% |
| Kastaros | 2005 | PLD (30 mg/m2) q 3 wk, vinorelbine (30 mg/m2) q 3 wk | 32 | 13 (43%) | 8 (26.7%) | 25% |
| Skarlos | 2005 | PLD (25 mg/m2) day 1, gemcitabine (650 mg/m2) day 1 and 8, every 28 days | 37 | 8 (22%) | 2 (5.5) | 22% |
| Nicoletto | 2006 | PLD (30–35 mg/m2), oxaliplatin (70 mg/m2) q 4 wk | 41 | 22 (54%) | NR | 28.6% |
| Petru | 2006 | PLD (30 mg/m2) day 1, gemcitabine (650 mg/m2) day 1 and 8, every 28 days | 30 | 10 (33%) | NR | 10 (33%) |
| Verhaar-Langereis | 2006 | PLD (30 mg/m2), topotecan (1 mg/m2) q 3 wk | 27 | 7 (28%) | 11 (44%) | NR |
| Valerio | 2006 | PLD (30 mg/m2) oxaliplatin (85 mg/m2) cyclophosphamide (750 mg/m2) | 49 | 18 (46%) | 9 (23%) | 37% |
Abbreviations: NR, not reported; PLD, pegylated liposomal doxorubicin.
Most of the trials have focused on platinum-resistant ovarian cancer patients or a mixture of platinum-sensitive and platinum-resistant patients. D’Agostino et al (2003) presented one of the first Phase II trials involving pegylated liposomal doxorubicin and gemcitabine in combination for recurrent ovarian cancer. The authors treated 67 evaluable patients who had failed at least one platinum and paclitaxel chemotherapy regimen with 30 mg/m2 pegylated liposomal doxorubicin on day 1 and 1000 mg/m2 gemcitabine on day 1 and day 8 every 21 days. There were 7 complete responses (10.4%) and 16 partial responses (23.9%) as well as 26 cases (38.8%) classified as stable disease. Among the 36 platinum-resistant patients there were 9 (25%) responses (8 presented one partial and 1 complete). The hematological complications were considerable, with 42.8% (30 patients) with grade 3/4 complications, but only 1 patient required hospitalization and there were no treatment-related deaths. There were 7 cases (10%) of grade 3 PPE. Ferrandina et al (2005) reported another Phase II trial using the same doses of pegylated liposomal doxorubicin and gemcitabine in a very similar recurrent ovarian cancer patient population. Of 111 patients, 45 patients were considered platinum-sensitive and 66 were platinum-resistant. Among 106 evaluable patients there were 9 (8.5%) complete responses and 27 (25.5%) partial responses for an overall response rate of 34%. Another 36 (34%) experienced stable disease (median duration of 34 weeks). Among the platinum-resistant patients, there were 2 complete and 12 partial responses for an overall rate of 21.6%. Among the platinum-sensitive patients there were 7 complete and 15 partial responses for an overall rate of 53.7%. Toxicities of 642 administered cycles were reported. Grade 4 hematological toxicity, most commonly leucopenia, affected 20 patients (18%). A dose reduction was required in 26% of patients due to PPE or mucositis and 11 patients (10%) had their chemotherapy discontinued due to toxicities. Subsequently, there were 2 studies that used the same drugs, but at lower doses. Skarlos et al (2005) reported on 37 platinum-resistant patients who received pegylated liposomal doxorubicin, 25 mg/m2 on day 1 and gemcitabine 650 mg/m2 on days 1 and 8 every 28 days. There was an overall response rate of 22% with an additional 5.5% having stable disease. The median survival was 8.4 months. Again myelosuppression was the most common toxicity and was found in 35% of patients. Only 1 case of PPE and 2 cases of severe stomatitis were reported. Petru et al (2006) studied 31 patients with this combination utilizing pegylated liposomal doxorubicin 30mg/m2 on day 1, and gemcitabine 650 mg/m2 on days 1 and 8 every 28 days. They reported a 33% response rate and a median overall survival of 15.8 months.
Campos et al (2003) studied 40 patients who received pegylated liposomal doxorubicin 30 mg/m2 every 3 weeks with weekly paclitaxel at 70 mg/m2 for 18 weeks. The group as a whole had a median platinum-free interval of 7 months, and 10 were classified as platinum–taxane resistant. An overall 29% response rate was observed with 4 complete responses and 7 partial responses. The median time to progression was 15 weeks overall. However, the median time to progression among the responders was 7 months. Among the platinum-resistant patients there was 1 complete and 3 partial responses for an overall rate of 17%. This compares with a 54% (7/13) response rate among the platinum-sensitive patients. Sixteen (41%) experienced grade 3/4 neutropenia and 21 (54%) experienced grade 3/4 PPE. The PPE rate is higher than in many other reports, possibly suggesting that the combination may increase the rate of PPE.
Katsaros et al (2005) published the results of a Phase II Italian multicenter trial in which patients previously treated with taxanes and platinum were treated with pegylated liposomal doxorubicin 30 mg/m2 and vinorelbine 30 mg/m2 on day 1 every 3 weeks. In 10 patients the order of drug administration was alternated for 5 patients each, and pharmacokinetics were calculated. Thirty-two patients who had received a median of 2 previous chemotherapy regimes were enrolled. A median of 4 cycles (range 1–9) were administered. There were 2 (6%) cases of grade 3 or 4 PPE, 4 (12%) cases of grade 3 or 4 neutropenia, 1 case of repeated grade 3 mucositis, and 1 case of grade 3 cardiac toxicity. Thirty patients were evaluable for response. After 3 cycles, there were 13 (43%) with a partial response, 8 (26.7%) with stable disease, and 9 (30%) patients with progressive disease. After 6 cycles, there were 11 (36.7%) with responses – 10 partial and 1 complete, 3 (10%) with stable disease, and 16 cases of progressive disease (53.5%). The overall median survival was 9 months. There was a similar frequency of response in platinum-sensitive and platinum-resistant patients. The pharmacokinetics demonstrated that administering pegylated liposomal doxorubicin first resulted in a significantly larger AUC for both drugs (p=0.0079), without increased toxicity. This combination produced fewer side-effects than previously studied combinations, suggesting that further study might be warranted.
Recently, Nicoleto et al (2006) published a trial of pegylated liposomal doxorubicin, dosed between 30 and 35 mg/m2 with oxaliplatin 70 mg/m2 every 28 days. The overall response rate was 54%, a disease stabilization rate of 29% with a median survival of 22.5 months. When broken down by sensitivity, there was a response rate of 66.7% among the 29 platinum-sensitive patients and 28.6% in the 14 platinum-resistant patients. There were 5 (12%) grade 3 or 4 toxicities, and only 3 patients (7%) required dose reduction. Neutropenia was the most common cause of severe toxicity.
Verhaar-Langereis et al (2006) recently reported a trial of pegylated liposomal doxorubicin at 30 mg/m2 on day 1 followed by topotecan at 1 mg/m2 daily for 5 days on a 21-day cycle. Twenty-seven patients were enrolled of which 14 of which were platinum resistant. Twenty-eight percent of patients responded with another 44% classified as having stable disease. The median time to progression was 30+ weeks and the overall survival was 41+ weeks. The most common toxicity was myelosuppression with >65% experiencing a grade 3 or 4 toxicity. There was 1 case of grade 4 PPE.
While the response rates of these combinations in platinum-resistant ovarian cancer seem higher than reported with single-agent pegylated liposomal doxorubicin, a randomized trial is necessary to evaluate the efficacy and toxicity of this approach.
Conclusions
Pegylated liposomal doxorubicin is a relatively new addition to the armamentarium in the treatment of recurrent ovarian cancer. While platinum should be considered in any patient in whom the disease may be sensitive, eventually most patients progress on treatment. Pegylated liposomal doxorubicin should be tried in the patient with recurrent disease at some point in the disease course. When used as a single agent, the incidence of response is similar to that with other drugs, and varies depending on the platinum-free interval. However, in addition to the number of patients with disease response, approximately twice as many will have stable disease and benefit from therapy with pegylated liposomal doxorubicin. In the patient who is not obstructed and has a good quality of life, yet has measurable disease, disease stabilization may be an acceptable outcome. Cesano et al (1999) evaluated patients randomized to topotecan or paclitaxel as second-line therapy for ovarian cancer. Patients who achieved a partial response had a very similar survival to patients with stable disease. Gronlund et al (2004) studied 100 recurrent ovarian cancer patients treated with topotecan or paclitaxel and carboplatin. This study demonstrated a survival benefit in patients with stable disease compared with those with progressive disease. It appears that a dose of 40 mg/m2 given every 28 days is the optimal dose to balance efficacy and side-effects. It should be emphasized that most reports show a median time to response of 8 weeks. The clinician must counsel the patient not to be discouraged if tumor markers progress during the first 2–3 cycles.
The low rate of severe neutropenia makes pegylated liposomal doxorubicin an ideal drug in patients who have received multiple courses of chemotherapy and possibly, even radiation. The lack of bone marrow suppression by this drug makes it easier to administer to patients who have been heavily pretreated. However, Lorusso et al’s (2004) data actually seem to suggest that pegylated liposomal doxorubicin should be used earlier in the course of treatment to derive maximal benefit. There are two major avenues of ongoing research. The first is the continued use of this drug in combination with other drugs in recurrent disease. As discussed above there are some promising combinations that warrant further investigation. The second is in its incorporation into primary therapy in the form of triplet combinations or sequential doublets or maintenance therapy. Success in either of these areas may lead to an even more prominent role for this novel delivery system of doxorubicin.
References
- American Cancer Society. Who gets ovarian cancer? [online] 2006 Accessed 3 March 2006. URL: http://www.cancer.org/docroot/CRI/content/CRI_2_2_1X_How_many_women_get_ovarian_cancer_33.asp?sitearea=
- Androulakis N, Kouroussis C, Mavroudis D, et al. Phase I study of weekly paclitaxel and liposomal doxorubicin in patients with advanced solid tumours. Eur J Cancer. 2002;38:1992–7. doi: 10.1016/s0959-8049(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Arcuri C, Sorio R, Tognon G, et al. A phase II study of liposomal doxorubicin in recurrent epithelial ovarian carcinoma. Tumori. 2006;90:556–61. doi: 10.1177/030089160409000604. [DOI] [PubMed] [Google Scholar]
- Campos SM, Penson RT, Mays AR, et al. The clinical utility of liposomal doxorubicin in recurrent ovarian cancer. Gynecol Oncol. 2001;81:206–12. doi: 10.1006/gyno.2000.5980. [DOI] [PubMed] [Google Scholar]
- Campos SM, Matulonis UA, Penson RT, et al. Phase II study of liposomal doxorubicin and weekly paclitaxel for recurrent Mullerian tumors. Gynecol Oncol. 2003;90:610–18. doi: 10.1016/s0090-8258(03)00373-1. [DOI] [PubMed] [Google Scholar]
- Cattel L, Ceruti M, Dosio F. From conventional to stealth liposomes: a new frontier in cancer chemotherapy. Tumori. 2003;89:237–49. doi: 10.1177/030089160308900302. [DOI] [PubMed] [Google Scholar]
- Cesano A, Lane SR, Poulin R, Ross G, Fields SZ. Stabilization of disease as a useful predictor of survival following second-line chemotherapy in small cell lung cancer and ovarian cancer patients. Int J Oncol. 1999;15:1233–8. doi: 10.3892/ijo.15.6.1233. [DOI] [PubMed] [Google Scholar]
- Chou HH, Wang KL, Chen CA, et al. Pegylated liposomal doxorubicin (Lipo-Dox(R)) for platinum-resistant or refractory epithelial ovarian carcinoma: A Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol Oncol. 2005 doi: 10.1016/j.ygyno.2005.10.027. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Copeland LJ, Bookman M, Trimble E. Clinical trials of newer regimens for treating ovarian cancer: the rationale for Gynecologic Oncology Group Protocol GOG 182-ICON5. Gynecol Oncol. 2003;90:S1–7. doi: 10.1016/s0090-8258(03)00337-8. [DOI] [PubMed] [Google Scholar]
- D’Agostino G, Ferrandina G, Ludovisi M, et al. Phase II study of liposomal doxorubicin and gemcitabine in the salvage treatment of ovarian cancer. Br J Cancer. 2003;89:1180–4. doi: 10.1038/sj.bjc.6601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RD, Lin WM, King M, et al. Oral dexamethasone attenuates Doxil-induced palmar-plantar erythrodysesthesias in patients with recurrent gynecologic malignancies. Gynecol Oncol. 2004;94:320–4. doi: 10.1016/j.ygyno.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Fracasso PM, Blum KA, Ma MK, et al. Phase I study of pegylated liposomal doxorubicin and the multidrug-resistance modulator, valspodar. Br J Cancer. 2005;93:46–53. doi: 10.1038/sj.bjc.6602653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracasso PM, Blum KA, Tan BR, et al. Phase I study of pegylated liposomal doxorubicin and gemcitabine in patients with advanced malignancies. Cancer. 2002;95:2223–9. doi: 10.1002/cncr.10937. [DOI] [PubMed] [Google Scholar]
- Fracasso PM, Rodriguez LC, Herzog TJ, et al. Phase I dose and sequencing study of pegylated liposomal doxorubicin and docetaxel in patients with advanced malignancies. Cancer. 2003;98:610–7. doi: 10.1002/cncr.11547. [DOI] [PubMed] [Google Scholar]
- Ferrandina G, Paris I, Ludovisi M, et al. Gemcitabine and liposomal doxorubicin in the salvage treatment of ovarian cancer: updated results and long-term survival. Gynecol Oncol. 2005;98:267–73. doi: 10.1016/j.ygyno.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Ferrero JM, Wever B, Lepille D, et al. Carboplatin (PA) and pegylated liposomal doxorubicin (CA:PACA regimen) in patients with advanced ovarian cancer in late relapse (>6 months) (AOCLR): results of a GINECO phast II trial. Proc Am Soc Clin Oncol. 2004;23:454s. [Google Scholar]
- Garcia AA, Roman L, Muderspach L, et al. Phase I clinical trial of topotecan and pegylated liposomal doxorubicin. Cancer Invest. 2005;23:665–70. doi: 10.1080/07357900500359877. [DOI] [PubMed] [Google Scholar]
- Gibbs DD, Pyle L, Allen M, et al. A phase I dose-finding study of a combination of Pegylated liposomal doxorubicin (Doxil), carboplatin and paclitaxel in ovarian cancer. Br J Cancer. 2002;86:1379–84. doi: 10.1038/sj.bjc.6600250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves A, Braud AC, Viret F, et al. Phase I study of pegylated liposomal doxorubicin (Caelyx) in combination with carboplatin in patients with advanced solid tumors. Anticancer Res. 2003;23:3543–8. [PubMed] [Google Scholar]
- Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–22. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- Gordon AN, Granai CO, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093–100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- Gordon AN, Tonda M, Sun S, et al. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Gronlund B, Hogdall C, Christensen IJ, et al. Is stabilization of disease a useful indicator of survival in second-line treatment of ovarian carcinoma pre-treated with paclitaxel-Platinum? Gynecol Oncol. 2004;94:409–15. doi: 10.1016/j.ygyno.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Katsaros D, Oletti MV, Rigault de la Longrais IA, et al. Clinical and pharmacokinetic phase II study of pegylated liposomal doxorubicin and vinorelbine in heavily pretreated recurrent ovarian carcinoma. Ann Oncol. 2005;16:300–6. doi: 10.1093/annonc/mdi055. [DOI] [PubMed] [Google Scholar]
- Kim RJ, Peterson G, Kulp B, Zanotti KM, et al. Skin toxicity associated with pegylated liposomal doxorubicin (40 mg/m2) in the treatment of gynecologic cancers. Gynecol Oncol. 2005;97:374–8. doi: 10.1016/j.ygyno.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Lorusso D, Naldini A, Testa A, et al. Phase II study of pegylated liposomal doxorubicin in heavily pretreated epithelial ovarian cancer patients. May a new treatment schedule improve toxicity profile? Oncology. 2004;67:243–9. doi: 10.1159/000081324. [DOI] [PubMed] [Google Scholar]
- Lyass O, Hubert A, Gabizon AA. Phase I study of doxil-cisplatin combination chemotherapy in patients with advanced malignancies. Clin Cancer Res. 2001;7:3040–6. [PubMed] [Google Scholar]
- Markman M, Kennedy A, Webster K, et al. Phase 2 trial of liposomal doxorubicin (40 mg/m(2)) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol. 2000;78:369–72. doi: 10.1006/gyno.2000.5921. [DOI] [PubMed] [Google Scholar]
- Mirchandani D, Hochster H, Hamilton A, et al. Phase I study of combined pegylated liposomal doxorubicin with protracted daily topotecan for ovarian cancer. Clin Cancer Res. 2005;11:5912–9. doi: 10.1158/1078-0432.CCR-04-1240. [DOI] [PubMed] [Google Scholar]
- Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol. 1997;15:987–93. doi: 10.1200/JCO.1997.15.3.987. [DOI] [PubMed] [Google Scholar]
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2000;1:225–34. doi: 10.2165/00128071-200001040-00004. [DOI] [PubMed] [Google Scholar]
- Nicoletto MO, Falci C, Pianalto D, et al. Phase II study of pegylated liposomal doxorubicin and oxaliplatin in relapsed advanced ovarian cancer. Gynecol Oncol. 2006;100:318–23. doi: 10.1016/j.ygyno.2005.08.020. [DOI] [PubMed] [Google Scholar]
- O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- O’Byrne KJ, Bliss P, Graham JD, et al. A Phase III study of Doxil/Caylex versus paclitaxel in platinum treated taxane naive relapsed ovarian cancer. Proc Am Soc Clin Oncol. 2002;21:203a nr 808. [Google Scholar]
- Petru E, Angleitner-Boubenizek L, Reinthaller A, et al. Combined PEG liposomal doxorubicin and gemcitabine are active and have acceptable toxicity in patients with platinum-refractory and -resistant ovarian cancer after previous platinum-taxane therapy: A phase II Austrian AGO study. Gynecol Oncol. 2006 doi: 10.1016/j.ygyno.2005.12.017. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Potamianou A, Androulakis N, Papakotoulas P, et al. Sequential combination of paclitaxel-carboplatin and paclitaxel-liposomal doxorubicin as a first-line treatment in patients with ovarian cancer. A multicenter phase II trial. Oncology. 2005;69:348–53. doi: 10.1159/000089767. [DOI] [PubMed] [Google Scholar]
- Recchia F, De Filippis S, Saggio G, et al. Phase I study of liposomal doxorubicin and oxaliplatin as salvage chemotherapy in advanced ovarian cancer. Anticancer Drugs. 2003;14:633–8. doi: 10.1097/00001813-200309000-00008. [DOI] [PubMed] [Google Scholar]
- Rose PG. Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarain cancer. The Oncologist. 2004;10:205–14. doi: 10.1634/theoncologist.10-3-205. [DOI] [PubMed] [Google Scholar]
- Rose PG, Greer BE, Markman M, et al. A phase I study of paclitaxel, carboplatin, and liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: A Gynecologic Oncology Group study. Proc Am Soc Clin Oncol. 2000;19:387a. [Google Scholar]
- Rose PG, Maxson JH, Fusco N, et al. Liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a retrospective comparative study of single-agent dosages. Gynecol Oncol. 2001;82:323–8. doi: 10.1006/gyno.2001.6272. [DOI] [PubMed] [Google Scholar]
- Rose PG, Rodriguez M, Walker J, et al. A phase I trial of prolonged oral etoposide and liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2002;85:136–9. doi: 10.1006/gyno.2002.6584. [DOI] [PubMed] [Google Scholar]
- Ryan CW, Fleming GF, Janisch L, et al. A phase I study of liposomal doxorubicin (Doxil) with topotecan. Am J Clin Oncol. 2000;23:297–300. doi: 10.1097/00000421-200006000-00019. [DOI] [PubMed] [Google Scholar]
- Skarlos DV, Kalofonos HP, Fountzilas G, et al. Gemcitabine plus pegylated liposomal doxorubicin in patients with advanced epithelial ovarian cancer resistant/refractory to platinum and/or taxanes. A HeCOG phase II study. Anticancer Res. 2005;25:3103–8. [PubMed] [Google Scholar]
- Tambaro R, Greggi S, Iaffaioli RV, et al. An escalating dose finding study of liposomal doxorubicin and vinorelbine for the treatment of refractory or resistant epithelial ovarian cancer. Ann Oncol. 2003;14:1406–11. doi: 10.1093/annonc/mdg364. [DOI] [PubMed] [Google Scholar]
- Uziely B, Jeffers S, Isacson R, et al. Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies. J Clin Oncol. 1995;13:1777–85. doi: 10.1200/JCO.1995.13.7.1777. [DOI] [PubMed] [Google Scholar]
- Valerio MR, Tagliaferri P, Raspagliesi F, et al. A phase II study of pegylated liposomal doxorubicin oxaliplatin and cyclophosphamide as second-line treatment in relapsed ovarian carcinoma. Int J Gynecol Cancer. 2006;16(Suppl 1):79–85. doi: 10.1111/j.1525-1438.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- Verhaar-Langereis M, Karakus A, van Eijkeren M, et al. Phase II study of the combination of pegylated liposomal doxorubicin and topotecan in platinum-resistant ovarian cancer. Int J Gynecol Cancer. 2006;16:65–70. doi: 10.1111/j.1525-1438.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Vorobiof DA, Rapoport BL, Slabber CF, et al. Phase 2 study of combination therapy with liposomal doxorubicin and carboplatin in patients with relapsed, platinum sensitive ovarian cancer. Proc Am Soc Clin Oncol. 2004;23:471s. [Google Scholar]
- Waterhouse DN, Tardi PG, Mayer LD, et al. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf. 2001;24:903–20. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- Wilailak S, Linasmita V. A study of pegylated liposomal Doxorubicin in platinum-refractory epithelial ovarian cancer. Oncology. 2004;67:183–6. doi: 10.1159/000081315. [DOI] [PubMed] [Google Scholar]


