Abstract
Objective
Evaluate the effects of core structure and storage conditions on the mechanical properties of acid-resin modified composites and a control material by three-point bending and conversion measurements 15 min and 24 h after curing.
Methods
The monomers pyromellitic dimethacrylate (PMDM), biphenyldicarboxylic-acid dimethacrylate (BPDM), (isopropylidene-diphenoxy)bis(phthalic-acid) dimethacrylate (IPDM), oxydiphthalic-acid dimethacrylate (ODPDM), and Bis-GMA were mixed with triethyleneglycol dimethacrylate (TEGDMA) in a 40/60 molar ratio, and photo-activated. Composite bars (Barium-oxide-glass/resin = 3/1 mass ratio, (2 × 2 × 25) mm, n = 5) were light-cured for 1 min per side. Flexural strength (FS), elastic modulus (E), and work-of-fracture (WoF) were determined in three-point bending after 15 min (stored dry); and after 24 h under dry and wet storage conditions at 37 °C. Corresponding degrees of conversion (DC) were evaluated by Fourier transform infrared spectroscopy. Data was statistically analyzed (2-way analysis of variance, ANOVA, Holm-Sidak, p < 0.05).
Results
Post-curing significantly increased FS, E and DC in nearly all cases. WoF did not change, or even decreased with time. For all properties ANOVA found significant differences and interactions of time and material. Wet storage reduced the moduli and the other properties measured with the exception of FS and WoF of ODPDM; DC only decreased in BPDM and IPDM composites.
Significance
Differences in core structure resulted in significantly different physical properties of the composites studied with two phenyl rings connected by one ether linkage as in ODPDM having superior FS, WoF and DC especially after 24 h under wet conditions. As expected, post-curing significantly contributed to the final mechanical properties of the composites, while wet storage generally reduced the mechanical properties.
Keywords: flexure strength, elastic modulus, work of fracture, dental composites, adhesive composites
Introduction
Currently, there are three main classes of polymerizable restoratives available: (1) resin composites, (2) resin-modified glass ionomer cements, and (3) (polyacid-modified) composite resins, also known as compomers. In contrast to resin composites, groups 2 and 3 contain, in addition to polymerizable methacrylate or acrylate groups, carboxylic acid groups that are attached either to a backbone (group 2) or to the center core of the monomer (group 3). These acid groups are capable of forming strong hydrogen bonds, for example to collagen, and thus promote adhesion to tooth structure.
Restorative and preventive dentistry is in need of materials that provide more-durable bonding of resin composites to both acid-etched dentin and enamel surfaces [1]. Although several polymerizable aliphatic and aromatic acid monomers are in use for bonding to dentin, the effort has been simply reduced to developing different acid monomers and choosing from the best. There is little or no information about the influence of chemical structure and the modification of a given acid monomer on the bond strength to dentin.
Acidic monomers of the type used in (polyacid-modified) composite resins are a significant component of a number of primers and bonding agents. They usually contain an aromatic center core. Dimethacrylates with aromatic core structures have been reported to produce rigid polymers, while dimethacrylates with aliphatic structures produce flexible polymers [2, 3] . It has also been suggested that dimethacrylates with “hard” segments (aromatic groups) and “soft” segments (aliphatic groups) will result in polymers with increased toughness and improved wear resistance in dental composites [4, 5]. For example the widely used dental monomer Bis-GMA (reaction product(s) of the diglycidyl ether of bisphenol A and methacrylic acid, primarily 2,2-bis[4-(2-hydroxy-3-methacryloxyprop-1-oxy)phenyl]propane) contains bulky aromatic groups that make it a rigid molecule and is commonly mixed with the flexible monomer TEGDMA (triethyleneglycol dimethacrylate). The flexibility of TEGDMA is related to the ether linkages of the molecule, with only a slight hindrance to free rotation about the bonds [6, 7]. While the structure of the “kinked” bisphenol A core imparts stiffness and strength, it hampers the polymerization and results in a higher amount of residual double bonds [8] and potentially leachable monomer [9] than, for example, an aliphatic core structure as in a urethane dimethacrylate. Work by Hammesfahr [10] indicated differences from various acidic monomer structures in dentin bond strength and cohesive (compressive) strength of compomer-type composite resin formulations.
Here, a number of acidic monomers with various core structures have been synthesized and the double bond conversion (DC), flexural strength (FS), elastic modulus (E) and work of fracture (WoF) under different storage conditions has been investigated. It has been hypothesized that both rigidity and flexibility within the same polymerizable monomer can be achieved by having aromatic groups interspaced with ether linkages. As a result, an increase in the number of aromatic groups and ether linkages within the monomer was expected to result in polymers with enhanced strength and work of fracture (as an indication of polymer toughness).
Materials and methods
Pyromellitic dimethacrylate (PMDM), biphenyl-dimethacrylate (BPDM), oxydiphthalic-dimethacrylate (ODPDM), and (isopropylidene-diphenoxy)bis(phthalic) dimethacrylate (IPDM) monomers were synthesized at the Paffenbarger Research Center laboratories following a procedure as described previously except using 2-hydroxyethyl methacrylate (HEMA) instead of glycerol dimethacrylate [11]. Briefly, stoichiometric amounts of one mole of each dianhydride and two moles of HEMA were dissolved in anhydrous acetone (dried previously over CaSO4) containing a small amount of butylated hydroxytoluene as stabilizer. Ten percent of poly 4-vinyl pyridine (Reillex 402, Aldrich Chemical Co.), based on the masses of the reactants, was added as catalyst. The mixture was stirred and heated to 50 °C to 53 °C until all dianhydride had reacted. Infrared (IR) spectroscopy was used to monitor the disappearance of the split C=O absorption, characteristic of aromatic anhydrides, at about (1785 and 1850) cm−1. When essentially all of the anhydride had reacted, the monomers were filtered and the solvent was removed. Further purification was needed to reduce the amount of unreacted HEMA to less than 3 % by mass fraction. Monomers were dissolved in diethyl ether and then transferred to a 500 mL separatory funnel. They were washed with 100 mL distilled water three times. The unreacted HEMA present in the aqueous phase was separated from the organic phase, and the diethyl ether solvent was then removed under vacuum.
The acid number of BPDM, IPDM, ODPDM and PMDM monomers was determined by acid-base titration (n ≥ 3). 50 mg to 60 mg of each acidic monomer, 10 mL of distilled water and 3 drops of bromothymol blue indicator (pH = 6.0 to 7.6) were transferred to a small beaker. The acid monomer was titrated against a 0.025 mol/L (N) potassium hydroxide (KOH) standard solution under constant stirring. The acid number was determined using the following equation:
| (1) |
where VKOH is the volume (mL) of 0.025 mol/L KOH standard solution used, CKOH is the KOH standard solution concentration (mol/L), and A is the amount (mmol) of the acidic monomer.
The monomers 2,2-bis[4-(2-hydroxy-3-methacryloxyprop-1-oxy)phenyl]propane (Bis-GMA) and triethyleneglycol dimethacrylate (TEGDMA) (Esstech, Essington, PA), the photo- and coinitiators camphorquinone (CQ) and ethyl 4-dimethylaminobenzoate (4-EDMAB), inhibitor BHT (2,6-di-tert-butyl-4-methylphenol, Aldrich Chemical Co., Milwaukee, WI) and solvents acetone (J.T. Baker, Phillipsburg, NJ) and diethyl ether (Sigma-Aldrich, St. Louis, MO) were used as received.
TEGDMA was mixed with Bis-GMA, BPDM, IPDM, ODPDM and PMDM in a 60-to-40 molar ratio. The resins were photo-activated with fixed concentrations of the initiators by incorporating a mole fraction of 0.6 % CQ and a mole fraction of 2.0 % 4-EDMAB. Resin composites were made by mixing silanated, milled, barium oxide containing glass filler (Dentsply Caulk, Milford, DE) with the monomer mixture at a 3-to-1 mass ratio.
Flexural strength specimens, 25 mm × 2 mm × 2 mm, were made by filling metal molds, clamping them between Mylar and glass slides and light curing them for 1 min per side using three side-by-side aligned curing units (Dentsply, York, PA). The edges were lightly polished. Three sets of each composite were prepared to conduct the following treatments: the first set was tested 15 min after the photo-curing at room temperature; the second set was stored dry in an oven at 37 °C for 24 h and then tested at room temperature; the third set was immersed in distilled water at 37 °C in a humidity chamber for 24 h and then tested wet at room temperature. Specimens (n = 5) were tested in three-point bending at a crosshead speed of 0.5 mm/min in a universal testing machine (Instron Corp., Canton, MA). The span length was 20 mm. The flexural strength (FS), elastic modulus (E) and work-of-fracture (WoF) were evaluated. The composite flexural strength was calculated by [12]
| (2) |
where Pmax is the maximum load on the load-displacement curve, L is flexure span, b is specimen width, and h is specimen thickness. Elastic modulus was calculated by
| (3) |
where load P divided by the corresponding displacement d is the slope of the load-displacement curve in the linear elastic region before the curve deviates from linearity.[12,13]
Work-of-fracture was calculated as a measure of toughness defined by the total area under the stress strain curve by [12]
| (4) |
where A is the area under the load-displacement curve, which is the work done by the applied load to deflect and fracture the specimen. With the unit of A being J (joules), the unit of WoF (or fracture resistance) is J/m2 or more conveniently, kJ/m2 (k = 103). [12,13]
Fourier transform infrared spectra (Nicolet Magna 550, Nicolet Inc., Madison, WI) in the near infrared (NIR) region were collected with 264 scans at 4 cm−1 resolution to measure bulk polymer cure (C=CH2 peak at 6164 cm−1) [14] using an area baseline technique with peak areas being normalized to the polymer thickness. The degree of conversion (DC) at 15 min or 24 h after curing was determined with respect to the uncured resin composite using the following equation:
| (5) |
where A0 is the peak area of the uncured composite, At is the peak area of the composite at 15 min or 24 h. Data was statistically analyzed by two-way analysis of variance (ANOVA) and an all pairwise multiple comparison procedure (Holm-Sidak, p < 0.05).
The standard deviations in tables and figures are given as a measure of standard uncertainty.
Results
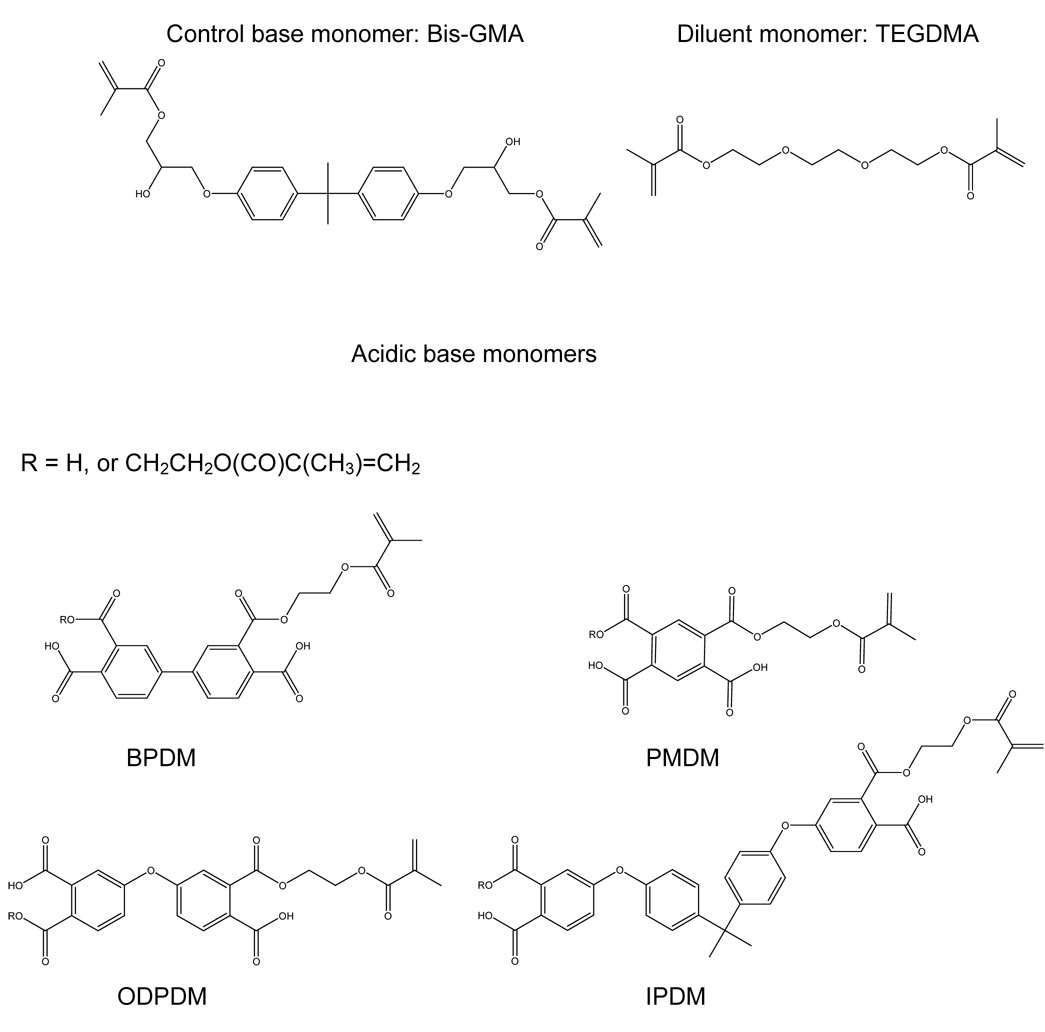
The acid numbers of the synthesized acid monomers (Table 1) varied from 2.13 (ODPDM) to 2.34 (BPDM). If the acid monomers were pure dimethacrylates they would contain two carboxylic acid groups (Figure 1) yielding an acid number of 2.0. On the other hand, pure monomethacrylates shown as an example for PMDM contain three carboxylic acid groups, which would result in an acid number of 3.0. Therefore, the acid monomers were mostly dimethacrylates.
Table 1.
Acid numbers (n ≥ 3) and standard deviations (in parentheses) of acidic base monomers and physical appearance of resin mixtures. Different superscripts indicate significant differences (One-way ANOVA, Sidak-Holmes, p < 0.05).
| Bis-GMA | BPDM | IPDM | ODPDM | PMDM | |
|---|---|---|---|---|---|
| Acid numbers | --- | 2.34 (0.04)d | 2.21 (0.02)b | 2.13 (0.01)a | 2.26 (0.02)c |
| Appearance of resin mixtures | clear, homogeneous | opaque, no phase separation | clear, homogeneous | clear, homogeneous | opaque, phase separation |
Figure 1.
Structures of Bis-GMA (acronyms see Introduction) and triethylene glycol dimethacrylate (TEGDMA) and of the acidic base monomers pyromellitic dimethacrylate (PMDM), biphenyl-dimethacrylate (BPDM), oxydiphthalic-dimethacrylate (ODPDM), and (isopropylidene-diphenoxy)bis(phthalic) dimethacrylate (IPDM). Other isomers with respect to the position of the acid groups, i.e., whether they are attached in meta or para position, are also possible.
Bis-GMA (control), IPDM and ODPDM monomers formed clear and homogeneous resin solutions when TEGDMA (diluent) was added (Table 1). BPDM monomer formed an opaque solution, though no phase separation was observed. PMDM showed poor miscibility with TEGDMA. Since the phase separation may have affected the physical properties, the PMDM composite was excluded from the overall statistical analysis. Additional reasons for eliminating PMDM from the general statistical analyses are presented in the discussion. The data for the PMDM composite are shown in Table 2.
Table 2.
Flexural strength, modulus, work of fracture, degree of conversion and standard deviations (in parentheses) of PMDM composites stored dry for 15 min or 24 h.
| Flexure Strength (MPa) | Elastic Modulus (GPa) | Work of Fracture (kJ/m2) | Degree of Conversion (%) | ||||
|---|---|---|---|---|---|---|---|
| 15 min | 24 h | 15 min | 24 h | 15 min | 24 h | 15 min | 24 h |
| 70 (5) | 84 (6) | 7.5 (0.8) | 11.9 (0.7) | 0.9 (0.1) | 0.7 (0.1) | 75 (3) | 83 (2) |
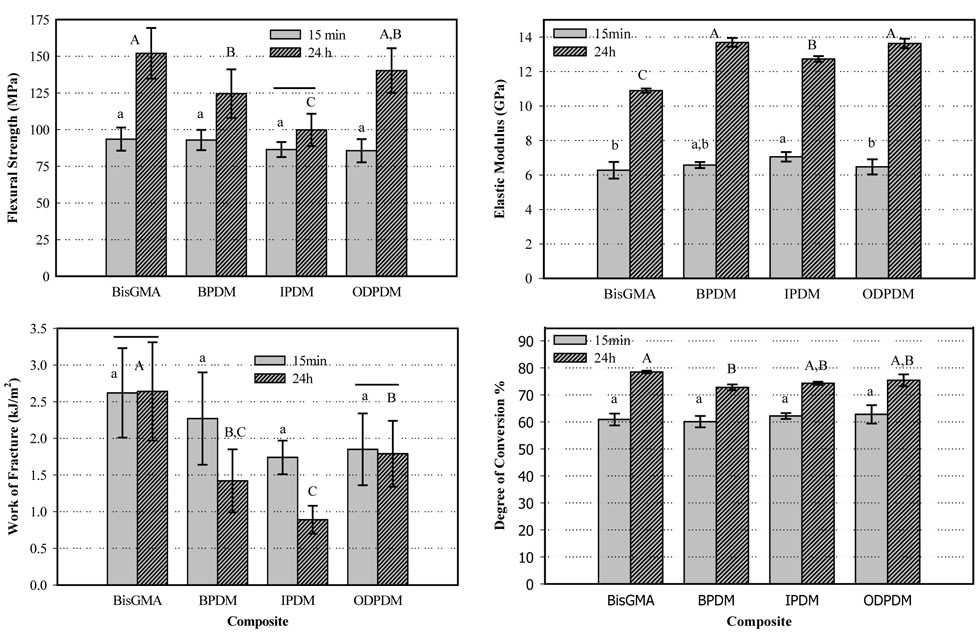
Two-way ANOVA (Holm-Sidak, p < 0.05) revealed that time and type of monomer had significant effects on all measured composite properties (FS, E, DC and WOF). Interactions between time and type of monomer were also found to be significant for all properties evaluated. In contrast to the 24 h data under dry conditions, at 15 min the properties with the exception of E were not significantly different (Figures 2). Significant chemical post-curing was observed for all composites within the first 24 h with DC increasing by about 12 % to 18 %. Accordingly, FS and E increased significantly for nearly all composites.
Figure 2.
Flexural strength, modulus, work of fracture and degree of conversion of composites stored dry for 15 min or 24 h. The horizontal bars indicate that no statistical differences exist between the 15 min and 24 h data. Lower case letters compare the 15 min data for a given property, upper case compare the 24 h data. (2-way ANOVA, Holm Sidak, p < 0.05). The vertical bars represent the standard deviations.
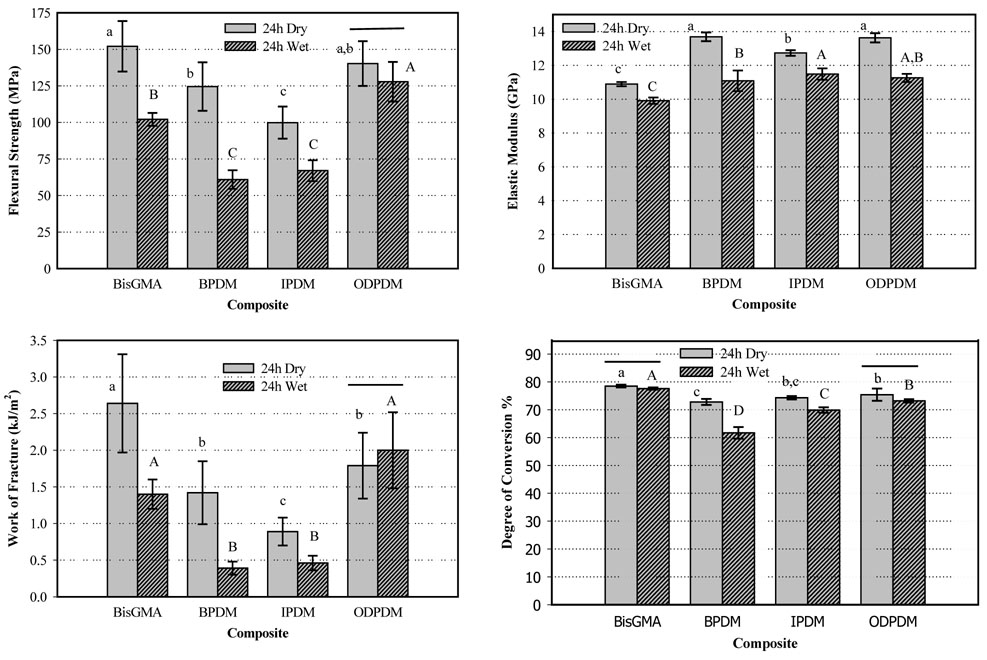
Also, 2-way ANOVA (Holm-Sidak, p<0.05) found that type of post-curing storage (dry or wet) and type of monomer had significant effects on all the composite properties (Figure 3). Interactions between type of post-curing treatment and type of monomer were significant for all the properties evaluated. Under wet conditions 24 h DC was similar to dry 24 h DC for Bis-GMA and ODPDM resin composites, but was significantly lower for IPDM and BPDM composites. 24 h water immersion also significantly reduced the FS and WoF for all but ODPDM composites, and lowered the moduli for all four composite resins.
Figure 3.
Flexural strength, modulus, work of fracture and degree of conversion of composites stored dry or wet for 24 h. The horizontal bars indicate that no statistical differences exist between the wet and dry data. Lower case letters compare the dry data of a given property, upper case compare the wet data (2-way ANOVA, Holm Sidak, p < 0.05). The vertical bars represent the standard deviations.
Discussion
This study was undertaken to systematically evaluate modifications in monomer core structures of acidic monomers used in a conventional manner combined with hybrid glass fillers to form acidic-resin modified composite. It is suggested that both rigidity and flexibility within the same polymerizable monomer can be achieved by having aromatic groups interspaced with ether linkages. All of the experimental monomers studied contained one or more aromatic rings, two or three carboxylic acid groups per molecule and polymerizable double bonds. When more than one ring was present in the structure the rings were connected by a straight bond, an ether linkage or an isopropylidene centric core structure. An increase in the number of aromatic rings and ether linkages within the monomer was then expected to increase strength and possibly the toughness measured by determining the work of fracture.
PMDM was originally part of the series representing an acidic monomer with only one phenyl ring as the center core as opposed to two and four phenyl rings in the other monomers. However, when it turned out that the PMDM acidic monomer did not form a homogeneous mixture with TEGDMA, we conducted all but the immersion experiments, but eliminated the results from the general data analysis. PMDM had nominally the highest degree of conversion after 15 min and 24 h when stored dry, yet FS and WoF were considerably lower than for the other composites. It is suggested that due to phase separation, TEGDMA and possibly a small fraction of PMDM cured predominantly to a fairly high degree of conversion leaving some uncured PMDM behind. Moreover, it is hypothesized that TEGDMA underwent cyclization forming microgel regions within the forming polymer. High cyclization and microgel formation has been reported for TEGDMA [15] and has been suggested as the reason for certain curing characteristics of TEGDMA-rich formulation studied by differential scanning calorimetry [8]. If TEGDMA formed predominantly primary cycles within the TEGDMA-rich phase after phase separation, the PMDM/TEGDMA resin could have relatively high conversion of double bonds but low FS or WoF, since primary cycles of poly(TEGDMA) would reduce the number of unreacted double bonds but would not contribute as much to the mechanical strength as crosslinked TEGDMA. The fact that PMDM/TEGDMA composites exhibited still relatively high moduli in comparison to the other composites may be attributed to the core structure, with one phenyl ring in PMDM conveying a tighter and more brittle network compared to the other acidic resins.
The results of the other four monomers studied, which showed significant differences for type of monomer, time after curing and immersion conditions, need to be considered in view of a number of parameters. Firstly, the acid number was not consistent for all three synthesized monomers; thus the average number of acid groups could have an effect on the results. As can be seen from Table 2, BPDM had the highest acid number and ODPDM the lowest. Under dry conditions, there were no significant differences between these two composite resins. Under wet conditions moisture could absorb more readily into the higher-acidic BPDM composite and could then contribute to the significant differences observed for FS and WoF between BPDM and ODPDM composites. The moisture effect and dependence on the acid number is further supported by the wet data for the IPDM composite, which also dropped significantly compared to the dry data, but less so than the wet BPDM composite. However, as the degree of conversion was lower for both IPDM and BPDM composites when stored wet, the reduction of FS and WoF under wet conditions could also have resulted from lower conversion.
The conversion of double bonds under dry conditions increased significantly over time with little statistical differences between the four composites. The phenomenon of “dark” post cure is in agreement with the findings of other authors [16,17], but is in contrast to conversion measurements performed by Raman spectroscopy, for which little increase in conversion within 24 h was reported [18,19]. Under wet conditions, the lower conversion in BPDM and IPDM composites is attributed to water absorbed in the composites, which may have poisoned the post-polymerization. Since IPDM and BPDM have higher acid numbers and thus higher potential for hydrogen bonding, more water may have been absorbed faster into these composites opposed to Bis-GMA and ODPDM, which showed similarly high conversion under dry or wet conditions.
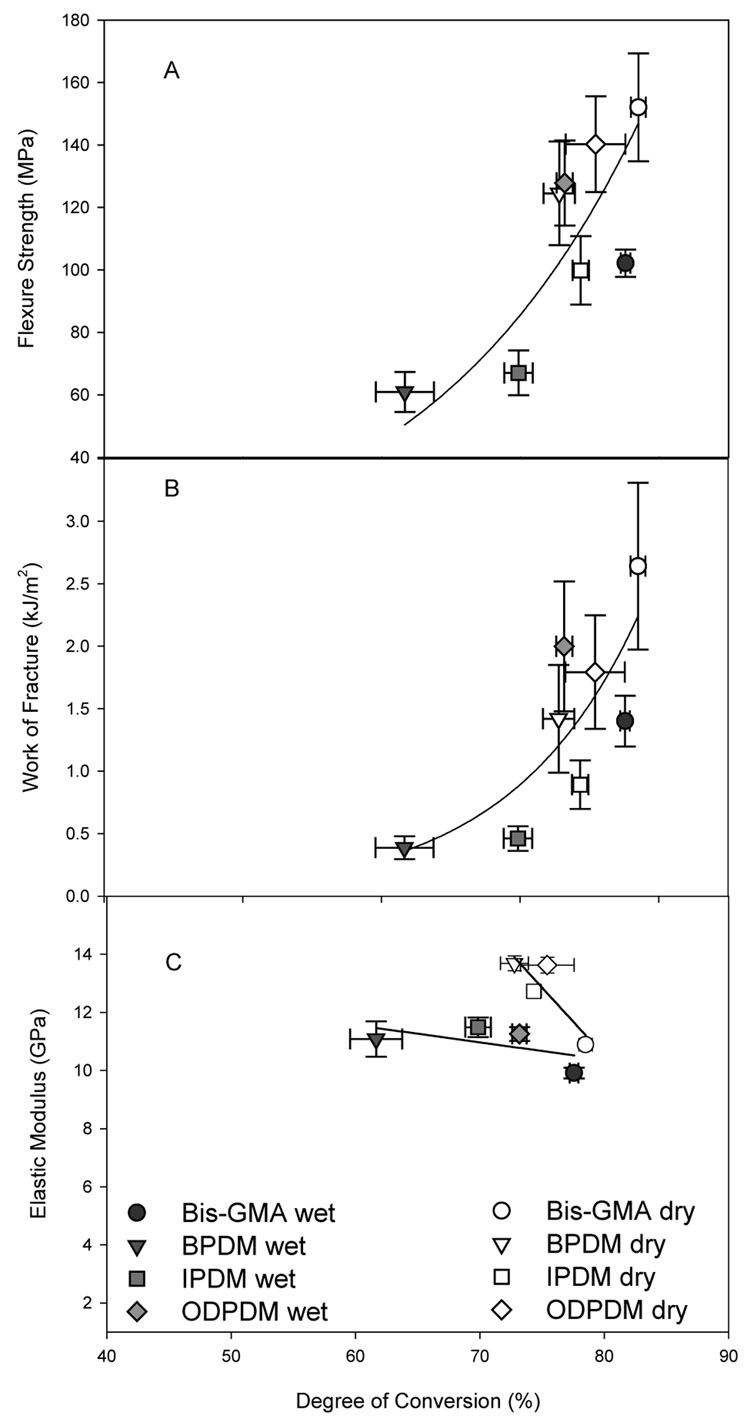
As expected, the conversion influenced the physical properties. Considering all 24 h wet and dry data, there is a positive correlation between conversion and FS and conversion and WoF. The degree of conversion is correlated with r = 0.75 to FS through the function of y = 2.84 e0.049x and to WoF with r = 0.78 through the function y = 0.0005 e0.104x. Firstly, this is somewhat surprising considering the differences in base monomer structure and storage conditions. This apparent correlation could suggest that the degree of conversion overrides the effects of monomer structure on FS. However, as depicted in Figure 4 the distinct base monomer distributions shown for both WoF and FS as a function of conversion indicate that different base monomer structures result in different conversions. Also shown in Figure 4 (C) are the elastic moduli as a function of conversion. Here we see a distinct difference between wet and dry data illustrating the strong effect of water as a plasticizer. Generally, under both wet and dry conditions acidic base monomers appear to have higher moduli than the neutral Bis-GMA base resin, but lower conversion (Figure 4). The lower conversion of the acidic monomers are most likely due to increased viscosities because of hydrogen bonding and dimerization, which then lead to lower polymerization rates and earlier network formation resulting in lower conversion as the gel forms earlier and reduces mobility of the monomer molecules [20]. Studies of polymerization kinetics of acidic monomers concluded that hydrogen bonding remains high throughout the polymerization process and influences the overall conversion rate [21]. Once water is in the polymer network the water molecules will hydrogen bond to the carboxylic acid groups, loosening the tighter carboxylic acid-carboxylic acid interactions.
Figure 4.
Dry and wet flexure strength (A) work of fracture (B), and elastic moduli (C) after 24 h as functions of the degree of conversion of Bis-GMA. The horizontal and vertical bars represent the standard deviations of degree of conversion and flexure strength (work of fracture), respectively. The standard errors of the estimate are: FS = 21.9 MPa; WoF = 0.52 kJ/m2; E dry = 0.71 GPa; E wet = 0.80 GPa.
As detailed above, and shown in Figure 4, the structure of the center core led to distinct group separation for the properties studied. Comparing BPDM and ODPDM composites, the higher flexibility of the ether linkage in ODPDM produced higher conversion, FS and WoF after 24 h, especially under wet conditions. In contrast to the conversion, the moduli of the three acidic-resin composites were not very different, which seems to indicate that the filler plays a greater role in the development of moduli, while the conversion may more strongly affect the WoF. Concurrently, use of IPDM resulted in a fairly high-modulus composite especially under wet conditions, but led to low FS and WoF, which was probably effected by its lower degree of conversion. Moreover, BPDM with even lower conversion also had a lower modulus when stored wet. However, in both cases an effect of the base monomer acidity cannot be completely excluded.
In summary, use of the acidic-resin monomers led to significant differences in the degree of conversion, which, in turn affected most strongly the work of fracture and flexural strength with ODPDM having significantly better properties, especially under wet conditions. To further elucidate the role of conversion a future study will evaluate resins and composites cured to similar degrees of conversion.
Acknowledgements
The authors are grateful to Esstech, Essington, PA, for the donation of the monomers and to Dentsply Caulk, Milford, DE, for the supply of the barium oxide glass fillers. This study was supported in part by the American Dental Association Foundation, the National Institute of Standards and Technology, and the National Institute of Dental and Craniofacial Research, Grant No. DE16298.
Footnotes
Disclaimer Certain commercial materials and equipment are identified in this paper for adequate definition of the experimental procedure. In no instance does such identification imply recommendation or endorsement by the National Institute of Standards and Technology or the ADA Foundation or that the material or equipment identified is necessarily the best available for the purpose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCoy RB, Anderson MH, Lepe X, Johnson GH. Clinical success of class V composite resin restorations without mechanical retention. J Am Dent Assoc. 1998;129:593–599. doi: 10.14219/jada.archive.1998.0277. [DOI] [PubMed] [Google Scholar]
- 2.Atsuta M, Nakabayashi N, Masuhara E. Hard methacrylic polymers. II. Copolymers of methyl methacrylate and 2,2-di-(4-methacryloxyphenyl) propane. J Biomed Mater Res. 1971;5:183–195. doi: 10.1002/jbm.820050306. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi M, Fukushima T, Horibe T. Effect of monomer structure on the mechanical properties of light-cured composite resins. Dent Mater J. 1989;8:40–45. doi: 10.4012/dmj.8.40. [DOI] [PubMed] [Google Scholar]
- 4.Matsukawa S, Hayakawa T, Nemoto K. Development of high-toughness resin for dental applications. Dent Mater. 1994;10:343–346. doi: 10.1016/0109-5641(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 5.Sue HJ, Puckett PM, Bertram JL, Walker LL, Garcia-Meitin EI. Structure and property relationships in model diglycidyl ether of bisphenol-A and diglycidyl ether of tetramethyl bisphenol-A epoxy systems. I. Mechanical property characterizations. J Polym Sci Polym Phys Ed. 1999;37:2137–2149. [Google Scholar]
- 6.Solomons TWG. Organic chemistry. 4th edition. New York: John Wiley & Sons, Inc.; 1988. p. 117. [Google Scholar]
- 7.March J. Advanced organic chemistry, reactions, mechanisms, and structure. 2nd edition. Tokyo, Japan: McGrawHill International Book Company; 1977. pp. 126–132. [Google Scholar]
- 8.Dickens SH, Stansbury JW, Choi KM, Floyd CJE. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules. 2003;36:6043–6053. [Google Scholar]
- 9.Dickens SH, Floyd CJE. Network formation of Bis-GMA and UDMA-based resin systems. Dent Mater. 2006;22:1143–1149. doi: 10.1016/j.dental.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Hammesfahr PD. Compomers and hydromers for use in restorative dental procedures. Am Chem Soc, Div Polym Chem, Polym Prepr. 1997;38(2):131–132. [Google Scholar]
- 11.Venz S, Dickens B. Modified surface-active monomers for adhesive bonding to dentin. J Dent Res. 1993;72:582–586. doi: 10.1177/00220345930720030501. [DOI] [PubMed] [Google Scholar]
- 12.Anusavice KJ, editor. Philipps’ Science of Dental Materials. 10th ed. Philadelphia: WB Saunders Company; 1996. Mechanical properties of dental materials; pp. 62–66. [Google Scholar]
- 13.Xu HHK, Ostertag CP, Braun LM, Lloyd IK. Short-crack mechanical properties and failure mechanisms of Si3N4-matrix/ SiC-fiber composites. J Am Ceram Soc. 1994;77:1889–1896. [Google Scholar]
- 14.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 15.Elliott JE, Lovell LG, Bowman CN. Primary cyclization in the polymerization of bis-GMA and TEGDMA: a modeling approach to understanding the cure of dental resins. Dent Mater. 2001;17:221–229. doi: 10.1016/s0109-5641(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 16.Halvorson RH, Erickson RL, Davidson CL. Energy dependent polymerization of resin-based composite. Dent Mater. 2002;18:463–469. doi: 10.1016/s0109-5641(01)00069-0. [DOI] [PubMed] [Google Scholar]
- 17.Ye Q, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dentin adhesives as a function of light source and irradiance. J Biomed Mater Res B Appl Biomater. 2007;80:440–446. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truffier-Boutry D, Demoustier-Champagne S, Devaux J, Biebuyck JJ, Mestdagh M, Larbanois P, et al. A physico-chemical explanation of the post-polymerization shrinkage in dental resins. Dent Mater. 2006;22:405–412. doi: 10.1016/j.dental.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Pianelli C, Devaux J, Bebelman S, Leloup G. The micro-Raman spectroscopy, a useful tool to determine the degree of conversion of light-activated composite resins. J Biomed Mater Res. 1999;48:675–681. doi: 10.1002/(sici)1097-4636(1999)48:5<675::aid-jbm11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Andrzejewska E, Andrzejewski M, Socha E, Zych-Tomkowiak D. Effect of polyacid aqueous solutions on photocuring of polymerizable components of resin-modified glass ionomer cements. 2003;19:501–509. doi: 10.1016/s0109-5641(02)00096-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Li Q, Lee TY, Guymon CA, Jonsson ES, Hoyle CE. Photopolymerization of acid containing monomers: Real-time monitoring of polymerization rates. 2006;39:8269–8273. [Google Scholar]