Abstract
Both the blood stage protein apical membrane antigen 1 (AMA1) and the 25 kDa sexual stage protein (Pfs25) of Plasmodium falciparum are two leading candidates in malarial vaccine development. We have previously demonstrated that conjugation of these malarial antigens to recombinant Pseudomonas aeruginosa ExoProtein A (rEPA) significantly increased the mean specific functional antibody responses in mice; however, some mice responded poorly and were unable to demonstrate a functional response. We hypothesized that the immunogenicities of these two malarial antigens could be further enhanced by inclusion of a CpG oligodeoxynucleotide in the formulation. Mice were immunized with either rEPA conjugated or unconjugated AMA1 and Pfs25 formulated on Alhydrogel with or without the addition of CPG 7909. Mice received the formulations on days 0 and 28, and mouse sera were collected on day 42. ELISA analyses on these sera showed that the addition of CPG 7909 to AMA1-rEPA and Pfs25-rEPA formulated on Alhydrogel induced significantly higher mean antibody titers than the formulations without CPG 7909, and led to a mixed Th1/Th2 response as demonstrated by the production of mouse IgG1 and IgG2a subclasses. The presence of CPG 7909 in the formulations of both conjugated antigens greatly increased the proportion of responders with antibody titers sufficient to inhibit blood-stage parasite growth in vitro or block transmission of sexual stage parasites to mosquitoes. The results obtained in this study indicate the potential use of a combination strategy to increase the number of responders to malarial antigens in humans.
Keywords: Malaria, AMA1, Pfs25, Conjugation, CPG 7909, rEPA, vaccine
Introduction
The blood stage antigen AMA1 and the sexual stage antigen Pfs25 are two leading malarial vaccine candidates of Plasmodium falciparum [1, 2]. Enhancing the immunogenicities of these antigens to obtain a protective and sustained response in humans remains a formidable task that confronts malarial vaccine researchers today[3, 4]. One strategy that we have used to increase immunogenicity was to chemically conjugate malarial antigens to a carrier protein [5–9]. Conjugation of Pfs25 to outer-membrane protein complex of Neisseria meningitidis (OMPC) greatly increased and sustained the specific antibody levels in animals [5]. Similar results were observed with the conjugation of Pfs25 to itself or to Pseudomonas aeruginosa ExoProtein A (rEPA) [6]. Our study showed that conjugation of AMA1 and Pfs25 to rEPA significantly enhanced the immune response against both malarial antigens, with a 3-fold increase for AMA1-rEPA/Alhydrogel and over a 1000-fold increase for Pfs25-rEPA/Alhydrogel compared to unconjugated antigens [7]. Analyses by an in vitro parasite growth inhibition assay (GIA) for AMA1 and its conjugates or by a transmission blocking assay (TBA) for Pfs25 and its conjugates demonstrated that conjugation did not significantly affect the B cell epitopes, which are critical to the induction of functional antibodies that recognize parasites.
Although the conjugation of these antigens markedly increased the antibody titers in mice for both the AMA1 and Pfs25 antigens, some responders had antibody titers that remained below the levels required for high levels of functional activities against malaria parasites [7]. We therefore sought to increase the immunogenicities of both rEPA conjugates of AMA1 and Pfs25 so that the antibody titers of most responders, if not all, would be above those required to achieve high levels of functional activity against blood stage or sexual stage parasites.
In our previous study, the addition of the TLR9 agonist CPG 7909 to the AMA1-C1/Alhydrogel formulation greatly increased the functional antibody responses in mice, rats and guinea pigs, and a mixed Th1/Th2 response was observed [10]. A strong positive correlation between GIA activity and anti-AMA1 specific antibody levels was displayed. In addition, an adult phase I trial in the U.S. with AMA1-C1, a mixture of AMA1-FVO and AMA1-3D7 alleles formulated on Alhydrogel with or without the addition of CPG 7909, established that this formulation was safe and well-tolerated [1, 4].
In this report, we demonstrate that the addition of CPG 7909 to the AMA1-rEPA and Pfs25-rEPA conjugates formulated on Alhydrogel further increased the specific antibody responses in mice, and led to the majority of responders attaining antibody levels required to achieve sufficient functional activities against malarial parasites as measured by either GIA or TBA.
Materials and Methods
Malarial antigen-rEPA conjugates
AMA1-rEPA and Pfs25-rEPA conjugates were prepared as previously described [7]. Briefly, malarial antigens (AMA1-FVO or Pfs25 NF54) [11, 12] were thiolated by DL-N-acetylhomocysteine thiolactone (Sigma Aldrich Inc., St Louis, MO) and carrier protein (rEPA) was modified with maleimide groups using Sulfo-EMCS (Pierce Inc., Rockford, IL). Conjugates were formed by incubation of the thiolated malarial antigens with the maleimide derivatized rEPA. Unconjugated proteins were removed by size exclusion chromatography for AMA1-rEPA conjugate or by Ni-NTA plus size exclusion chromatography for Pfs25-rEPA conjugate. Three fractions from high to low molecular weight, AMA1-rEPA F1 (> ~ 190 kDa), AMA1-rEPA F2 (~ 130– 240 kDa) and AMA1-rEPA F3 (~ 130– 190 kDa), were obtained for AMA1-rEPA by pooling the appropriate fractions from the size exclusion chromatography, whereas two fractions of Pfs25-rEPA F1 (> ~ 100 kDa) and Pfs25-rEPA F2 (~ 90– 120 kDa) were obtained for Pfs25-rEPA.
Formulation and animal immunization
Vaccine proteins were formulated on 1600 μg/mL aluminum hydroxide gel (Alhydrogel®, Brenntag Biosector, Denmark) with or without the addition of 20 μg/dose CPG 7909 (Coley Pharmaceutical Group, Wellesley, MA). For the formulations with CPG 7909, the vaccine proteins were first formulated on Alhydrogel followed by the addition of CPG 7909 to the formulation [10, 13, 14]. Animal studies were conducted in compliance with the National Institutes of Health guidelines and with an Animal Care and Use Committee-approved protocol. These studies were carried out in BALB/c mice (Charles River Laboratories, Frederick, MD) with each group containing 10 mice. Vaccine formulations were administered intramuscularly on Days 0 and 28. On day 42, two weeks after the second immunization, the mice were bled and the sera were collected for serology. The AMA1 doses used for AMA1-rEPA/Alhydrogel with or without CPG 7909 were 0.01, 0.03 and 0.1 μg, whereas for Pfs25-rEPA/Alhydrogel with or without CPG 7909 the Pfs25 doses were 0.01, 0.05 and 0.25 μg. The antigen concentrations of AMA1 and Pfs25 within the conjugates were calculated based on the amino acid analysis of the conjugate. Comparison of antibody responses between the formulations with or without CPG 7909 was only performed with the 0.03 μg dose for the AMA1-rEPA conjugate, which is considered to be the middle of the dose response curve for AMA1/Alhydrogel, AMA1/Alhydrogel + CPG and AMA1-rEPA/Alhydrogel [7, 10]. For the Pfs25-rEPA conjugate, this comparison was evaluated at two doses, 0.05 and 0.25 μg, as the exact middle of the dose response curve was not known. Unconjugated antigens formulated with CpG were included in the 0.03 μg dose group for the AMA1 formulations and in the 0.05 and 0.25 μg dose groups for the Pfs25 formulations.
Enzyme-linked immunosorbent assay and IgG subclass analysis
ELISA was performed following the standardized protocol used in the laboratory [10]. Experimental serum samples and anti-AMA1-FVO or anti-Pfs25 mouse reference standards were used as primary antibodies; alkaline phosphatase-labeled goat anti-mouse IgG (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD) was used as secondary antibody. Bound antibodies were visualized by adding p-nitrophenyl phosphate substrate solution (Sigma Aldrich Inc., St Louis, MO) and absorbance was read at 405 nm. Serial dilutions of anti-AMA1-FVO or anti-Pfs25 mouse reference standard were included on each test plate to generate a standard curve, which was used to convert the absorbance of test sera into antibody units. One ELISA unit represents the reciprocal of the dilution required to attain an O.D.=1 in the standardized assay.
Analysis of antigen-specific IgG subclasses was performed with ELISA using goat anti-mouse IgG subclasses as secondary antibody. Mouse sera from each immunization group were pooled to obtain equivalent ELISA units from each individual serum. Alkaline phosphatase conjugated goat anti-mouse IgG1, IgG2a, IgG2b, IgG3 (Southern Biotech, Birmingham, AL) were normalized for their activity on mouse hybridomas (Sigma Aldrich Inc., St Louis, MO) before use in the analysis [10].
Growth inhibition assay
GIA was performed on total IgG purified from pooled immune sera [11, 15]. The mouse sera from each immunization group were pooled and the total IgG purified. The GIA was performed using human RBCs parasitized with late trophozoite and schizont stages of P. falciparum FVO strain parasites. Parasite growth was determined by a biochemical assay specific for parasite lactate dehydrogenase (LDH). Absorbance at 650 nm of the enzymatic product was read. Results were expressed as percent inhibition calculated as follows: 100 − [(A650 of infected RBCs with test IgG − A650 of normal RBCs only)/(A650 of infected RBCs without any IgG − A650 of normal RBCs only) × 100].
Transmission blocking assay
TBA was performed on pooled mouse immune sera [5, 16], which were mixed with in vitro cultured mature P. falciparum NF54 gametocytes. The sera-gametocyte mixture was fed to Anopheles stephensi (Nijmegen strain) mosquitoes through a membrane-feeding apparatus. These mosquitoes were maintained for 8 days prior to dissection and staining of their midguts to obtain oocyst counts. Inhibition of oocyst development by the test sera was calculated by comparing the oocyst numbers obtained with the test sera to those obtained with the naïve mouse sera at the same concentration. Results were expressed as percent oocyst reduction calculated as follows: 100 − [(oocyst number with tested sera/oocyst number with naïve mouse sera) × 100].
Statistical analysis
Effect of antigen dose on antibody response was tested by Spearman Rank Correlation for the data obtained from day 42 sera. A ρ value > 0 and significance of P value < 0.05 were required for a dose response. To test for a significant level of enhancement of average antibody titers among the groups of conjugated or unconjugated antigens formulated on Alhydrogel with or without the addition of CPG 7909, a Kruskal-Wallis One-Way ANOVA was performed; P values of < 0.025 were considered significant. If the Kruskal Wallis test was significant, then a post hoc analysis was performed with Student-Newman-Keuls comparison; P values of < 0.05 were considered significant.
Results
CPG 7909 enhances specific anti-AMA1 and anti-Pfs25 antibody responses
Immunization studies in mice were conducted to compare the immune responses induced by conjugated or unconjugated antigens formulated on Alhydrogel, with or without the addition of CPG 7909. AMA1-rEPA/Alhydrogel + CPG 7909 formulations were administered with AMA1 doses of 0.01, 0.03 and 0.1 μg, whereas Pfs25-rEPA/Alhydrogel + CPG 7909 formulations were administered with Pfs25 doses of 0.01, 0.05 and 0.25 μg. The immune serum obtained from each individual mouse was assayed by ELISA to determine the specific anti-AMA1 or anti-Pfs25 antibody titers. Significant dose responses were observed for all three conjugate fractions of the AMA1-rEPA formulated on Alhydrogel + CPG 7909 (AMA1-rEPA F1: ρ = 0.7028 and P < 0.0001; AMA1-rEPA F2: ρ = 0.8871 and P < 0.0001; AMA1-rEPA F3: ρ = 0.8018 and P < 0.0001), as well as for the two conjugate fractions of the Pfs25-rEPA/Alhydrogel + CPG 7909 (Pfs25-rEPA F1: ρ = 0.6509 and P < 0.0001; Pfs25-rEPA F2: ρ = 0.7264 and P < 0.0001).
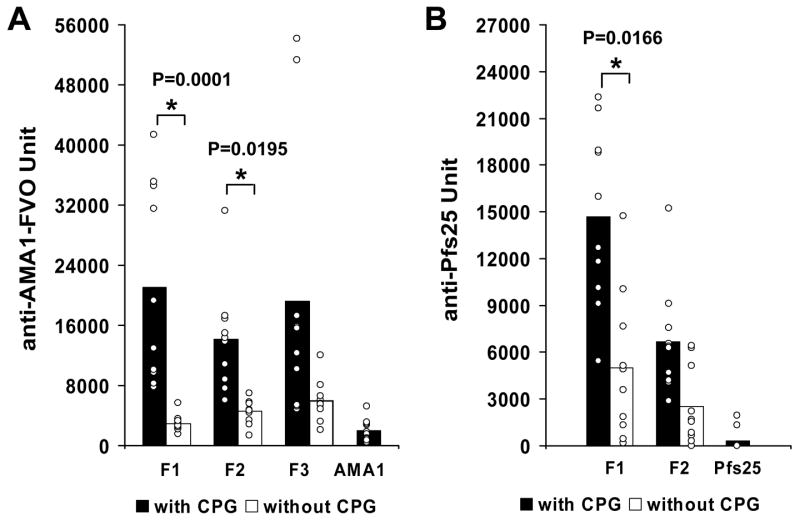
Comparisons of anti-AMA1 titers were performed for the 0.03 μg dose groups, comparing AMA1-rEPA/Alhydrogel with or without CPG 7909 and AMA1/Alhydrogel with CPG 7909. As shown in Fig 1A, higher antibody responses were elicited by each fraction of the AMA1-rEPA/Alhydrogel when CPG 7909 was added. The average antibody titer induced by AMA1-rEPA F1/Alhydrogel + CPG 7909 was 7-fold higher than that induced by AMA1-rEPA F1/Alhydrogel (Student-Newman-Keuls, P = 0.0001). A similar result was observed in the AMA1-rEPA F2/Alhydrogel + CPG 7909 group, inducing significantly 3-fold higher antibody titers than AMA1-rEPA F2/Alhydrogel (Student-Newman-Keuls, P = 0.0195). There was no significant difference between AMA1-rEPA F3/Alhydrogel with or without CPG 7909 (Student-Newman-Keuls, P = 0.0545). The addition of CPG 7909 to the unconjugated AMA1 at the 0.03 μg dose induced a relatively low mean antibody response (Fig 1A). Our previous mouse study showed that a higher dose of 0.1 μg was necessary for AMA1 to display a significant increase in antibody titer upon the addition of CPG 7909 [10].
Figure 1.
Comparison of antigen specific antibody responses in mice immunized with Alhydrogel formulations with or without the addition of CPG 7909. A. Mice were immunized with the AMA1-rEPA F1 (F1), AMA1-rEPA F2 (F2), AMA1-rEPA F3 (F3) or unconjugated AMA1 (AMA1) formulated on Alhydrogel with (black bars) or without (white bars) the addition of CPG 7909 at a dose of 0.03 μg of AMA1. B. Mice immunized with the Pfs25-rEPA F1 (F1), Pfs25-rEPA F2 (F2) and unconjugated Pfs25 (Pfs25) formulated on Alhydrogel with (black bars) or without (white bars) CPG 7909 at the dose of 0.25 μg of Pfs25. All ELISA results are presented as units compared to the anti-AMA1-FVO or Pfs25 mouse reference standards. Results are expressed as arithmetic mean of ELISA units of the group with each animal represented by an open circle. The asterisks indicate that the antigen specific antibodies are significantly higher for AMA1- or Pfs25-rEPA formulated on Alhydrogel with CPG 7909 than without CPG 7909.
The antibody titers against Pfs25 were compared at the dose levels of 0.25 and 0.05 μg. At the 0.25 μg dose level, as shown in Fig 1B, Pfs25-rEPA F1/Alhydrogel + CPG 7909 significantly increased the specific anti-Pfs25 antibody titer, resulting in a 3-fold difference compared to Pfs25-rEPA F1/Alhydrogel (Student-Newman-Keuls, P = 0.0166). However the difference in antibody titer between Pfs25-rEPA F2/Alhydrogel with and without CPG 7909 was not significant (Student-Newman-Keuls, P = 0.1517). Only 2 mice out of 10 in the 0.25 μg dose group of unconjugated Pfs25 developed detectable antibody responses, even when the antigen was formulated in the presence of CPG 7909 (Fig 1B). For Pfs25-rEPA F1/Alhydrogel with and without CPG 7909 at the 0.05 μg dose level, the immune enhancement of the CPG 7909 was significant with an average antibody titer of 7,399 and 1,422 respectively (Student-Newman-Keuls, P = 0.0478); while for Pfs25-rEPA F2/Alhydrogel with and without CPG 7909 the average antibody titer was 11,330 and 1,146 respectively (Student-Newman-Keuls, P = 0.0196). At the 0.05 μg dose level, no mice in the Pfs25/Alhydrogel + CPG 7909 group developed detectable antibody responses.
Alhydrogel + CPG 7909 formulation elicits antibody responses with multiple IgG subclasses
Individual mouse sera from the 0.03 μg dose groups of AMA1-rEPA/Alhydrogel with or without CPG 7909 or the 0.25 μg dose groups of Pfs25-rEPA/Alhydrogel with or without CPG 7909 were pooled, respectively. IgG subclass analysis was performed on each sample of the pooled sera to characterize the type of antibody responses elicited with and without CPG 7909. Consistent with the previous results observed [7], Alhydrogel formulations lacking the CpG 7909 immunostimulant predominately elicited an antibody response with a single IgG subclass in all relevant immunization groups (Fig 2). Almost all of the anti-AMA1 and anti-Pfs25 antibodies were of the IgG1 subclass with little or no IgG2a and IgG2b subclasses. When CPG 7909 was added to the Alhydrogel formulations, an antibody response with multiple IgG subclasses was induced, as determined by the increase in IgG2a as well as in IgG2b subclasses while the IgG1 subclass was maintained (Fig 2).
Figure 2.
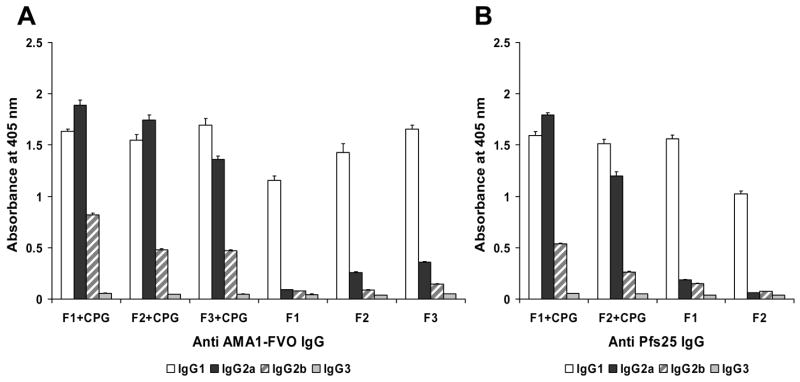
IgG subclass analysis of AMA1 and Pfs25-specific mouse antibodies. Mouse sera in each group were combined by pooling equivalent ELISA units of each individual serum sample and tested at 1: 5000 dilutions. Results are presented as arithmetic mean of OD value of the IgG subclasses plus standard deviation in four tested wells. A. IgG subclasses in mice immunized with AMA1-rEPA/Alhydrogel with and without CPG 7909 at a dose of 0.03 μg. B. IgG subclasses in mice immunized with Pfs25-rEPA/Alhydrogel with and without CPG 7909 at a dose of 0.25 μg. F1: conjugate fraction 1; F2: conjugation fraction 2; F3: conjugate fraction 3.
Alhydrogel + CPG 7909 formulation elicits functional antibodies against malaria parasites
In order to evaluate functional activities of the anti-AMA1 and anti-Pfs25 antibodies elicited by the addition of CPG 7909, GIA and TBA were performed. The mouse sera from groups receiving AMA1-rEPA F1/Alhydrogel with and without CPG 7909 at the 0.03 μg dose were each pooled. The total IgGs were purified from the pooled sera and tested in the GIA to evaluate their ability to inhibit malarial parasite invasion of human red blood cells. The GIA results demonstrated that IgGs elicited by AMA1-rEPA F1/Alhydrogel + CPG 7909 were functionally active, as 53% inhibition of in vitro parasite growth was displayed at an antibody titer of 6397 units (Table 1––1:10 dilution). This approximates a plateau value of inhibition by rodent anti-AMA1 IgGs against FVO parasites. For example, in previous studies it was observed that approximately 55% in vitro inhibition was attained when the antibody titers of the mouse derived IgGs to AMA1-FVO increased to 6000 units, which is close to the maximum level currently observed against the FVO strain of P. falciparum with rodent immunoglobulins (66% GIA activity at 7283 units given by AMA1-rEPA F2/Alhydrogel elicited antibodies) [7, 10]. To analyze the effect of CPG 7909 on minimizing the number of functional non- or low-responders, the antibody titer of 6000 units was chosen as an arbitrary demarcation line for comparing animal groups receiving antigen formulations with or without CPG 7909. Twenty-seven out of 30 mice (90%) in the three AMA1-rEPA/Alhydrogel + CPG 7909 groups (F1, F2 and F3 group) had antibody levels above this mark, whereas only 5 out of 30 mice (16.7%) in the three AMA1-rEPA/Alhydrogel groups had antibody levels above this mark.
Table 1.
In vitro growth inhibition assay with anti-AMA1 antibodies against P. falciparum FVO parasitesa
| AMA1-rEPA F1/Alhydrogel
|
AMA1-rEPA F1/Alhydrogel + CPG 7909
|
|||||
|---|---|---|---|---|---|---|
| Concentration in the Well (mg/ml) | 0.8 | 0.4 | 0.2 | 0.8 | 0.4 | 0.2 |
| ELISA Units in the Well | 4521 | 2260 | 1130 | 12794 | 6397 | 3198 |
| % Inhibition | 66%b | 52% | 19% | 58%b | 53% | 29% |
Mouse sera from the groups immunized with AMA1-rEPA F1/Alhydrogel with and without CPG 7909 at the dose of 0.03 μg were pooled, respectively. Total IgGs were purified from the pooled sera, adjusted to a concentration of 4 mg/ml of IgG and used in the standardized GIA, tested in three 2-fold serial dilutions.
This is approximately the plateau value for rodent anti-AMA1 IgGs against FVO parasites since the error in these determinations is ± 10%. Including the data of two previous studies, approximately 55% GIA activity against FVO strain of P. falciparum was achieved when the antibody titers of the mouse derived IgGs to AMA1-FVO was 6000 units, which was close to the maximal GIA level observed (66% GIA activity at 7283 units given by AMA1-rEPA F2/Alhydrogel elicited antibodies) [7, 10].
The mouse sera from the groups immunized with Pfs25-rEPA F1/Alhydrogel with and without CPG 7909 at the 0.25 μg dose were each pooled, and adjusted to equivalent ELISA titers. The TBA was performed on the pooled sera to evaluate transmission blocking activities. Both pooled sera exhibited almost the same functional activities, giving 98% and 99% oocyst reduction, respectively, when the antibody titers of both pooled sera were at 2600 units (Table 2). Further dilutions of the sera also showed that the two sets of antisera displayed comparable TBA. Based on the TBA results obtained from this and a previous study [7], a demarcation titer for Pfs25, corresponding to a greater than at least 90% reduction of oocysts, was determined to be 2600 units. At the 2600 units value chosen for comparison, every individual mouse (100%) in the two Pfs25-rEPA/Alhydrogel + CPG 7909 groups (F1 and F2 group) at the 0.25 μg dose level had antibody levels above this mark. In contrast, without the addition of CPG 7909, only 9 out of 20 mice (45%) in the two Pfs25-rEPA/Alhydrogel groups had antibody levels above this mark. At the 0.05 μg dose level, the values were 75% and 25%, respectively, for the groups receiving the formulations with and without CPG 7909.
Table 2.
Transmission blocking assay with anti-Pfs25 antibodiesa
| Pfs25-rEPA F1/Alhydrogel
|
Pfs25-rEPA F1/Alhydrogel + CPG 7909
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Sample Dilution | 1: 4.4 | 1: 8.8 | 1: 17.6 | 1: 35.2 | 1: 4.4 | 1: 8.8 | 1: 17.6 | 1: 35.2 |
| ELISA Units in the Feeder | 2600 | 1300 | 650 | 325 | 2600 | 1300 | 650 | 325 |
| % Oocyst Reduction | 99% | 95% | 83% | 53% | 98% | 90% | 80% | 68% |
Mouse sera from the groups immunized with Pfs25-rEPA F1/Alhydrogel with and without CPG 7909 at the 0.25 μg dose were pooled, respectively. The ELISA units of the pooled sera were measured and adjusted with naïve mouse sera to give the same initial values before the sera were tested in four 2-fold serial dilutions with naïve human sera in the assay.
Discussion
Both AMA1 and Pfs25 antigens of P. falciparum are considered leading malaria vaccine candidates directed to parasite blood stages or mosquito stages, respectively. Many studies have shown that the ability of these antigens to inhibit or block the development of malarial parasite is antibody dependent [15, 17–20]. Thus, the induction of immune responses with high antibody titers is thought to be critical for both candidates to be effective vaccines in humans. Unfortunately, Pfs25 is known to be a poor immunogen and AMA1, when formulated on Alhydrogel, failed to induce high levels of antibody responses in naïve volunteers for efficient GIA [3]. In order to enhance their immunogenicities, these two antigens were chemically conjugated to rEPA and a dramatic increase in the mean antibody titers was observed [7]. However, despite the titer enhancement observed in most animals, there were still animals that developed no or low responses to the vaccinations [7]. In this paper we have presented a strategy to further enhance the immunogenicity of the conjugates by addition of CPG 7909 to the formulation.
As a widely used adjuvant for various antigens, alum has been demonstrated to be safe in humans. However, it is a strongly Th2 biased adjuvant, mainly stimulating antibodies containing IgG1 as the predominant subclass in mice with little or no additional IgG subclasses. Unlike alum, CpG ODN has been shown to be a strongly Th1 biased immunostimulant, showing synergy with alum to promote a mixed Th1 and Th2 pathway. This balancing of antibody responses leads to an increase in total antibody titers [10, 21–24]. In this study, the comparison of antibody responses between Alhydrogel formulations with and without CPG 7909 was performed with the malarial antigen conjugated to rEPA. The ELISA results showed that the rEPA conjugated AMA1 and Pfs25 formulated on Alhydrogel + CPG 7909 elicited a significant increase in antibody levels as compared to the same formulation without the addition of CPG 7909. The analysis of IgG subclasses showed that both conjugates formulated on Alhydrogel + CPG 7909 elicited a mixed Th1 and Th2 type antibody response in mice, as both the IgG1 and IgG2a were the dominant IgG subclasses.
While the antibody titers were greatly increased for the groups receiving the conjugated antigens with the addition of CPG 7909, the antibody responses to the unconjugated antigens in the same formulation were still low, especially for the Pfs25. At the 0.25 μg dose, only two mice receiving Pfs25/Alhydrogel + CPG 7907 responded, with low antibody titers, whereas at the 0.05 μg dose no mice responded. This observation indicates that the effect of CPG 7909 on poor immunogens may be limited unless they are conjugated to a carrier.
To test whether the increased antibodies induced by the AMA1-rEPA and Pfs25-rEPA conjugates formulated on Alhydrogel + CPG 7909 were functional, the GIA and TBA were performed, respectively, on the purified IgGs or on the pooled sera. The GIA results showed that the antibodies elicited by the AMA1-rEPA F1/Alhydrogel + CPG 7909 and the AMA1-rEPA F1/Alhydrogel were both effective against parasites. The comparison of the GIA data obtained in this study with those obtained in our previous study [7] indicates that the difference observed with and without CPG is within the experimental error of the assay (~ ± 10%). The GIA activity of the antibodies elicited by the formulation with CPG 7909 fell within the same range as those of unconjugated AMA1 and AMA-rEPA conjugates formulated on Alhydrogel without CPG 7909 observed in a previous study [7]. However in this study, a trend towards higher GIA activities were displayed in the antibodies elicited by the formulation without CPG 7909 than in the antibodies elicited by the formulation with CPG 7909 or by the same formulation in a previous study [7] at the same ELISA units. The antibodies elicited by Pfs25-rEPA F1/Alhydrogel + CPG 7909 displayed almost the same activity in TBA as the antibodies elicited by Pfs25-rEPA F1/Alhydrogel. The TBA activities at 2600 units of antibody were also comparable to those previously published [6, 7]. These results demonstrated that the immune sera induced by the formulation with CPG 7909 were as functional as the immune sera induced by the formulation without CPG 7909 at the same ELISA units.
The addition of CPG 7909 into the conjugates not only significantly increased the overall antibody responses in animals, but also effectively minimized the number of non- or low-responders. It is desirable that the target antibody responses should be at a level sufficient to effectively inhibit the growth of either blood-stage or sexual stage parasites. Addition of CPG 7909 to the formulation significantly increased the proportion of animals (from 16.7% to 90% for the AMA1-rEPA conjugates, and from 45% to 100% for the Pfs25-rEPA conjugates) that developed antibody levels beyond what was required for high levels of GIA or TBA, demonstrating overall improvement in vaccine efficacy by the addition of CPG 7909.
In this study, the data obtained have demonstrated the value of the addition of CpG ODN to Alhydrogel formulations of conjugated malarial antigens, and have presented an encouraging prospect for this strategy to be used in humans to increase the number of responders to malarial antigens.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIAID. The GIA Reference Center is supported by the PATH Malaria Vaccine Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. Malaria vaccines: are we getting closer? Curr Opin Mol Ther. 2007;9(1):12–24. [PubMed] [Google Scholar]
- 2.Girard MP, Reed ZH, Friede M, Kieny MP. A review of human vaccine research and development: Malaria. Vaccine. 2007;25(9):1567–80. doi: 10.1016/j.vaccine.2006.09.074. [DOI] [PubMed] [Google Scholar]
- 3.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73(6):3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. doi: 10.1016/j.vaccine.2007.10.064. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci U S A. 2006;103(48):18243–8. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, et al. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci U S A. 2007;104(1):293–8. doi: 10.1073/pnas.0609885104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, et al. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007;25(20):3923–33. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Que JU, Cryz SJ, Jr, Ballou R, Furer E, Gross M, Young J, et al. Effect of carrier selection on immunogenicity of protein conjugate vaccines against Plasmodium falciparum circumsporozoites. Infect Immun. 1988;56(10):2645–9. doi: 10.1128/iai.56.10.2645-2649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanisic DI, Martin LB, Liu XQ, Jackson D, Cooper J, Good MF. Analysis of immunological nonresponsiveness to the 19-kilodalton fragment of merozoite surface Protein 1 of Plasmodium yoelii: rescue by chemical conjugation to diphtheria toxoid (DT) and enhancement of immunogenicity by prior DT vaccination. Infect Immun. 2003;71(10):5700–13. doi: 10.1128/IAI.71.10.5700-5713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullen GE, Giersing BK, Ajose-Popoola O, Davis HL, Kothe C, Zhou H, et al. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24(14):2497–505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70(12):6948–60. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai CW, Duggan PF, Shimp RL, Jr, Miller LH, Narum DL. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J Biotechnol. 2006;121(4):458–70. doi: 10.1016/j.jbiotec.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Aebig JA, Mullen GE, Dobrescu G, Rausch K, Lambert L, Ajose-Popoola O, et al. Formulation of vaccines containing CpG oligonucleotides and alum. J Immunol Methods. 2007;323(2):139–46. doi: 10.1016/j.jim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullen GE, Aebig JA, Dobrescu G, Rausch K, Lambert L, Long CA, et al. Enhanced antibody production in mice to the malaria antigen AMA1 by CPG 7909 requires physical association of CpG and antigen. Vaccine. 2007;25(29):5343–7. doi: 10.1016/j.vaccine.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Miura K, Zhou H, Muratova O, Keegan B, Miles A, et al. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect Immun. 2006;74(8):4573–80. doi: 10.1128/IAI.01679-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139(12):4213–7. [PubMed] [Google Scholar]
- 17.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69(5):3286–94. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaslow DC, Bathurst IC, Lensen T, Ponnudurai T, Barr PJ, Keister DB. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994;62(12):5576–80. doi: 10.1128/iai.62.12.5576-5580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura K, Zhou H, Muratova OV, Orcutt AC, Giersing B, Miller LH, et al. In immunization with Plasmodium falciparum apical membrane antigen 1, the specificity of antibodies depends on the species immunized. Infect Immun. 2007;75(12):5827–36. doi: 10.1128/IAI.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura K, Keister DB, Muratova OV, Sattabongkot J, Long CA, Saul A. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs25 is a direct and predictable function of antibody titer. Malar J. 2007;6:107. doi: 10.1186/1475-2875-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogg CN, Americo JL, Lustig S, Huggins JW, Smith SK, Damon I, et al. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007 doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Near KA, Stowers AW, Jankovic D, Kaslow DC. Improved immunogenicity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by the addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect Immun. 2002;70(2):692–701. doi: 10.1128/IAI.70.2.692-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugai T, Mori M, Nakazawa M, Ichino M, Naruto T, Kobayashi N, et al. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine. Vaccine. 2005;23(46–47):5450–6. doi: 10.1016/j.vaccine.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 24.Coban C, Ishii KJ, Stowers AW, Keister DB, Klinman DM, Kumar N. Effect of CpG oligodeoxynucleotides on the immunogenicity of Pfs25, a Plasmodium falciparum transmission-blocking vaccine antigen. Infect Immun. 2004;72(1):584–8. doi: 10.1128/IAI.72.1.584-588.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]