Abstract
Objective
To integrate: (1) the neuroanatomical model of affect regulation; (2) a functional model of affect regulation; and (3) the evolving picture of affect dysregulation as exemplified by bipolar disorder.
Methodology
A computerized search for articles on related topics was augmented by additional selected studies.
Results
Subdivision between the orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC) is defined by distinct cytoarchitecture, corticocortical and subcortical connectivity, and function. The hierarchical relationship between OFC and amygdala is not resolved. Positive and negative emotions appear to have differential effect on cognitive ability. It is possible that cognitive and affective stimuli activate DLPFC and OFC respectively in a see-saw like manner. This complementary activation may be out of sync in disease models.
Conclusion
It is critical to account for: (1) differential anatomy and corresponding functions of various parts of the prefrontal cortex as opposed to treating it as a single entity; (2) complexity of clinical presentation of bipolar disorder that involves affect dysregulation, cognitive erosion, and motoric disinhibition in functional imaging studies; and (3) the mutual influence of affect and cognition. Future studies focusing on pharmacological effects using functional magnetic neuroimaging techniques will inform us if the affective circuitry dysfunction is reversible, and if so, what are the predictors of response.
Keywords: bipolar disorder, child, affect, functional magnetic neuroimaging, neuroanatomy, mania, prefrontal cortex, amygdala
Introduction
Voluntary self-regulation of negative affect is essential to a healthy psyche. A chronic inability to regulate affect is commonly associated with major mood disorders like depression and bipolar disorder. Numerous behavioral and cognitive models implicate poor negative affect regulation as a major factor contributing to vulnerability in bipolar and unipolar disorders. Emotional affect regulation in healthy individuals facilitates goal-directed activities and helps individuals to recover from negative life events and stressors.
Investigating children and adults with a spectrum of mood disorders, in addition to normal subjects, may provide a valuable window into neurodevelopmental aspects of affect regulation (Davidson et al 2002). The fundamental areas associated with affect regulation are the prefrontal cortex (PFC) and the amygdala. However, there are many other unresolved pieces of the puzzle of affect regulation. The delineation of brain regions involved in the experience vs the control of emotional responses, specification of what areas within the PFC are involved in affective modulation, separating emotional and cognitive responses to stimuli and how they interact, and clarification of the different functional roles in the reciprocal connectivity of PFC and amygdala are some of the unresolved pieces of the puzzle in accounting for affect regulation.
Several brain structures are implicated in bipolar (Blumberg et al 1999; Yurgelun-Todd et al 2000) and unipolar disorders (Mayberg et al 1997; Drevets 2000; Davidson et al 2002). For example, overactivity of the PFC seen in pediatric bipolar disorder (PBD) (Adleman et al 2001) contrasts with underactivity of the PFC seen in adult bipolar disorder. Both show a corresponding increase in reactivity of the amygdala (Blumberg et al 1999; Yurgelun-Todd et al 2000). We are far from having a unified model for understanding pathophysiology of mood, its regulation, and its developmental maturation. Given the pendulum-like mood swings seen in bipolar disorder, this apparent age-related divergence in functional brain organization offers a window of opportunity to study affect dysregulation in a “disease model” meanwhile, studies of unaffected humans and nonhuman primates facilitate the study of normal affect regulation.
It is also imperative to understand the mutual influence of cognition and emotion in constructing models of affect regulation. Further, the ability to integrate functional and structural neuroanatomy is essential to interpreting results of imaging studies involving activation in multiple related areas of affective circuitry. This integration will also guide us to an understanding of the functional connectivity of these discrete regions. Therefore, the focus of this paper is to examine: (1) the neuroanatomical model of affect regulation; (2) a functional model of affect regulation; and (3) the evolving picture of affect dysregulation exemplified by bipolar disorder.
Neuroanatomical basis of affective circuitry and related cognitive circuitry
The frontal cortex in humans is an extensive area of the brain extending from the central sulcus to the frontal pole. The caudal portion of the frontal cortex includes primary motor and premotor areas. The rostral portion is referred to as the PFC, a term that is used to distinguish it from the general term of “frontal lobe” that also includes the caudal half (Barbas 2000). The PFC consists of the lateral (Brodmann’s areas 46, 9, and 10), medial (lateral parts of areas 11 and 13, and all of area 12), and orbital areas (medial parts of areas 11 and 13, and all of area 14) anterior to the premotor cortices. Each of these areas has its own characteristic structural and functional attributes. Distinction between the heterogeneous areas of the PFC is based upon their structural and functional characteristics. The commonly held view of the function of the PFC is that it plays a major organizational or executive role in cognitive processes and executive functions (Goldman-Rakic 1988; Fuster 1993; Petrides 1996). A major advance in the study of the prefrontal cortex was the discovery that the limbic system extended to the medial (Broca 1878; Papez 1937) and basal parts of the PFC (Yakovlev 1948; Nauta 1979). This essentially provides the anatomic basis for the PFC to serve as a center for integrating emotional and cognitive processes, which is dependent upon the interconnectedness of the limbic brain (medial and orbitofrontal) and the dorsal neocortex (dorsolateral PFC; DLPFC). There are also corticocortical connections between these three major prefrontal areas as well as other areas of the neocortex. In addition, there are corticosubcortical connections, which, in part, constitute affective circuitry. An illustrative presentation of major components of affective circuitry including corticocortical and corticosubcortical connectivity is shown in Figure 1.
Figure 1.
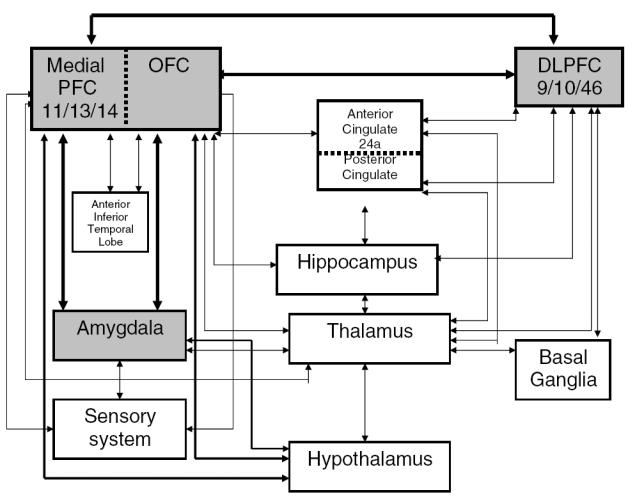
Affect regulation circuitry: relationship between cognition and emotion. Abbreviations: PFC, prefrontal cortex; OFC, orbitofrontal cortex; DLPFC, dorsolateral prefrontal cortex.
Subdivision between the PFC is defined by distinct: (1) cytoarchitecture; (2) corticocortical and subcortical connectivity; and (3) function.
Cytoarchitecture
There are three or four cellular layers that are continuous with the subcortical limbic area radially extending to the basoventral (orbitofrontal) and mediodorsal (medial) PFC. Layer 4 is typically poorly developed or absent in the prefrontal limbic or transitional cortex. These prefrontal limbic cortices issue widespread projections from their deep layers and reach eulaminate areas by terminating in superficial layers. The 6-layered eulaminate areas in the DLPFC communicate with limbic cortices from their upper layers and terminate in a columnar pattern (Barbas 1995). The limbic cortices are therefore distinct in their structural architecture and connectivity. The laminar architecture that is appropriately termed the “transitional cortex” is typical of limbic areas and is widespread in different areas of the brain. This may explain the plastic nature of the limbic cortex in learning and memory, as well as its vulnerability to pathology that contributes to neurologic and psychiatric disorders.
At the subcortical level, two cytoarchitectonic divisions are evident. These are most apparent in the thalamus. The larger cell bodies of the neurons are seen in the magno-cellular area, and smaller cell bodies are seen in the parvocellular regions. Magnocellular parts are seen in older regions of the thalamus and limbic cortex. Parvocellular regions are seen in areas that appeared more recently in phylogenetic development and are involved in more recently acquired cognitive functions such as those executed by the DLPFC. Reflecting these differences, the DLPFC receives projections from the mediodorsal nucleus. In contrast, the nuclear origin of projections to the orbitofrontal cortex (OFC) and medial limbic cortices is more diverse within the thalamus. Further, the amygdala is heavily connected to the OFC and medial PFC in that order, with very limited connectivity to the DLPFC.
Connectivity
A well delineated pattern of anatomic connectivity defines functional capabilities of the prefrontal cortices. The core model of affective circuitry is illustrated in Figure 1. The roles of the OFC and DLPFC along with their connections appear to be complementary and interdependent. As mentioned, OFC is further divided into medial and lateral divisions. Medial OF is concerned with internal control of mood and neurovegetative functions. Lateral OFC is believed to mediate empathic socially appropriate behavior and integrates external object-affect analysis. Another closely linked area that plays an important role in regulating motivational state is the anterior cingulate cortex. The OFC, DLPFC, and anterior cingulate have their individual parallel circuits, which involve the same structures including the frontal lobe, striatum, globus pallidus, substantia nigra, and thalamus. The anatomical segregation of each circuit supports the concept of circuit specific potential for the affective modulation of behavior.
Further, it is critical to understand the connectivity of PFC with posterior sensory and perceptual systems. This is crucial for executive and working memory functions so that affective state can influence higher cognitive abilities, and be influenced by them.
Sensory system connections of the PFC
DLPFC receives connections primarily from visual, auditory, and somatosensory cortices (Barbas 1995). In contrast, OFC receives robust projections from all the sensory modalities that include gustatory and olfactory cortices. Thus, there are intricate differences in the nature and function of each sensory modality connected to DLPFC and OFC.
The nature of visual input differs across the DLPFC and the OFC. Occipital and parietal cortices send spatial location information to the DLPFC. The DLPFC differs in its analysis and use of this visual information relative to the intraparietal visuomotor region in its role in making voluntary choices about focusing visual attention and holding visual information online over time for later action in working memory. In the case of the OFC, input is received from the inferior temporal cortices that specialize in processing input needed to recognize objects in the environment (Desimone and Gross 1979). The amygdala is connected to the OFC, and the connectivity and function of this network is concerned with determining the emotional significance of sensory stimuli and their representation in memory systems. The ability to appreciate emotional expressions on human faces is dependent on this circuitry – the amygdala in particular (Adolphs et al 1995). The medial OFC has reciprocal connections to the medial, magnocellular division of the amygdala, and with the cingulate, rostral insula, temporal pole, and entorhinal areas. This shared connectivity helps in conveying information about the visceral state of the organism to the OFC. In summary, the OFC receives input from the external (sensory system, directly and via amygdala) as well as internal (through amygdala) environment (Figure 1). It therefore has an extensive integrated capacity to capture the emotional significance of events.
Neuroanatomical basis of cognitive/behavioral functions including memory, motivation, attention, motoric activity, and emotional response
Memory
The DLPFC plays a crucial role in working memory, both in maintaining internal representations about the spatial location of visual targets, and also in manipulating them for planning adaptive behavior. The most significant connection of the DLPFC with the limbic cortices is with the posterior part of the cingulate cortex that is involved in attention and eye movements. The OFC, in contrast, has significant connections with anterior inferior temporal cortices and sensory information from rhinal cortices, and is associated with long-term memory (Zola-Morgan and Squire 1993). The OFC and the medial prefrontal area adjacent to it are well aligned with medial temporal structures involved in long-term memory, including visual memory. The OFC is also connected to hypothalamic autonomic centers. Therefore, the OFC is potentially influential in physiological responses to emotional reactivity. The medial PFC has even stronger connections to the hypothalamic autonomic centers and maintains a strong role in emotional communication. This is further underlined by the medial PFC connection to brainstem structures that are directly linked to laryngeal muscles in phonation (Vogt and Barbas 1988).
Anterior cingulate cortex (Brodmann’s area 24, aCC) and its subcortical circuitry mediate motivated behavior and are intricately related to executive function through its outflow to areas 9, 10, and 46. There is a functional subdivision within the anterior cingulate (Whalen et al 1998) as emotional tasks or manipulations activate the rostral anterior cingulate affective division which has extensive connections with the amygdala and orbitofrontal cortex. This is also termed the “pregenual” region of the anterior cingulate (Mayberg 1997) and is considered to be the affective regulatory area where pretreatment activity has been shown to predict treatment response in depression (Mayberg 1997). Cognitive tasks typically activate the dorsal anterior and posterior cingulate or “supragenual” cognitive division. This area has extensive connections to prefrontal, premotor, and supplementary motor areas and may be involved in attentional and motoric responses (Bush et al 1998).
Link to motor control systems: basal ganglia and thalamus
Basal ganglia are connected to the PFC via the thalamus to regulate motor behavior associated with functions of the DLPFC and limbic cortices. Mania has been observed in patients with lesions in the OFC, with caudate dysfunction in basal ganglia, and with lesions of the thalamus (Starkstein et al 1987; Jorge et al 1993).
Functional basis of affective responses on cognitive function
A number of studies with healthy individuals have now shown the importance of the reciprocal relationship between emotional and cognitive functions subserved by OFC and DLPFC using functional magnetic resonance imaging (fMRI) (Perlstein et al 2002). Strong positive emotions enhanced the task-related activation at DLPFC, while strong negative emotions activated OFC, with reduced responsivity of DLPFC. In other words, DLPFC appears to function efficiently with working memory task under the influence of desirable emotions but functions less efficiently during negative affective states, while OFC becomes more active during emotionally unpleasant situations. It is possible that activation of OFC/amygdala and DLPFC are typically inversely proportional in relation to emotional state, and together are responsible for the integration of emotion and cognition.
Functional studies of affect regulation
There is burgeoning literature attempting to delineate neural mechanisms of affect regulation using fMRI techniques to probe the relevant circuitry. This is adding to our knowledge base of brain areas involved in suppression, enhancement, and maintenance of emotions.
Levesque et al (2003) examined the voluntary ability to suppress negative emotion, ie, sadness. Healthy female subjects (n = 20) were shown neutral and sad excerpts of film. For example, sad excerpts depicted the death of a family member while neutral ones were an interview or carpentry. In case of the sad films, subjects were instructed to react normally to some stimuli and voluntarily suppress responses to other comparable stimuli. The scanning was followed by subjects completing a visual analogue scale (0–8) of intensity of sadness ever felt as well as that felt during the film. They also completed the strategy questionnaire, including the degree to which they were able to suppress. Their results have shown that transient sadness activated bilateral anterior temporal pole (imparting affective tone to subjective experience) and midbrain (autonomic nervous system (ANS) responses in the mesencephalic region), left amygdala and insula (recall induced sadness plus neural correlate of ANS responses), and right ventrolateral prefrontal cortex (VLPFC) (processing emotions). Positive correlation was seen between self-reported ratings of sadness and BOLD signal changes of right VLPFC (brain area (BA) A 47), left insula, and left aCC (BA 24/32; emotional detection/awareness). Suppression of sad emotion activated right DLPFC (BA 9 in all and 10 in some; explained as an objective observer sending commands to OFC to suppress) and OFC (BA 11). They conclude that there is a key role played by DLPFC and OFC on the right side in suppressing negative emotion. Limitations of this study are the use of nonstandardized film excerpts, but the creative study design brings the scientific examination of processes of affect regulation closer to real life context.
Conscious, voluntary suppression of negative emotion is associated with smaller startle eyeblinks and enhancement with larger startle eyeblinks (Jackson et al 2000). Given that the amygdala is purported to be a key structure for emotional perception and production (Davidson and Irwin 1999), Schaefer et al (2002) studied modulation of amygdala activity during conscious regulation of negative emotion. International Affective Pictures (CSEA-NIMH 1995) were used in 7 healthy women. Results indicated that negative stimuli activated the amygdala. They postulate that conscious activation/modulation was in part orchestrated by the amygdala. Their data, however, do not lead to determine directionality and control of affect modulation beyond the participatory role of the amygdala.
Hariri et al (2003) took a step further to examine the relationship between the PFC (VLPFC and DLPFC) and amygdala function. They looked at how cognitive evaluation of facial expressions can influence areas involved in emotion such as the amygdala. They also examined the influence of this network on peripheral ANS responses. Stimuli consisted of standardized threatening and fearful non-facial stimuli such as sharks and pointed guns selected from International Affective Picture System (IAPS). Simple geometric shapes served as control stimuli. Their results indicated that perceptual processing of IAPS stimuli (matching two out of three) was associated with bilateral amygdala response. Cognitive evaluation (labeling the scene as an “artificial” or “natural disaster”) of the same stimuli was associated with attenuation of amygdala response and an increase in response of the right PFC and anterior cingulate cortex (conscious evaluation and appraisal, and regulating emotion). This pattern is also reflected in skin conductance.
While the majority of the direct neural connections are between the OFC and amygdala, the more lateral PFC (ventral and dorsal PFC) is also connected to the amygdala via the OFC and thalamic and striatal circuits. The OFC is believed to be present in more “low level” reward or punishment values (O’Doherty et al 2001), while the more lateral PFCs have been implicated in the facilitation of more complex behavioral responses involved in evaluating reward and punishment values (Cools et al 2002). They suggest that modulation of emotional responses involves the interaction of the amygdala and the lateral PFCs. The ACC and VLPFC are both activated in labeling tasks. The ACC is well connected to both limbic cortex and PFC (Barbas and Pandya 1989). This is implicated in top-down regulation of the amygdala during cognitive evaluation. Amygdala response paralleled the responses of the ANS.
Functional MRI studies investigating the appraisal and evaluation of stimuli (Nakamura et al 1999; Narumoto et al 2000) or self regulation of emotional responses (Beauregard et al 2001) have implicated right VLPFC and ACC in emotional modulation. Right PFC is also involved in response inhibition (Garavan et al 1999; Konishi et al 1999) and set shifting in Wisconsin card sorting test (Konishi et al 1999). Probably this area is involved in behavioral inhibition along with emotional modulation. This is probably why children with PBD are impulsive and have a hard time transitioning, with poor emotional regulation.
Functional studies of affect dysregulation: bipolar disorder as a model
Based on the neuroanatomical knowledge presented above, functional studies in mood disorders are examining affect dysregulation as a primary focus of investigation. The data from adults with bipolar disorder indicate an important alteration in the functional linkage between the amygdala and the DLPFC, with reduction in activation of the DLPFC and increased amygdala activation in response to emotional provocation (Yurgelun-Todd et al 2000). Positron emission tomography (PET) and single photon emission tomography (SPECT) studies in adult bipolar disorder indicate increased temporal activity or decreased OFC activity (Blumberg et al 1999). Additionally, in adults with bipolar disorder, limited structural imaging data indicates increased amygdala volume (Altshuler et al 1998); functional imaging data also shows increased activity in the prefrontal cortex post treatment (Manji et al 1999). Exaggerated activity in the amygdala is believed to be reversed by treatment through an increased modulation from the OFC in depressed adults (Mayberg 1997; Drevets 2000), but whether there are similar effects in bipolar disorder is unknown. There are interesting leads from studies of adult bipolar patients indicating that increased amygdala reactivity is a fundamental aspect of the disorder, and that treatments may be effective by increasing prefrontal modulation of this reactivity. Given the continuity of cytoarchitecture between the amygdala and the OFC, it is difficult to develop a precise conceptualization of how this higher cortical center influences subcortical systems based on an analysis of anatomy alone.
Research and clinical implications: future direction
In summary, there appear to be several brain systems involved in affect regulation. Neuroanatomical studies offer a framework for understanding the anatomy and connectivity of brain areas that influence and modulate affective responses. Functional studies define the interconnections and sequence of events beyond identifying the structures involved. The central pathway is marked by the connectivity between the PFC and the amygdala. The relationship between cognition and emotion is multifold: at the DLPFC/OFC level, the amygdala and the OFC with the hippocampus and anterior cingulate gyrus, and interconnectivity across related circuitry also involving motoric functions represented by basal ganglia. Positive and negative experiences and emotional stimuli seem to have varying effect on cognition, and vice versa. The actual process of emotional regulation is also multifold involving recognition of faces/stimuli, interpretation of the emotions on faces/stimuli, and processing these emotions and interacting with the cognitive functions that are interrelated. Understanding the normal processes of affect regulation will contribute to our knowledge of areas and function affected in mood disorders, such as bipolar disorder, where severe mood swings are a hallmark. Understanding the pathophysiology of mood disorders, alterations resulting from medications, and what predicts the outcome are some of the pertinent questions that have yet to be addressed in future studies.
References
- Adleman N, Chang KD, Dienes K, et al. FMRI of emotional processing in pediatric bipolar disorder. Poster presented at the 48th annual meeting of the American Academy of Child and Adolescent Psychiatry; 2001 Oct 23–28; Honolulu, HI. 2001. [Google Scholar]
- Adolphs R, Tranel D, Demasio H, et al. Fear and the human amygdala. J Neurosci. 1995;15:5879–91. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Greider T, et al. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–4. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Barbas H. Pattern in the cortical distribution of prefrontally directed neurons with divergent axons in the rhesus monkey. Cereb Cortex. 1995;5:158–65. doi: 10.1093/cercor/5.2.158. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–30. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–75. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–8. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- Broca P. Anatomie comparee’ des enconvolutions cerebrales: Le grand lobe limbique et la scissure limbique dans la serie des mammiferes. Revue d’Anthropologie. 1878;1:385–498. [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, et al. The counting Stroop: an interference task specialized for functional neuroimaging – validation study with functional MRI. Hum Brain Mapp. 1998;6:270–82. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [CSEA-NIMH] Center for the Study of Emotion and Attention. The International Affective Picture Systemc (IAPS; photographic slides) Gainesville: The Center for Research in Psychophysiology, University of Florida; 1995. [Google Scholar]
- Cools R, Clark L, Owen AM, et al. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–7. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, et al. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178:363–80. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobes. Curr Opin Neurobiol. 1993;3:160–5. doi: 10.1016/0959-4388(93)90204-c. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Ann Rev Neurosci. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, et al. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–22. [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Starkstein SE, et al. Depression and anxiety following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 1993;5:369–74. doi: 10.1176/jnp.5.4.369. [DOI] [PubMed] [Google Scholar]
- Konishi S, Kawazu M, Uchida I, et al. Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex. 1999;9:745–53. doi: 10.1093/cercor/9.7.745. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biol Psychiatry. 1999;46:929–40. doi: 10.1016/s0006-3223(99)00165-1. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Ito K, et al. Activation of the right inferior frontal cortex during assessment of facial emotion. J Neurophysiol. 1999;82:1610–14. doi: 10.1152/jn.1999.82.3.1610. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Yamada H, Iidaka T, et al. Brain regions involved in verbal or non-verbal aspects of facial emotion recognition. Neuroreport. 2000;11:2571–6. doi: 10.1097/00001756-200008030-00044. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Expanding borders of the limbic system concept. In: Rasmussen T, Marino R, editors. Functional neurosurgery. New York: Raven Pr; 1979. pp. 1–23. [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch neurol psychiatry. 1937;38:725–43. [Google Scholar]
- Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. PNAS. 2002;99:1736–41. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc London B Biol Sci. 1996;351:1455–61. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, et al. Modulation of amygdalar activity by the conscious regulation of negative emotion. J Cogn Neurosci. 2002;14:913–21. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG, Price TR. Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain. 1987:1045–59. doi: 10.1093/brain/110.4.1045. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Barbas H. Structure and connections of the cingulate vocalization region in the rhesus monkey. In: Newman JD, editor. The physiological control of mammalian vocalization. New York: Plenum; 1988. pp. 203–25. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–18. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI. Motility, behavior and the brain: stereodynamic organization and neurocoordinates of behavior. J Nerv Ment Dis. 1948;107:313–35. doi: 10.1097/00005053-194810740-00001. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, et al. FMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–84. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Neuroanatomy of memory. Ann Rev Neurosci. 1993;16:547–63. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]


