Abstract
Unilateral damage to sensorimotor cortical (SMC) regions can profoundly impair skilled reaching function in the contralesional forelimb. Such damage also results in impairments and compensatory changes in the less-affected/ipsilesional forelimb, but these effects remain poorly understood. Furthermore, anesthetization of the ipsilesional hand in humans with cerebral infarcts has been reported to produce transient functional improvements in the paretic hand [14,48]. One aim of this study was to sensitively assay the bilateral effects of unilateral ischemic SMC damage on performance of a unimanual skilled reaching task (the single pellet retrieval task) that rats had acquired pre-operatively with each forelimb. The second aim was to determine whether partially recovered contralesional reaching function is influenced by anesthetization of the ipsilesional forelimb. Unilateral SMC lesions were found to result in transient ipsilesional impairments in reaching success and significant ipsilesional abnormalities in reaching movements compared with sham-operates. There were major contralesional reaching impairments which improved during a 4 week training period, but movements remained significantly abnormal. Anesthetization of the ipsilesional forelimb with lidocaine at this time attenuated the contralesional movement abnormalities. These findings indicate that unilateral ischemic SMC lesions impair skilled reaching behavior in both forelimbs. Furthermore, after partial recovery in the contralesional forelimb, additional improvements can be induced by transient anesthetization of the ipsilesional forelimb. This is consistent with the effects of unilateral anesthetization in humans which have been attributed to the modulation of competitive interhemispheric interactions. The present findings suggest that such interactions are also likely to influence skilled reaching function in rats.
Keywords: motor cortex, ischemia, behavioral compensation, skilled reaching, motor learning, less-affected forelimb, interhemispheric competition
1. Introduction
Unilateral lesions to sensorimotor cortical (SMC) regions in rats result in sensory and motor impairments in the contralesional forelimb, including impairments in the fine movements used in reaching and grasping [51,54]. Such damage also results in complex behavioral changes in the “less-affected”, ipsilesional forelimb. Subtle deficits in the ipsilesional forelimb have been documented using sensitive measures of skilled reaching movements [18,31,33]. Animals also increase reliance on the ipsilesional forelimb for many behaviors including postural support [25], coordinated limb placement during locomotion [45], reaching and food handling [54,55] in a manner which compensates for impairments in the contralesional forelimb. Because skilled reaching tests in rodents are thought to assay impairments homologous to upper extremity impairments in humans with cerebral strokes, it seems important to understand the nature of the functional impairments and improvements that occur in this task in rats. Whishaw [55] found that recovery of reaching ability in the contralesional forelimb is dependent upon whole-body compensatory changes and this includes compensatory ways of using the ipsilesional forelimb to assist in movements of the impaired forelimb. However, it is unknown whether compensation with the ipsilesional forelimb is a necessary chronic contributor to improvements in reaching performance with the contralesional forelimb. Furthermore, recent findings in humans suggest that sensory and motor function of one hand can be improved by ischemic nerve block of the opposite hand [48,50]. This effect is thought to be due to modulation of interhemispheric inhibition and it points to a potential target for therapeutic interventions [49], but it is has not been established to occur in rats. Thus, one purpose of the present study was to test whether partially recovered reaching performance with the contralesional forelimb after unilateral SMC lesions is enhanced or impaired by peripheral anesthetization of the ipsilesional limb.
Another purpose of the present study was to sensitively analyze bilateral changes in reaching performance after unilateral SMC lesions. Focal SMC lesions can enhance skilled reaching in a task that animals learn to perform with the ipsilesional forelimb after the lesion compared to intact animals learning the same task [5,10,22,29]. This effect co-exists with subtle ipsilesional abnormalities in the movements used for reaching [23] and may be linked to neuronal growth promoting effects of denervation and enhanced excitability in the contralesional motor cortex [3,5,10,22,23,29]. In humans, transient virtual lesions of the motor cortex created using repetitive transcranial magnetic stimulation also enhance the performance of a motor task with the ipsilesional hand [27,47]. However, Gonzalez et al. [18] found reductions in skilled reaching success rates in the forelimb ipsilateral to relatively large unilateral frontal cortex lesions using a task that the animals had acquired prior to the injury [see also 33,55]. It may be that enhancement in ipsilesional forelimb reaching function can only be detected after small lesions that do not produce very major ipsilesional deficits [23]. It is also possible that lesion-induced facilitation in skill learning does not generalize to performance of skills that were well learned prior to the injury, even after relatively small cortical lesions. This latter possibility was tested in the present study.
In this study, rats were trained to be proficient in a unimanual skilled reaching with each forelimb. Endothelin-1, a vasoconstricting peptide, was then used to induce ischemic SMC lesions of the forelimb representation region of one hemisphere. Quantitative and qualitative changes in reaching performance were then assessed in each forelimb. To promote improvements in the reaching function of the contralesional forelimb, rats received a period of rehabilitative reach training of this limb, an approach that has previously been found to be effective in improving function [7,13,37,42] and promoting reorganization of movement representations in the peri-lesion cortex [28,36]. The ipsilesional forelimb was then anesthetized to test its effects on reaching performance of the impaired forelimb. Figure 1 summarizes the experimental design.
Figure 1.
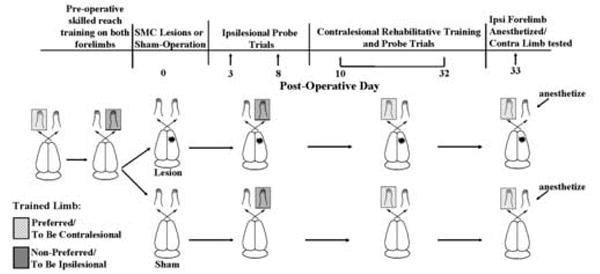
Schematic diagram illustrating the experimental design and the time course of behavioral testing.
2. Materials and Methods
2.1 Animals
Seventeen male Long-Evans hooded rats were used in this study. Rats were obtained at 1 month of age from a breeding colony of the Animal Resources Center at the University of Texas at Austin (n=7) or from Charles Rivers Laboratories (n=10). Rats were housed in clear Plexiglas cages in pairs or triplets, were on a 12:12 h light:dark cycle and were given water ad libitum. Rats were ~ 100 days old at the onset of training. Beginning a few days before training, rats were restricted to 14-15 grams of chow per animal given at the end of each day to ensure that they were not sated at the time of the reach training. Animals were assigned to two groups: one group received unilateral ischemic lesions in the forelimb representation area of the motor cortex (n=9) and the second group received a sham operation (n=8). The mean ± SEM weekly weight change during the experiment was -0.27 ± 0.33 % in the lesion group and 1.58 ± 1.52% in the sham group. Assignments were random with the exception that groups were matched as closely as possible for pre-operative reaching performance and for breeding colony source. The protocol for this study was approved by The University of Texas at Austin Animal Care and Use Committee.
2.2 Surgeries
Unilateral ischemic lesions of the forelimb area of the SMC were made using endothelin-1 (ET-1), a vasoconstricting peptide, using methodology adapted from Fuxe et al. [15; see also 2,3]. Lesions were made contralateral to the forelimb rats preferred to use for reaching. Prior to surgery, rats were anesthetized using Equithesin (approximately 140 mg/kg chloral hydrate and 35 mg/kg sodium pentobarbital). Atropine (0.25 mg/kg) was also administered to negate the depressive respiratory effects of Equithesin. The skull was removed between 1.5 mm anterior and 2.5 mm posterior to bregma and 1.5 mm medial and 4.5 lateral to bregma. The dura underlying the craniectomy was carefully removed and 80μM ET-1 (Peninsula Laboratories, Inc.) was administered to the cortical surface with a Hamilton microsyringe. The total amount of ET-1 administered was 4.0 μl, delivered in two 2.0 μl applications separated by 2 min and followed by an 8 minute wait time, after which the skin was sutured. Sham animals were treated to the same conditions up to the removal of skull. Craniectomies were not performed in sham-operates because this procedure has been found to cause behavioral and neurochemical asymmetries [1] and it was important in this study for this group to serve as intact controls.
2.3 Reach Training and Testing on the Single Pellet Retrieval Task
Animals were trained on the single pellet retrieval task, adapted from Whishaw [32,52] and others [19,39], as previously described by Bury and Jones [10]. The apparatus was a Plexiglas reaching chamber (26cm long × 34 cm high × 16 cm wide) with a tall narrow window (1 cm wide × 23 cm high) that was located in the middle of the 16 cm wide wall. The animals were trained to reach with one forelimb through the window in order to retrieve a banana flavored pellet (Bio-Serve, Inc., Frenchtown, NJ) placed in a well on a 3cm high shelf (Fig. 2). The well was 1 cm from the window opening. In order to prevent the animal from scraping the food pellet into the reaching chamber, a lip consisting of a 2mm diameter metal rod was placed on the shelf in front of the window. Animals were trained pre-operatively to reach with each forelimb. Previous research has shown that most rats develop a preferred limb for performing unilateral reaching tasks [52]. The preferred limb was determined using shaping trials in which rats were permitted to use either forelimb to retrieve centrally placed pellets. Once the animal made 15 out of 20 reaches with one forelimb and, on the following day, made 10 consecutive reaches with the same forelimb, this limb was designated as the animals’ preferred limb. Shaping typically required 3-5 training sessions.
Figure 2.
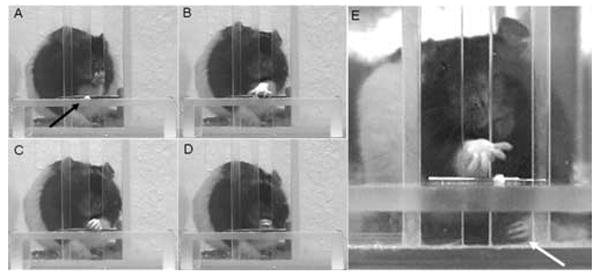
Single pellet retrieval task. Sequential photographs of a rat A) aiming, B) grasping, C) retrieving and D) eating a palatable food pellet (arrow). E) White arrow shows the ipsilesional forelimb following anesthetization.
Once a preferred limb was established, rats began training on this limb. A Plexiglas wall (34 cm high) was inserted into the reaching chamber ipsilateral to the preferred limb and pellets were placed, one at a time, in a well positioned contralateral to this limb (aligned with the edge of the reaching window). This configuration effectively enforced the use of the preferred forelimb for reaching. Rats then received 16 consecutive days of training on the preferred forelimb. They were then required to switch to using the non-preferred limb, by switching the position of the wall and pellet, and were trained for 9 days. Each day’s training session consisted of 60 trials or 15 minutes, whichever came first. For each trial, a pellet was placed in the well and rats were permitted to make up to 5 attempts to retrieve it. Animals were prompted to turn around before starting each new trial by placing a pellet at the back of the cage. Performance was based on the percentage of successful retrievals per reach attempt. A successful reach was one in which the animal grasped the pellet and ate it without dropping it. Unsuccessful reaches included ones in which the pellet was missed, knocked from the well or dropped.
Post-operatively, performance in both the ipsilesional and contralesional forelimb was assessed. Animals were tested in probe trials of reaching performance with the less-affected, ipsilesional limb (in sham-operates, the non-preferred limb) on day 3 and day 8 post-lesion. More extensive testing of the ipsilesional limb was avoided because this has been found to exacerbate functional deficits in the contralesional forelimb [6]. Rats were tested with the contralesional (pre-operatively preferred) limb on day 10, prior to the onset of rehabilitative training, three times during the rehabilitative training period (Days 15-17, 20-22 and 25-27 postoperatively) and then after the rehabilitative training period without (Days 29-32) and with (Days 32-34) anesthetization of the ipsilesional forelimb (described below). To simplify the presentation, the testing sessions are referred to as post-operative Days 16, 21, 26, 30 and 33. The post-operative data are presented as the difference in performance compared with the last pre-operative test day for each limb.
2.4 Post-Lesion Training on a Tray Reaching Task
To promote improved function of the impaired forelimb, rats under went 12 consecutive days of rehabilitative training on a tray-reaching task beginning on post-lesion Day 11. This task was used because it has previously been found to be effective in improving performance on the single-pellet retrieval task of the forelimb contralateral to SMC lesions in comparison to untrained controls [30]. In this task, 100 pellets were placed on an inclined tray and rats were permitted to reach using the impaired (lesion group) or pre-operatively preferred (sham group) forelimb for them for 10 minutes. The metal tray was 7.5 cm long × 5.2 cm high × 6 cm wide and had a 25 degree incline toward the reaching window. The task uses the same Plexiglas chamber with an inner chamber wall as used for the single-pellet retrieval task. Rats in both groups were able to retrieve all 100 pellets in all but the first 2 days of tray reaching, when the lesion group retrieved slightly less than this (98.2 ± 1.0, 99.9 ± 0.06 mean ± S.E.M pellets retrieved on day 1 and 2 of training, respectively).
2.5 Anesthetization of the Ipsilesional Forelimb
Rats were assessed on the single pellet retrieval task (40 trials), using their contralesional/pre-operatively preferred limb, after a subcutaneous injection of lidocaine (~0.2cc, 10 mg/ml) into the proximal ipsilesional forelimb. The lidocaine test is based on the one used by Schallert et al [44] and the dosage was based on a previous study by Bury and Jones [10]. The lidocaine injections made the forelimb limp (Fig.2). Immediately following the lidocaine administration animals were observed in their home cage to confirm that the injection sufficiently anesthetized the ipsilesional forelimb to make it appear limp. Approximately 10 minutes after the lidocaine injection, rats were observed in a transparent cylinder to verify that they were either completely disusing or slipping when attempting to use the ipsilesional limb for upright postural support. Animals then received 40 trials on the single-pellet retrieval task. Postural support with the anesthetized forelimb appeared highly abnormal in all animals during these trials.
2.6 Analysis of reaching movements
An adaptation of the Whishaw rating scale of reaching movements [18,53,56] was used to analyze the movements used to perform the single pellet retrieval task. A Canon 3CCD digital camera was used to videotape animals as they were reaching with the ipsilesional forelimb on post-lesion Day 9 and with the contralesional forelimb during post-lesion week 4 (with and without the non-trained/ipsilesional forelimb anesthetized). Animals’ reaching behavior was assessed during 5 trials in which the animal successfully retrieved the pellet. The 10 components assessed were 1) Digits semi-flexed: the paw is aligned with the midline of the body and digits are together, 2) Aim: the elbow is adducted and the forelimb is aligned with the reaching window, 3) Advance: the head is lifted and the limb is advanced directly toward the pellet, 4) Digits Open: the digits are extended and opened at the end of the advance, 5) Pronation: the digits are open and the paw is directly over the pellet, 6) Grasp: digits are closed to secure the pellet, 7) Withdrawal: the paw is withdrawn at a slight elevation through the reaching window, 8) Supination I: the paw is dorsiflexed and supinated 90° as the limb is withdrawn, 9) Supination II: the paw is supinated again by approximately 45° to bring the pellet to the mouth, 10) Release: the digits are opened and the pellet is released into mouth. The use of the inner chamber wall in the present study invalidates one movement component (“digits to midline”) of the original Whishaw Rating Scale and this component was omitted from the analysis. In addition “withdrawal” is an addition to the rating scale because observations in other studies indicated that this is a movement in which the ipsilesional forelimb sometimes provides assistance. Each movement was rated on a scale of 0 (normal), 0.5 (mildly abnormal), or 1.0 (absent or highly abnormal). The mean score of the 5 trials was recorded for each movement component. A cumulative score was also calculated by summing the 10 components into a score between 0 (no abnormalities) and 10 (severe abnormalities). In other studies, we have seen the ipsilesional forelimb assist the contralesional forelimb in the movements needed to withdraw the forelimb, supinate the wrist and to open the digits and release the food pellet into the mouth (unpublished data). Therefore, during the movement analysis of the contralesional forelimb, any movements made by the ipsilesional forelimb to assist in withdrawal, supination I and II and release were also recorded.
2.7 Measurement of Forelimb Asymmetry
The Schallert cylinder test [45] was used to measure asymmetrical forelimb use for postural support to verify that the lesions resulted in increased reliance on the ipsilesional forelimb. Animals were placed in the Plexiglas cylinder (19 cm diameter) and digitally video-recorded for 2 minutes. The cylinder encourages animals to explore the environment in an upright position in which they support themselves and move along the cylinder wall using the forepaws. This sensitively reveals asymmetries in the use of the forepaws for postural-support. In slow-motion playbacks, each instance of forelimb use was recorded. The asymmetry score was calculated by the formula: (ipsilateral + 1/2 simultaneous bilateral use) / total limb use × 100.
2.8 Histology and Lesion Analysis
On post-lesion day 46-51, rats were given a lethal overdose of sodium pentobarbital and transcardially perfused with 0.1M sodium phosphate buffer, followed by a fixative solution containing 4% paraformaldehyde in the same buffer. Brains were removed and placed in the fixative solution and were sliced on a vibratome within 24 hours of removal. Six rostral to caudal tissue sections were taken and stored in 0.1M sodium phosphate buffer and saline. One set of 50-μm coronal sections was Nissl stained with Toluidine Blue and used to determine lesion placement and size.
The volume of remaining cortex in the SMC region was measured to indirectly determine lesion size. The area of the remaining cortex from sections within the SMC region were obtained using Neurolucida perimeter tracing software (Microbrightfield, Inc.) at a final magnification of X 17. A total of six sections, 400 μm apart, moving in a caudal direction were measured beginning with the appearance of the head of the caudate. The Cavalieri method [20] was used to calculate volume as the product of the summed areas and the distance between section planes (400 μm). The extent and placement of each lesion was also reconstructed onto schematic cortical coronal sections adapted from Paxinos and Watson [38].
2.9 Statistical Analyses
To analyze the reaching and cylinder test data, SPSS (SPSS, Inc.) program for general linear models for repeated measures analysis of variance (ANOVA) was used to determine the effects of Day, Group and Group by Day interactions. Post hoc T tests were used to further analyze reaching data. The apriori α level for all comparisons was 0.05. To analyze the reaching movement analysis data, SPSS general linear model multivariate analysis was used. Bivariate correlations were used to assess the relationship between reaching performance and cortical volume.
3. Results
3.1 Lesion Reconstruction and Volume
Figure 3 shows the reconstruction of the unilateral endothelin-1 induced lesions aimed at the forelimb area of the sensorimotor cortex. All lesions appeared to result in major damage in the forelimb area of the SMC. Most of the animals had cortical damage that extended deep into layer V. Two of the animals had damage that extended into the corpus callosum. It appeared that no animals had any direct damage to the striatum.
Figure 3.

Reconstruction of the extent and placement of focal unilateral forelimb region SMC lesions. The areas in black (common) are regions of damage common to all FLsmc lesions, areas in dark gray (representative) is a representative lesion and the areas in light gray show the largest extent of all SMC lesions combined (range). The numbers on the right indicates the approximate coordinate in mm relative to bregma.
Volume measurements of the remaining SMC revealed a loss of volume in the infarcted hemisphere compared with sham-operates. For the lesion group, the mean ± SEM remaining SMC region volume in mm3 was 90.60 ± 1.96 in the damaged hemisphere and the interhemispheric difference in volume (contra - ipsi) in the SMC region was -13.15 ± 3.20, which was significantly reduced compared with sham operates (P=.001). In the sham-control group, the volume of this region of sensorimotor cortex was 103.06 ± 1.87 in the hemisphere contralateral to the preferred forelimb and the interhemispheric cortical volume difference in the SMC region was 0.71 ± 1.80.
3.2 Pre-Lesion Skilled Reaching Performance
Rats in the lesion and sham groups were matched as closely as possible for pre-lesion success rates with both forelimbs on the skilled reaching task, as shown in Figure 4. Using repeated measures ANOVAs, there were significant effects of Day for both the preferred (F(15,225)=6.37, P=.001) and non-preferred (F(8,120)=7.99, P<.00000001) forelimbs reflecting that, over time, rats improved in their reaching performance. There was no significant Group or Group by Day interaction effects in pre-operative reaching performance with either forelimb between the pre-sham and pre-lesion groups (P’s > 0.05).
Figure 4.
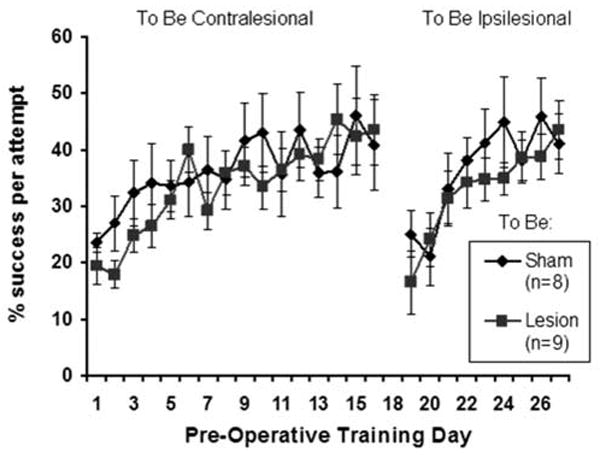
Pre-operative performance on the single pellet retrieval task on the to-be-contralesional and to-be-ipsilesional forelimbs. Rats were trained to criterion using each forelimb and groups were matched for reaching performance prior to surgery. Data are means ± S.E.M.
3.3 Lesion Effects on Reaching with the Ipsilesional Forelimb
As shown on Figure 5A, after unilateral SMC lesions, there was a transient decrease in reaching success rates with the ipsilesional forelimb compared with pre-lesion performance. Using a repeated measures ANOVA, there were significant effects of Group by Day (F(1,15)=4.82, P=.044) and Day (F(1,15)=11.64, P=.004) but no significant main effect of Group (F(1,15)=.676, P=.424). In post hoc analyses, the difference in reaching performance between the sham-operated and lesion groups on post-lesion day 3 failed to reach significance (P=.16). However, in the lesion group, there was a significant difference between post-operative day 3 and both day 0 (pre-operative, P=.038) and day 8 (P=.005), whereas the sham-operated group did not show a significant difference in reaching performance between days.
Figure 5.

Post-operative reaching performance on the single pellet retrieval task using the ipsilesional (A) and contralesional (B) forelimb. There was a significant decrease in reaching performance of the ipsilesional forelimb on day 3 († P<.05), but not day 8, after SMC lesions compared to pre-operative performance. The contralesional forelimb was also impaired compared to shams as assessed on post-lesion day 10 (* P<.05). Contralesional performance improved over days of rehabilitative training. Anesthetization of the ipsilesional forelimb (the non-trained limb of shams) did not significantly change reaching success. Black arrow indicates onset of rehabilitative training. Data are means ± S.E.M.
As shown on Figure 6 and Table 1, the SMC lesions also resulted in significant abnormalities in movements of the ipsilesional forelimb. As assessed on day 9 post-lesion, lesion animals had an increase in the cumulative movement abnormality score based on an adaptation of Whishaw’s Rating Scale. A one-way ANOVA revealed a significant increase in the movement abnormality score in the lesion group compared to the sham group in the ipsilesional limb (F(1,16)=8.25, P=.012). Multivariate analyses including each movement component as a dependent variable revealed a significant increase in abnormalities in digits semi-flexed (P=.041) and pronation (P=.011), but not the other movement components, in the lesion group compared to the sham group.
Figure 6.
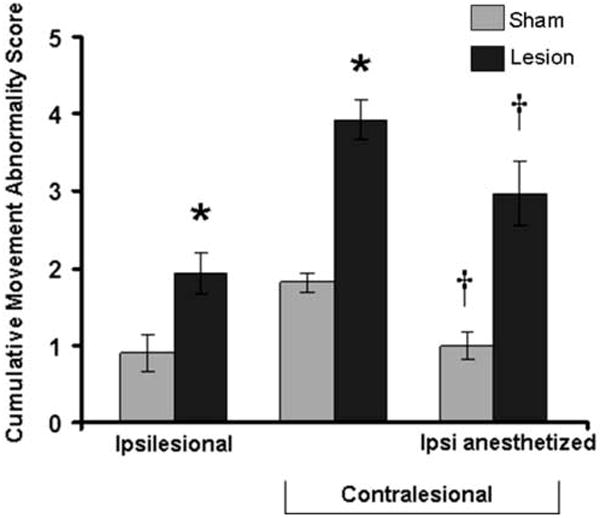
Cumulative movement abnormality scores in the ipsilesional and contralesional forelimbs. In the ipsilesional limb the cumulative abnormality score was significantly different between lesion and sham groups (* P=.012). As expected, unilateral lesions resulted in significant movement abnormalities in the contralesional forelimb compared with the sham group (*P<.002). Anesthetization of the ipsilesional/non-trained forelimb significantly reduced the abnormality score of the unanesthetized forelimb in both groups (†P<. 05 pre- vs. post-lidocaine).
Table 1.
| Ipsilesional | Contralesional | Contralesional Ipsi anesthetized | ||||
|---|---|---|---|---|---|---|
| Movement Category | Sham | Lesion | Sham | Lesion | Sham | Lesion |
| 1) Digits Semi-Flexed | .03 ± .02 | * .28 ± .11 | .26 ± .12 | * .76 ± .13 | .03 ± .03 | .46 ± .15 |
| 2) Aim | .01 ± .01 | .11 ± .07 | .10 ± .06 | * .29 ± .07 | .04 ± .03 | .18 ± .08 |
| 3) Advance | .04 ± .02 | .02 ± .02 | .06 ± .02 | .13 ± .05 | † .00 ± .00 | .04 ± .02 |
| 4) Digits Open | .00 ± .00 | .00 ± .00 | .00 ± .00 | .00 ± .00 | .00 ± .00 | .00 ± .00 |
| 5) Pronation | .28 ± .10 | * .64 ± .08 | .62 ± .10 | .79 ± .09 | .35 ± .09 | .70 ± .11 |
| 6) Grasp | .10 ± .05 | .13 ± .03 | .12 ± .02 | * .29 ± .05 | .01 ± .04 | .20 ± .05 |
| 7) Withdrawal | .10 ± .03 | .12 ± .04 | .12 ± .04 | * .34 ± .06 | .09 ± .05 | † .17 ± .03 |
| 8) Supination I | .00 ± .00 | .00 ± .00 | .00 ± .00 | .07 ± .05 | .00 ± .00 | .06 ± .03 |
| 9) Supination II | .14 ± .00 | .18 ± .09 | .38 ± .09 | .61 ± .14 | † .03 ± .03 | .43 ± .14 |
| 10) Release | .21 ± .10 | .44 ± .11 | .16 ± .07 | * .66 ± .13 | .25 ± .12 | .73 ± .09 |
Data are ± SEM,
p<.05 significantly different from sham,
p<.05 significantly different from unanesthetized.
3.4 Lesion Effects on Reaching with the Contralesional Forelimb
As first assessed on post-lesion Day 10, rats with a unilateral lesion had significant impairments in contralesional reaching performance in the single pellet retrieval task compared to the sham-operated group (Fig. 5B). The greatest severity of impairment was evident on Day 10. In repeated measures ANOVA, there was a significant effect of Group (F(4,60)=5.02, P=.041) but no significant Group by Day interaction effect (F(1,15)=.73, P=.576). There was also a significant effect of Day (F(4,60)=5.05, P=.001), which reflects that both the sham-operated and the lesion animals improved in the reaching performance over the days of testing (during which time they were receiving training on the tray reaching task).
As shown in Figure 6 and Table 1, SMC lesions also resulted in major abnormalities in reaching movements in the contralesional forelimb as assessed using the Whishaw Rating Scale during post-lesion week 4. In One-Way ANOVA, lesion animals had a significantly higher cumulative movement abnormality score than the sham group (F(1,16)=45.69, P=.000006). Multivariate analysis revealed significantly greater abnormalities in the lesion group compared to the sham group in the following individual movement components: digits semi-flexed (P=.007), aim (P=.047), grasp (P=.003), withdrawal (P=.004), and release (P=.000000002). In rats with lesions, there was a significantly greater cumulative abnormality score during performance with the contralesional forelimb compared to the ipsilesional forelimb (P=.001). However, there was also a significant difference in the cumulative abnormality score in sham-operates reaching with the preferred versus non-preferred forelimb (P=.004). Nevertheless, the magnitude of the difference between the contralesional and the ipsilesional cumulative movement abnormality score in the lesion group was significantly greater than the interlimb difference in sham-operates (P=.031). In lesion rats, the mean ± SEM percentage of trials in which the ipsilesional forelimb was observed assisting the contralesional forelimb in the retrieval and/or consumption of the banana pellet was 23.33 ± 11.13. In sham-operates the percentage of trials in which the non-trained limb was observed assisting the trained limb was 16.67 ± 10.04. There was no significant difference between lesion and sham operates in the frequency of non-trained forelimb assistance (P=.46).
3.5 Effects of Anesthetizing the Ipsilesional Forelimb on Reaching with the Impaired Forelimb
Lidocaine was peripherally administered to the ipsilesional limb to anesthetize it and then the reaching performance of the contralesional limb was assessed. As shown in Figure 5B, neither group had a significant change in reaching performance as a result of lidocaine injection into the ipsilesional and/or non-reaching forelimb compared with the prior test of performance (t-test for Day 30 versus 33: P =.474 lesion group, P=.998 sham group).
As shown on Figure 6, following peripheral administration of lidocaine into the ipsilesional/non-reaching forelimb, both the lesion (P=.032) and sham (P=.005) group had significantly reduced cumulative movement abnormality scores with the unanesthetized forelimb compared to the pre-lidocaine assessment of this limb. There was no difference in the magnitude of the change in the cumulative movement abnormality scores in the lesion versus sham-operate group (P=.926). However, there were some group difference in the specific movements most affected (Table 1). Multivariate analyses revealed a significant decrease in abnormalities in the individual movement components pre-lidocaine versus post-lidocaine. This was found in the sham group in advance (P=.025) and supination II (P=.002) and in the lesion group in withdrawal (P=.012). As with the pre-lidocaine test, ANOVA revealed a significant difference between the sham and lesion group in the cumulative movement abnormality score during the lidocaine test (F(1,16)=19.19, P=.001).
3.6 Increased Reliance on the Ipsilesional Forelimb for Postural Support Behavior
Unilateral SMC lesions resulted in an increased reliance on the ipsilesional forelimb in the Schallert cylinder test. Pre-operatively, the asymmetry score (%ipsi + ½ bilateral use/ total use observations) was 48.89 ± 4.58 in the lesion group and 46.67 ± 2.76 in the sham group. Post-operatively (Day 4), the asymmetry score was 73.52 ± 4.43 in the lesion group and 51.67 ± 2.64 in the sham group. A repeated measures ANOVA revealed a significant Group (F(1,15)=26.64, P<.001) and Group by Day effect (F(2,30)=3.40, P=.047) and post-hoc analysis indicated significantly increased asymmetry in the lesion group compared with the sham-operates at the post-operative (P = .001), but not pre-operative time point.
4. Discussion
In summary, unilateral ischemic lesions of the forelimb representation region of the SMC resulted in major reaching impairments and movement abnormalities in the contralesional forelimb and less major, but significant, impairments in the ipsilesional limb on a skilled reaching task that had been well-learned with both forelimbs prior to the injury. Peripheral anesthetization of the ipsilesional/non-preferred forelimb failed to have a significant effect on reaching success rates with the impaired forelimb, but it significantly decreased movement abnormalities in the unanesthetized forelimb in both the sham and lesion group.
The ipsilesional effects of the SMC lesions were revealed as a minor, transient, reduction in reaching success rates and more notable abnormalities in reaching movements. These ipsilesional effects are consistent with the findings of Gonzalez et al. [18] in which rats that pre-operatively learned a skilled reaching task had reduced success rates and movement abnormalities in the ipsilesional forelimb following unilateral ischemic lesions of the motor cortex produced by pial strip or of the laterofrontal cortex following distal middle cerebral artery occlusion (MCAO). The impairments in the present study appear to be more minor and transient compared to the findings in Gonzalez et al. [18] which might be attributed to the differences in methods of lesion induction, lesion size and the reaching task. Nevertheless, both of these studies suggest that a previously acquired motor skill is disrupted in the ipsilesional forelimb following unilateral cerebral infarcts. Lidocaine injections into the motor cortex have also been found to impair reaching function in the ipsilateral forelimb of intact animals and to reinstate deficits in an impaired forelimb when injected contralateral to unilateral MCAO [8].
The ipsilateral ventral corticospinal tract provides about 5% of the corticospinal tract and may contribute to movements of the ipsilateral body side. However, this pathway seems unlikely to be a major contributor to the ipsilesional impairments found in the present study because Whishaw and Metz [56] found that unilateral corticospinal tract lesions at the medullary level, rostral to the decussation, produced no impairments in reaching with the ipsilateral forelimb. Another potential explanation is that unilateral lesions result in degenerative effects in the contralateral cortex that disrupt the neural circuits contributing to the performance of pre-learned motor movements and/or movement sequences. The forelimb representation regions of either hemisphere are interconnected via transcallosal fibers [e.g., 4,21] and denervation of transcallosal fibers is associated with reactive astrocytic changes [9,17,34] and loss of axonal processes [26] in the cortex. Partial deafferentation causes cellular and structural changes, such as increases in neurotrophic factors [24] and reactive synaptogenesis [12]. Unilateral cortical lesions also result in increased excitability in the contralateral cortex [40,41,46,58]. It is possible that these relatively subtle degenerative effects and excitability changes disrupt previously established motor skills while facilitating acquisition of new skills. Compared to intact animals, unilateral lesions of the SMC also result in better performance of a skilled reaching task that rats learned after the lesion [5,10,22,29]. This effect was muted in animals with larger lesions and greater of ipsilesional movement abnormalities [23].
Consistent with many previous studies [33,51,54], unilateral SMC lesions resulted in reaching impairments of the contralesional forelimb. By Day 32, rats had returned to pre-lesion reaching success levels with the contralesional forelimb. However, analysis using Whishaw’s rating scale of reaching movements revealed movement abnormalities compared to the sham-operated group at this time point, also consistent with previous findings [16,55,57]. Anesthetization of the ipsilesional/non-preferred forelimb at this stage partially normalized movements in the other limb on this rating scale in both groups of rats. It did not interfere with reaching performance of the contralesional limb as would be expected if animals had been significantly relying on the anesthetized limb for compensation. The overt use of this limb to assist in movements made with the contralesional forelimb, such as assistance in limb withdrawal and release of the pellet into the mouth, was not significantly increased in frequency relative to sham operates at this time point (Day 32-34 post infarct). The ipsilesional/non-reaching forelimb continued to be used for postural support in both lesion and sham-operates, as animals stand on this limb while reaching with the other forelimb. This postural support was highly abnormal after lidocaine administration indicating that it is unlikely to be an important contributor to successful reaching (at least in this task design, see Miklyaeva et al. [32]). It cannot be ruled out that any detriment of having the non-reaching forelimb anesthetized is masked by the enhancing effect of unilateral anesthetization or that there is a rapid development of alternative compensatory strategies. However, the failure to observe increased use of the ipsilesional forelimb to aid the contralesional limb in movements used during reaching provides further support that the recovered contralesional reaching ability is likely to be largely independent of the contribution of ongoing ipsilesional compensation.
The functional improvements resulting from peripheral anesthetization are consistent with research in humans in which anesthetization of one hand in healthy subjects [50] or of the non-paretic hand of human stroke survivors [14,48] using ischemic nerve block is found to result in transient sensory and motor functional improvements of the non-anesthetized hand. Furthermore, this effect is associated with increased somatosensory evoked potentials contralateral to the non-anesthetized hand [50]. These authors have suggested that these effects may occur because the loss of sensory input from one hand decreases interhemispheric competition and inhibitory interactions [35,49; see also Clarey et al. [11]]. In the present study, although the magnitude of reduction in the cumulative movement abnormality score was similar in the lesion versus sham group, there were differences in the specific movements most affected by the anesthetization. Furthermore, in both groups some movements were relatively unaffected by the lidocaine injection, such as the abnormalities in grasp and release in the lesion group. These differences cannot be easily attributed to the magnitude of the pre-anesthesia abnormality score, to distal versus proximal movements or to phases of the reach. A better understanding of the neural mechanisms of these effects may shed light on why some movement abnormalities are most sensitive to the effects of peripheral anesthetization.
The failure to finding reductions in reaching success following anesthetization of the ipsilesional forelimb should not be taken to suggest that compensatory use of the ipsilesional forelimb is not an important contributor during earlier phases of recovery on this task. Previously, rats have been reported to use this forelimb in compensatory ways to assist the contralesional/reaching forelimb in earlier post-lesion periods [55]. It is also likely that larger lesions than produced in the present study promote greater reliance on the ipsilesional forelimb. Restraining the ipsilesional forelimb in a sling early after large (but not small) lesions of the motor cortex during practice of the reaching task was been found to impair reaching performance [55]. Thus, anesthetization of the ipsilesional forelimb at an earlier time point might have impaired skilled reaching function, especially in animals with more severe contralesional impairments. Thus, if there are facilitating effects of forelimb anesthetization at earlier time points post-injury, these may require alternative measures to detect. It is also possible that the extensive pre-operative training, or the 2 post-injury probe sessions of performance with the ipsilesional limb, tended to suppress the rats’ tendency to use this forelimb in a different way in the reaching apparatus, i.e., to assist movements of the contralesional forelimb.
In conclusion, unilateral ischemic SMC lesions of the forelimb representation region resulted in minor ipsilesional and more considerable contralesional impairments in skilled reaching performance that had been established prior to the injury. Anesthetization the ipsilesional forelimb did not interfere with the reaching performance of the contralesional forelimb suggesting that, at least at later stages of recovery after relatively small cortical infarcts, it is possible for reaching movements in the contralesional forelimb to recover in this task without ongoing compensatory reliance on the ipsilesional forelimb. The finding that skilled reaching movements were, instead, partially normalized by anesthetization of the other forelimb indicates that the interhemispheric and bimanual effects of peripheral anesthetization reported in humans most likely extend to rat reaching function. This seems important in light of recent findings indicating competitive interactions between the hemispheres that become unbalanced by unilateral brain damage and which might be targeted for therapeutic interventions [43,47,48,49]. The present results suggest that rat models of skilled reaching may be useful for studying the role of interhemispheric interactions in recovery of somatomotor function after unilateral brain damage.
Acknowledgments
The authors would like to thank Dr. DeAnna Adkins, Rachel Allred, Monica Maldonado and Edward Hsu for assistance with surgical and behavioral procedures and Brian Bernier for help with skilled reaching. We would also like to thank Kellan Schallert, Pete Campos, David Quach, and Toral Parikh for their assistance with vibratome slicing, Michael Smith and Toral Parikh for cylinder tape rating and Allison Ahrens for assistance with movement analysis. Supported by MH64586 and RR020700.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams FS, Schwarting RK, Huston JP. Behavioral and neurochemical asymmetries following unilateral trephination of the rat skull: is this control operation always appropriate? Physiol Behav. 1994;55:947–952. doi: 10.1016/0031-9384(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 2.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–8. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 3.Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004128:473–86. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Akers RM, Killackey HP. Organization of corticocortical connections in the parietal cortex of the rat. J Comp Neurol. 1978 Oct 1;181:513–37. doi: 10.1002/cne.901810305. [DOI] [PubMed] [Google Scholar]
- 5.Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage in female rats: forelimb behavioral effects and dendritic structural plasticity in the contralateral homotopic cortex. Exp Neurol. 2004;190:433–45. doi: 10.1016/j.expneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Allred RP, Maldonado MA, Hsu JE, Jones TA. Training the “less-affected” forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restor Neurol Neurosci. 2005;23:297–302. [PubMed] [Google Scholar]
- 7.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–80. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–99. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 9.Bury SD, Eichhorn AC, Kotzer CM, Jones TA. Reactive astrocytic responses to denervation in the motor cortex of adult rats are sensitive to manipulations of behavioral experience. Neuropharmacology. 2000;39:743–55. doi: 10.1016/s0028-3908(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 10.Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarey JC, Tweedale R, Calford MB. Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somatosensory cortex. Cereb Cortex. 1996;6:196–206. doi: 10.1093/cercor/6.2.196. [DOI] [PubMed] [Google Scholar]
- 12.Cotman CW, Nieto-Sampedro N, Harris EW. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981;6:684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- 13.DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–6. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- 14.Floel A, Nagorsen U, Werhahn KJ, Ravindran S, Birbaumer N, Knecht S, Cohen LG. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56:206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- 15.Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. Neuroreport. 1997;8:2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- 16.Gharbawie OA, Gonzalez CL, Whishaw IQ. Skilled reaching impairments from the lateral frontal cortex component of middle cerebral artery stroke: a qualitative and quantitative comparison to focal motor cortex lesions in rats. Behav Brain Res. 2005;156:125–37. doi: 10.1016/j.bbr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Gomide VC, Chadi G. The trophic factors S-100beta and basic fibroblast growth factor are increased in the forebrain reactive astrocytes of adult callosotomized rats. Brain Res. 1999;835:162–74. doi: 10.1016/s0006-8993(99)01557-7. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur J Neurosci. 2004;20:3442–52. doi: 10.1111/j.1460-9568.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- 19.Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–14. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- 20.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Miscosc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 21.Henry EC, Catania KC. Cortical, callosal, and thalamic connections from primary somatosensory cortex in the naked mole-rat (Heterocephalus glaber), with special emphasis on the connectivity of the incisor representation. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:626–45. doi: 10.1002/ar.a.20328. [DOI] [PubMed] [Google Scholar]
- 22.Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur J Neurosci. 2005;22:2069–80. doi: 10.1111/j.1460-9568.2005.04370.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less- affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479–94. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Isackson PJ. Trophic factor response to neuronal stimuli or injury. Curr Opin Neurobiol. 1995:350–357. doi: 10.1016/0959-4388(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 25.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–60. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 26.Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- 27.Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- 28.Liepert J, Hamzei F, Weiller C. Lesion-induced and training-induced brain reorganization. Restor Neurol Neurosci. 2004;22:269–77. [PubMed] [Google Scholar]
- 29.Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–99. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado MA, Allred RP, Jones TA. Exercise and rehabilitation training effects on behavioral outcome after a focal unilateral sensorimotor cortex lesion in rats. Soc Neurosci Abstr. 2004:1004.3. [Google Scholar]
- 31.Marston HM, Faber ES, Crawford JH, Butcher SP, Sharkey J. Behavioural assessment of endothelin-1 induced middle cerebral artery occlusion in the rat. Neuroreport. 1995;6:1067–71. doi: 10.1097/00001756-199505090-00029. [DOI] [PubMed] [Google Scholar]
- 32.Miklyaeva EI, Whishaw IQ. HemiParkinson analogue rats display active support in good limbs versus passive support in bad limbs on a skilled reaching task of variable height. Behav Neurosci. 1996;110:117–25. doi: 10.1037//0735-7044.110.1.117. [DOI] [PubMed] [Google Scholar]
- 33.Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–28. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- 34.Moumdjian RA, Antel JP, Yong VW. Origin of contralateral reactive gliosis in surgically injured rats cerebral cortex. Brain Res. 1991;547:223–8. doi: 10.1016/0006-8993(91)90965-x. [DOI] [PubMed] [Google Scholar]
- 35.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 36.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;5:2144–9. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 37.Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain stereotaxic coordinates. 2. Sydney: Academic Press; 1986. [Google Scholar]
- 39.Peterson GM, Devine JV. Transfer of handedness in the rat resulting from small cortical lesions after limited forced practice. J Comp Physiol Psychol. 1963;56:752–56. [Google Scholar]
- 40.Que M, Schiene K, Witte OW, Zilles K. Widespread up-regulation of N-methyl-D-aspartate receptors after focal photothrombotic lesion in rat brain. Neurosci Lett. 1999;273:77–80. doi: 10.1016/s0304-3940(99)00598-4. [DOI] [PubMed] [Google Scholar]
- 41.Reinecke S, Lutzenburg M, Hagemann G, Bruehl C, Neumann-Haefelin T, Witte OW. Electrophysiological transcortical diaschisis after middle cerebral artery occlusion (MCAO) in rats. Neurosci Lett. 1999;261:85–8. doi: 10.1016/s0304-3940(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 42.Riolobos AS, Heredia M, de la Fuente JA, Criado JM, Yajeya J, Campos J, Santacana M. Functional recovery of skilled forelimb use in rats obliged to use the impaired limb after grafting of the frontal cortex lesion with homotopic fetal cortex. Neurobiol Learn Mem. 2001;75:274–92. doi: 10.1006/nlme.2000.3979. [DOI] [PubMed] [Google Scholar]
- 43.Rushmore RJ, Valero-Cabre A, Lomber SG, Hilgetag CC, Payne BR. Functional circuitry underlying visual neglect. Brain. 2006;129:1803–21. doi: 10.1093/brain/awl140. [DOI] [PubMed] [Google Scholar]
- 44.Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229–238. [PubMed] [Google Scholar]
- 45.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–6. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 48.Voller B, Floel A, Werhahn KJ, Ravindran S, Wu CW, Cohen LG. Contralateral hand anesthesia transiently improves poststroke sensory deficits. Ann Neurol. 2006;59:385–8. doi: 10.1002/ana.20689. [DOI] [PubMed] [Google Scholar]
- 49.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–8. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–8. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- 51.Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. 1991;42:77–91. doi: 10.1016/s0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 52.Whishaw IQ. Lateralization and reaching skill related: results and implications from a large sample of Long-Evans rats. Behav Brain Res. 1992;52:45–48. doi: 10.1016/s0166-4328(05)80323-7. [DOI] [PubMed] [Google Scholar]
- 53.Whishaw IQ, Pellis SM, Gorny B, Kolb B, Tetzlaff W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav Brain Res. 1993;56:59–76. doi: 10.1016/0166-4328(93)90022-i. [DOI] [PubMed] [Google Scholar]
- 54.Whishaw IQ, Coles BL. Varieties of paw and digit movement during spontaneous food handling in rats: postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav Brain Res. 1996;77:135–48. doi: 10.1016/0166-4328(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 55.Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled forelimb reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 56.Whishaw IQ, Metz GA. Absence of impairments or recovery mediated by the uncrossed pyramidal tract in the rat versus enduring deficits produced by the crossed pyramidal tract. Behav Brain Res. 2002;134:323–36. doi: 10.1016/s0166-4328(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 57.Whishaw IQ, Piecharka DM, Zeeb F, Stein DG. Unilateral frontal lobe contusion and forelimb function: chronic quantitative and qualitative impairments in reflexive and skilled forelimb movements in rats. J Neurotrauma. 2004;21:1584–600. doi: 10.1089/neu.2004.21.1584. [DOI] [PubMed] [Google Scholar]
- 58.Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1149–1165. doi: 10.1097/00004647-200008000-00001. [DOI] [PubMed] [Google Scholar]


