Abstract
The purpose of this review is to present the recent evidence linking the family of ubiquitous proteases called calpains (EC 3.4.22.17) to neuropathologies of the retina. The hypothesis being tested in such studies is that over-activation of calpains by elevated intracellular calcium contributes to retinal cell death produced by conditions such as elevated intraocular pressure and hypoxia. Recent x-ray diffraction studies have provided insight into the molecular events causing calpain activation. Further, x-ray diffraction data has provided details on how side chains on calpain inhibitors affect docking into the active site of calpain 1. This opens the possibility of testing calpain-specific inhibitors, such as SJA6017 and SNJ1945, for human safety and as a site-directed form of treatment for retinal pathologies.
Keywords: calpain, calpain activation, calpain inhibitors, glaucoma models, human disease, molecular genetics, molecular modeling, neurochemistry, neuropathology, retina, retinitis pigmentosa models, x-ray diffraction
Calpains in Retina
The purpose of this review is to present the recent evidence linking the family of proteases called “calpains” (EC 3.4.22.17) to neuropathologies of the retina. Ubiquitous calpains are distributed throughout the layers of retina. They are present in the cytoplasm and nucleus of retinal ganglion cells (Fig. 1).
Fig. 1.
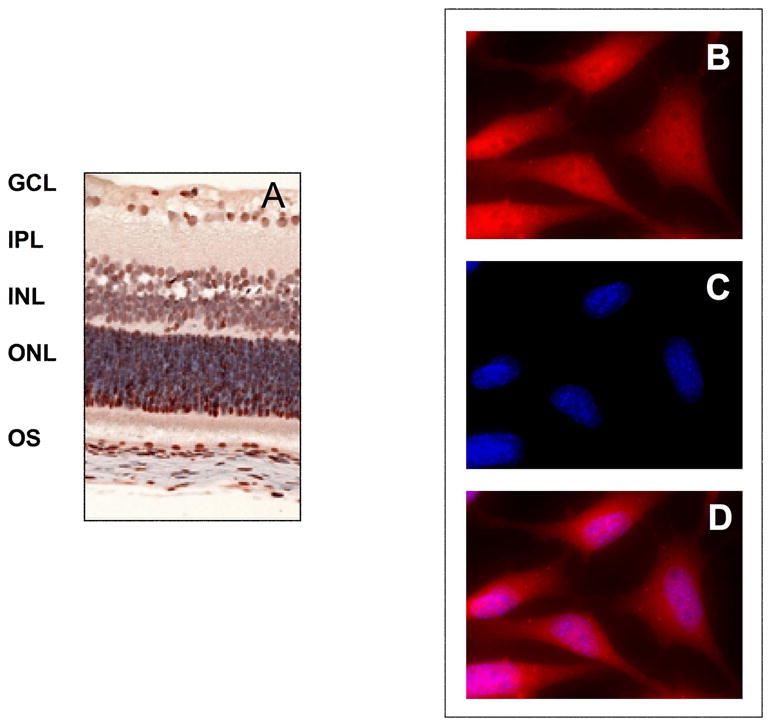
(A) Brown immunostaining for calpain 2 protein in layers and cells of retina. Layer designations are GCL = ganglion cells, IPL = inner plexiform, INL = inner nuclear, ONL = outer nuclear, OS = outer segment. (B) Red staining for calpain proteins in rat retinal ganglion cells (primary polyclonal antibody reactive with calpains 1 & 2, secondary antibody conjugated with Alexa fluo-594), (C) blue DNA staining in the cell nuclei (Hoechst 33342), and (D) merged images from (B) and (C), showing positive staining for calpain proteins 1 & 2 in the cytoplasm and nucleus.
THE CALPAIN FAMILY OF PROTEASES
Calpains comprise a family of calcium-activated, cysteine proteases derived from 14 genes for the 80 kDa “catalytic” subunit and two genes for the 30 kDa “regulatory” subunit55 (Fig. 2). Calpains exist ubiquitously in cells from humans to microorganisms. While calpains cleave consistently at the same sites on a specific protein, they do not recognize consensus amino acid cleavage sites. Rather, they cleave at exposed regions between domains of proteins and thus produce large, usually bioactive, fragments under physiologic conditions. At normal cellular calcium levels (<0.05 μM), calpains are biomodulators of calcium-regulated events such as signal transduction, cell proliferation, cell cycle progression, differentiation, apoptosis, membrane fusion, processing of arrestin, and platelet activation. 3,20 A classic example is the action of calpain on protein kinase C to release an intact kinase domain that is active without calcium or diacylglycerol.55 Over-activation of calpains after pathological elevation of cellular calcium has been associated with such conditions as cataract in experimental animals, neuronal degeneration, Alzheimer disease, and metastasis.33
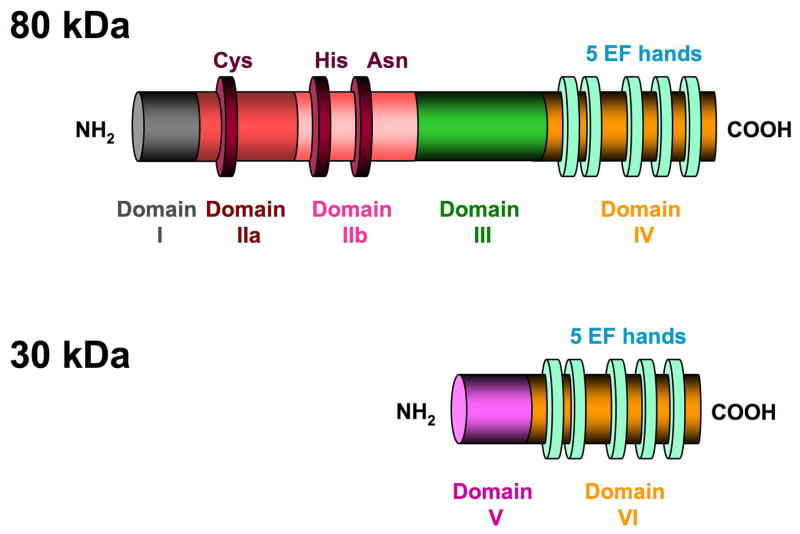
Fig. 2.
Canonical structure of a typical calpain showing the six domains found in the 80 and 30 kDa subunits of the ubiquitous, heterodimeric calpains 1 and 2. A deep, wide substrate-binding cleft separates the active site cysteine residue in subdomain IIa from the histidine and asparagine residues in subdomains IIb in the inactive molecule. The light green ovals represent EF-hand structures in domains IV and VI. The highly flexible domain V (N-terminal domain) of the 30 kDa regulatory domain contains clusters of non polar glycine residues, and is one of the sites for calcium-induced autolysis as is domain I of the 80 kDa subunit. Interactions between the two C-terminal EF structures stabilize the dimer.
STRUCTURE OF TYPICAL CALPAINS
The most abundant and well-studied calpains are the ubiquitous typical calpains 1 & 2, which exist as dimers of the 80 and 30 kDa subunits. The canonical domain structure for the calpains consists of domains I-IV in the catalytic subunit and domains V and VI in the regulatory subunit (Fig. 2).53
CALPAIN ACTIVITY IN RETINA
Although the proteins or genes have been described for 14 different calpains among various species, the zymograms of whole retinas from both human and monkey retinas show only calpain 1 & 2 activities, which respond to various neurodegenerative conditions such as hypoxia (Fig. 3).
Fig. 3.
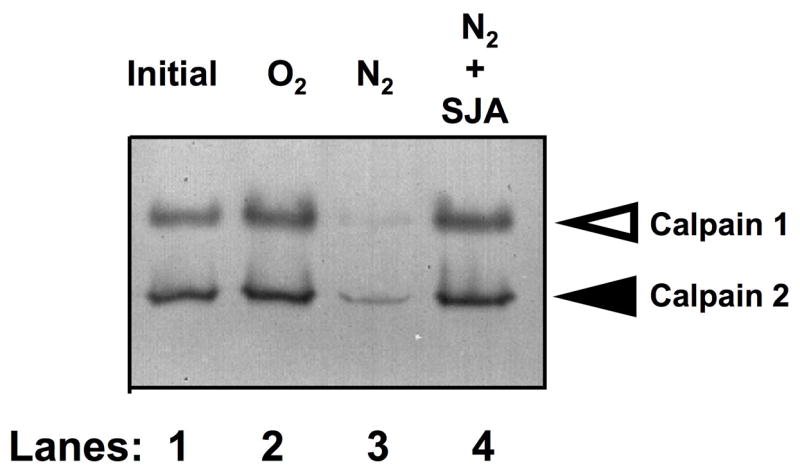
Casein zymogram with image inverted to show dark areas of lysis due to calpain activities in soluble proteins from normal monkey retinas (lane 1). The retinas were also cultured under normal oxygen conditions (lane 2) and under hypoxic treatment (lanes 3 & 4). Open arrowhead indicates calpain 1 activities, and solid arrowhead indicates calpain 2 activities. Retinas were incubated for ten hours under normoxia (O2), hypoxia (N2), or N2 plus 100 μM calpain inhibitor SJA6017 (SJA) (modified with permission from Nakajima E et al27).
CALPAIN 3 VARIANTS IN RETINA
In contrast to the ubiquitous calpains described above, the most studied of the tissue-preferred calpains are monomeric calpain 3 (muscle-preferred localization) and its splice variants, such as Rt88 in retina7, 29 and Lp82 in lens25 (Fig. 4). mRNA for atypical calpain 10 has also been found in rat retina,8 but enzymatic activity for calpain 10 has only been demonstrated in kidney mitochondria.1 Calpain 3 and its variants differ from the canonical structure of calpains 1 & 2 in three ways: the presence of a novel N-terminus and two insert regions 1 & 2. These calpains do not associate with a 30 kDa subunit. Although mRNAs for calpain 3 splice variants have been described in monkey and human retinas,29 their enzyme activities have not been observed in these tissues. The calpain 3 variants are probably in such small amounts that they are undetectable, and their physiologic and pathological roles in retina are unknown at the present. Thus, most of the studies reported below are concerned with changes in calpains 1 & 2. But note that other calpain activities could possibly be present at undetectable levels in retina, concentrated in a particular location and performing very specialized functions.
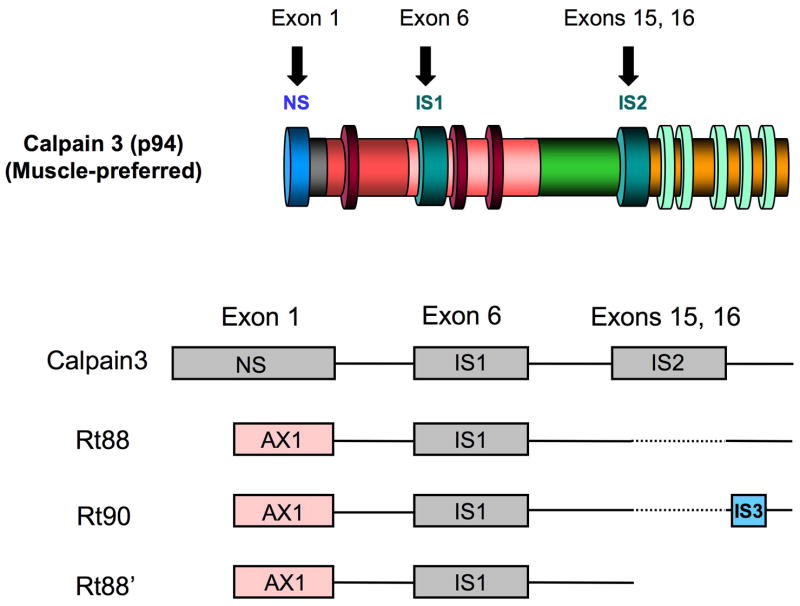
Fig. 4.
Gene diagrams (lower) for calpain 3 variants found in retina compared to the parent protein molecule, muscle-preferred calpain 3 (upper). NS, novel sequence; IS1, insert region 1; IS2 insert region 2; IS3, insert region 3; AX1, alternative exon 1 coding region, dashed lines are deleted sequences. Due to a stop codon, AXI is not found in human or monkey retinas, where it is replaced with the NS region.
Studies Linking Calpains to Retinal Neurodegeneration
CALPAIN ACTIVATION AND DRUG INHIBITION IN OTHER DISEASES AND CONDITIONS
During aging, caspase proteases, lysosomal cathepsins, and calpains lead to a combination of apoptosis and necrosis during degeneration of neurons.33 Activation of calpains have been associated with pathologies in neuronal conditions such as Alzheimer disease, stroke, trauma, CNS excitotoxicity, Huntington disease, and Parkinson’ disease.2, 20
Activation of calpains has been well documented during neuronal cell death in the ischemic brain, and calpain inhibitor SJA6017 partially protected against the loss of ganglion cells.9, 19, 43, 44, 47, 62 Topical SJA6017, also slowed, but did not prevent, progression of hereditary cataracts in ovine lenes.42 Infusion of SJA6017 was partially effective in reducing α-spectrin breakdown in a testicular torsion model of ischemia/reperfusion injury.50 Functional outcome (ability to hang on a string with paws) was improved in rats after delayed systemic injection of SJA6017 following diffuse brain injury.23 Thus, action of calpains also seems likely during neuronal cell death in retina. Partial improvement of biochemical parameters or functionality by calpain inhibitors, even when applied after injury, opens the possibility of a “therapeutic window” for their use after diagnosing retinal pathology.
PRESENCE OF A CALPAIN SYSTEM IN RETINAS FROM VERTEBRATE RETINAS
Calpains 1 & 2 and their endogenous tissue inhibitor calpastatin (CS) were partially purified in 1984 from pig retina61 and from rat retina in 1985.58 The calpain activity levels were higher than rat brain, calpain II was higher than calpain I, and calpain II levels were higher than CS, presumably allowing ready activation in vivo. In rabbit retina, calpain II was heavily immunostained in the pigment epithelium and outer and inner plexiform layers.40 The outer segments of photoreceptor cells stained heavily for calpains 1 & 2 and CS. Normal rat retinas contained 12 times more mRNA for calpain 2 than calpain 1, and calpain enzymatic activity could be easily demonstrated on casein zymograms.57 In the optic tract of mice, intact axons of retinal ganglion cell neurons contained more calpain activity than in the optic glia.32
Calpain II immunostaining was most pronounced in the plexiform layers and the photoreceptor outer segments of bovine retinas.4 The localization of calpain II was also observed in the connecting cilium after light exposure, and myosin was proteolyzed in vitro of by calpain II. This suggested that calpain II could be involved in light-dependent regulation of disk membrane morphogenesis by proteolysis of myosin II.
Rat retina also contains a splice variant of muscle-preferred calpain p94, called Rt88 (Fig. 4), which contained an alternative exon 1 (AX1, as in rat lens Lp82), and deletion of exons 15 and 16 removed the unique insertion region IS2.56 mRNA levels for Rt88 in rat retina were comparable to calpain II mRNA levels, and recombinant Rt88 was enzymatically active, but rapidly truncated to an enzymatically active form.7 No Rt88 is present in primates due to a stop codon, but calpain 3 variants with the NS region replacing the AX1 region, plus calpains 1 and 2, are found in both human and monkey retinas.29 Expression patterns for ubiquitous calpains 1 & 2 were conserved among various mammalian species, while expression patterns for calpain 3 variants varied widely. Thus, ubiquitous calpains 1 & 2 may have the same general functions in the retinas of most animal species, while calpain 3 variants may have specialized functions depending on the animal species.
ASSAY METHODOLOGY
Comments on the methods used to detect or infer calpain activation in retina are useful because the expected results are not always obvious and results are sometimes overextended.
Calcium levels
Since calpains require calcium for activation, the first question usually asked when determining if calpain could be active in tissues is if tissue calcium levels are elevated enough to activate calpains. The half maximal calcium requirements for calpains 1 & 2 determined in vitro are approximately 3–50 μM and 400–800 μM, respectively.20 Calcium levels as high as 16,000 μM calcium (wet weight) have been reported in pathologic whole retinas.45 However, elevated retinal calcium does not automatically prove calpain activation because: a) Half maximal activation levels were usually determined in vitro using purified calpains. The in vivo calcium activation requirements for calpains are largely unknown, although they are considered to be lower than those determined in vitro. b) A low ratio of calpain enzyme activity to calpastatin inhibitor activity (< 1) may inhibit activation. c) Retinal pathologies are usually layer specific, but calcium levels are not known for each layer of the retina. d) We do not know how much of elevated retinal calcium is extracellular, and calpains are nearly always intracellular enzymes. e) Calcium determinations are frequently performed by such methods as atomic absorption spectrometry that measures the total of free and bound calcium, whereas free calcium is needed to activate calpains.
Calpain-specific Substrates
One of the most powerful and definitive techniques for proving calpain activation is based on identifying unique, calpain-specific cleavage sites on signature proteins. For example, calpain-specific, α-spectrin breakdown products (SBDPs) can be detected by immunoblotting. A 145 kDa SBDP is produced exclusively by calpains and can be detected using a commercially available antibody to non-erythroid alpha-spectrin (BIOMAL International, catalogue # FG6090-0500). Multiple SBDPs migrating at 150 kDa are produced by both calpains and caspase 3.31 An antibody for the calpain-specific fragment at the 150 kDa has been produced,44 and the antibody has been frequently used to confirm calpain activity.
A more global approach has been recently employed to identify other calpain-specific cleavage sites in retina.27 This is performed by two-dimensional electrophoresis (2DE) of retina treated with exogenous calpain followed by mass spectrographic analyses of the new termini (cleavage sites) of fragments excised from the 2DE gels. The cleavage sites or the migration positions of calpain-produced fragments could then be used as biologic markers for calpain activation in retina under normal or pathologic conditions, as has been performed in experimental cataract formation.59 Towards that end, we are developing proteome maps of rat retinal soluble proteins before and after treatment with exogenous calpain 2 (Fig. 5, Tables 1 & 2). These could be compared to disease models. Such an approach, not involving calpain, as has been performed to understand the proteomic changes in human age-related macular degeneration.
Fig. 5.

Proteome map of the soluble proteins in normal rat retina on Coomassie blue-stained, two dimensional gel (2DE) showing more than 80 spots – many of which were identified by mass spectrometry. For example, one of the most concentrated retinal proteins was enolase, the protein spots labeled “ENOA.” The proteins with abbreviated labels marked on the gels were used as landmark proteins because their migration positions were not obviously altered by treatment with calpain 2 (Table 1). Treatment with activated calpain 2 lead to decreased amounts of proteins circled and numbered 1–27; identities are listed in Table 2. The horizontal axis of the 2DE gel is the first dimension with pH 3 on the left to pH 10 on the right. The second dimension was SDS-PAGE in the vertical axis with molecular mass markers in kDa on the left.
Table 1.
Marker retinal proteins
| Abbreviation | Protein Name | Accession Number | Mass (whole protein, Da) |
|---|---|---|---|
| HSP7C | Heat shock cognate 71 kDa protein, rat | P63018 | 70,871 |
| ALBU | Serum albumin precursor, rat | P02770 | 68,718 |
| CRTC | Calreticulin precursor, rat | P18418 | 47,995 |
| ENOA | Alpha-enolase (EC 4.2.1.11), rat | P04764 | 46,996 |
| PGK1 | Phosphoglycerate kinase 1 (EC 2.7.2.3), rat | P16617 | 44,423 |
| ALDOA | Fructose-bisphosphate aldolase C (EC 4.1.2.13), rat | P09117 | 39,152 |
| G3P | Glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12), rat | P04797 | 35,705 |
| CALB2 | Calretinin, rat | P47728 | 31,405 |
| UCHL1 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 (EC 3.4.19.12), rat | Q00981 | 24,838 |
| TPIS | Triosephosphate isomerase (EC 5.3.1.1), rat | P48500 | 26,790 |
| PRDX2 | Peroxiredoxin-2 (EC 1.11.1.15), rat | P35704 | 21,652 |
| COF1 | Cofilin-1, rat | P45592 | 18,401 |
| PPIA | Peptidyl-prolyl cis-trans isomerase A, rat | P10111 | 17,743 |
| SODC | Superoxide dismutase, rat | P07632 | 15,780 |
| FABPE | Fatty acid-binding protein, epidermal, rat | P55053 | 14,928 |
| MIF | Macrophage migration inhibitory factor (MIF) (Phenylpyruvate tautomerase) (EC 5.3.2.1), rat | P30904 | 12,346 |
Table 2.
Proteins decreased after addition of activated calpain 2
| Spot Number. on Fig. 5 | Protein Name (abbreviation, species) | Accession Number | Mass (Whole protein, Da) |
|---|---|---|---|
| 1 | 78 kDa glucose-regulated protein precursor (GRP78, rat) | P06761 | 72,346 |
| 2 | Heterogeneous nuclear ribonucleoprotein K (HNRPK, rat) | P61980 | 50,976 |
| 3 | Heterogeneous nuclear ribonucleoprotein K (HNRPK, rat) | P61980 | 50,976 |
| 4 | Protein disulfide-isomerase precursor (EC 5.3.4.1) (PDIA1, rat) | P04785 | 56,951 |
| 5 | Vimentin (VIME, rat)
Tubulin beta-5 chain (TBB5, rat) |
P31000
P69897 |
53,601
49,671 |
| 6 | Tubulin beta-5 chain (TBB5, rat) | P69897 | 49,671 |
| 7 | Gamma-enolase (EC 4.2.1.11) (ENOG, rat) | P07323 | 47,009 |
| 8 | Hsc70-interacting protein (F10A1, rat) | P50503 | 41,279 |
| 9 | Heterogeneous nuclear ribonucleoprotein L (HNRPL, mouse) | Q8R081 | 60,123 |
| 10 | Heterogeneous nuclear ribonucleoprotein L (HNRPL, mouse) | Q8R081 | 60,123 |
| 11 | Protein NDRG1 (NDRG1, mouse) | Q62433 | 43,008 |
| 12 | Fructose-bisphosphate aldolase C (EC 4.1.2.13) (ALDOC, rat) | P09117 | 39,152 |
| 14 | Clathrin light chain A (CLCA, rat) | P08081 | 26,980 |
| 17 | Heterogeneous nuclear ribonucleoproteins A2/B1 (ROA2, mouse) | O88569 | 35,993 |
| 18 | Malate dehydrogenase, mitochondrial precursor (EC 1.1.1.37) (MDHM, rat) | P04636 | 35,655 |
| 19 | Heterogeneous nuclear ribonucleoproteins A2/B1 (ROA2, mouse) | O88569 | 35,993 |
| 20 | Proteasome subunit alpha type 5 (EC 3.4.25.1) (PSA5, rat)
Heterogeneous nuclear ribonucleoproteins A2/B1 (ROA2, mouse) |
P34064
O88569 |
26,391
35,993 |
| 21 | Ran-specific GTPase-activating protein (RANG, mouse) | P34022 | 23,596 |
| 22 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 (EC 3.4.19.12) (UCHL1, rat) | Q00981 | 24,838 |
| 23 | Guanylate kinase (EC 2.7.4.8) (KGUA, mouse) | Q64520 | 21,787 |
| 24 | Eukaryotic translation initiation factor 5A-1 (IF5A1, rat) | Q3T1J1 | 16,701 |
| 25 | Glia maturation factor beta (GMFB, rat) | Q63228 | 16,605 |
| 26 | Stathmin (Phosphoprotein p19) (STMN1, rat) | P13668 | 17,157 |
| 27 | Stathmin (Phosphoprotein p19) (STMN1, rat) | P13668 | 17,157 |
Calpain-specific Inhibitors
Inhibition of proteolysis (e.g., inhibition of production of the 145 kDa SBDP) by calpain-specific inhibitors has been used to confirm calpain activation. For example, SJA6017 is a potent inhibitor of calpains 1 & 2 and does not inhibit other calcium-activated, cysteine proteases.21 Cathepsins B and L are inhibited, but they do not require calcium for their activation, allowing differentiation of enzyme inhibition by comparing samples incubated with and without calcium. Methods (2) and (3) have thus been utilized to provide direct evidence of calpain activation in retina in the studies described below.
Casein Zymography and Immunoblotting
Calpain zymography and immunoblotting have been used to provide indirect evidence for calpain activation. Results are usually contrary to first expectations. That is, zymograms and immunoblots often show a loss of the intact 80 kDa catalytic subunit of calpain after activation by elevated calcium (Fig. 3, lane 3). This is because, after calpain activation, not only do calpains hydrolyze other protein substrates, but they also hydrolyze themselves (autolysis). Thus, intact calpain 1 rapidly autolyzes to active calpain 1 fragments at 78 and 76 kDa (not readily detected on native zymograms, but the fragments can be observed on immunoblots).48 Calpain 2 autolyzes to a 43 kDa fragment,6 and then these fragments are further degraded. Thus, loss of the 80 kDa calpain band is considered indirect evidence of calpain activation. Of course, a decrease in the 80 kDa band could be due to action of other proteases. But the concomitant presence calpain 1 fragments at 78/76 kDa and at 43 kDa for calpain 2 on immunoblots confirms calpain activation.
MODELS OF GANGLION CELL DEATH INDUCED BY HYPOXIA PERTINENT TO GLAUCOMA
In Vitro Models
Rat Retina Culture with N2
Ca2+ overload activates calpain and caspase cascades leading to apoptotic death in RGC-5 cells, following a 24-hour exposure to 250 nM ionomycin.14 Also, a tissue culture model of hypoxia was produced by incubating rat retinas in culture media bubbled with 95% N2/5%CO2.56 Compared to normoxic control retinas, hypoxia for 12 hours caused leakage of large amounts of lactate dehydrogenase (LDH) from retinas into the medium, indicating significant cell damage. Hypoxia caused production of calpain-specific SBDPs at 145 and 150 kDa, and decreased calpain 1 and 2 activities. These markers of calpain activity were reduced by calpain-inhibitor SJA6017. These data suggested that calpains in tissue-cultured retinas were activated under hypoxic conditions. The tissue culture procedure caused retinas in all treatment groups to be become thin and fragile, probably due to the mechanical action of the bubbling gasses. Although this adds mechanical trauma along with hypoxia as a potential initiating factor in the model, the normoxic controls did not show appreciable α-spectrin breakdown, LDH leakage, or calpain 1 & 2 activation. These data suggested that the tissue culture model was a reasonable, although not ideal protocol for demonstrating involvement of calpain in neuronal cell damage in hypoxic retinas.
A recent follow up study using the above protocol, showed that hypoxic rat retinas cultured under 95% N2 also showed breakdown of a group of microtubule-associated proteins called tau, conversion of pro-caspase-3 to 30 kDa caspase, and proteolysis of p35 regulator to p25 suggesting prolonged activation of cyclin dependent protein kinase-5 (Cdk5).57 These hydrolytic changes were partially inhibited by calpain inhibitor SJA6017. Thus, another consequence of in vitro calpain activation in hypoxic retinas was the breakdown of critical cytoskeletal and neuronal proteins associated with retinal cell death (Fig. 6).
Fig. 6.
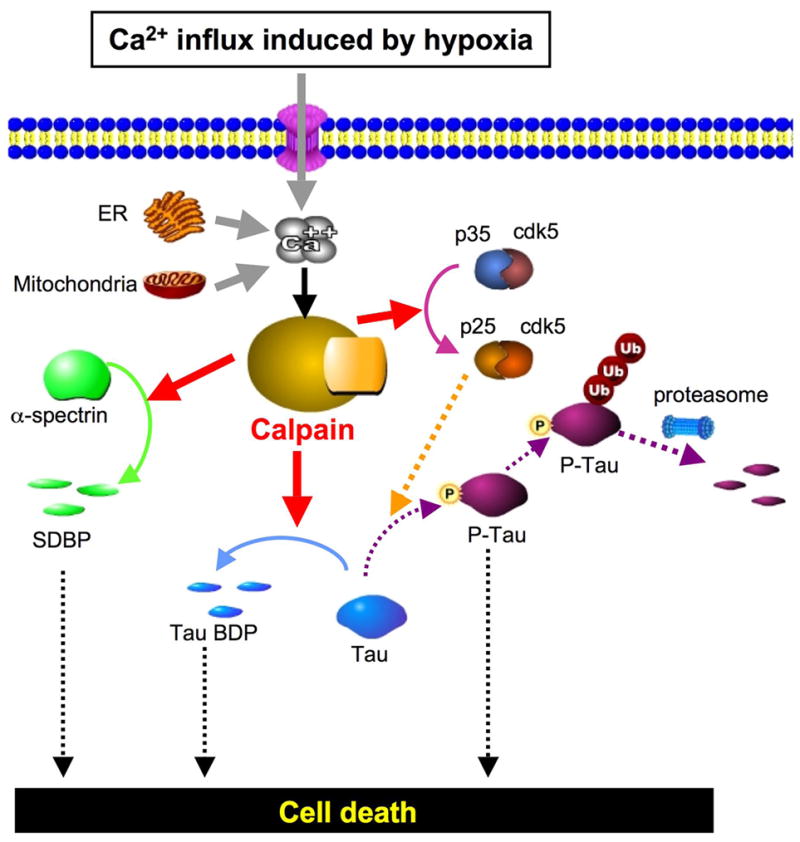
Proposed pathways leading to cell death in hypoxic rat retina due to calpain-mediated modifications in α-spectrin and tau. Solid lines show confirmed pathways, and dotted lines show the pathways reported in the literature (reprinted with permission from Tamada Y et al57).
Monkey Retina Cultures
In order to determine the possible relevance of the above in vitro data from rats to human retinal diseases, monkey retinas were cultured in 95% N2, and they reacted similarly as rat retinas.28 That is, LDH leaked into the medium, calpain autolysis was observed, and α-spectrin was degraded to the calpain-specific fragment at 145 kDa, and calpain inhibitor SJA6017 partially inhibited these changes (Fig 3).
Total retinal soluble proteins were also isolated by centrifugation after homogenization of normal human and monkey retinas.27 The soluble proteins were then incubated with 2.5 mM calcium to directly activate endogenous calpains. Protein fragments were separated by two-dimensional electrophoresis and then identified by mass spectrometry and confirmed by immunoblotting. Potential calpain substrates included retinal vimentin, β-tubulin, Hsp70, and α-enolase. Thus, despite the presence of endogenous inhibitor calpastatin in the soluble proteins of human and monkey retina,27 calpains were activated by calcium and caused proteolysis of endogenous retinal proteins in primates. Depending on the tissue, this result is not always the case. For example, in some tissues, such as aged human lenses, the presence of a high ratio of calpastatin to low levels of endogenous calpains resulted in only minimal calpain activation, even when large amounts of exogenous calcium were added to the lens total soluble proteins.27 The ratio of mRNAs for calpastatin/calpains is 1.1 and 0.6 for human and monkey retinas, respectively (Fig. 7). Our data indicated that human and monkey retinas were apparently able to escape the action of calpastatin when calcium was highly elevated. Although these in vitro studies used extreme conditions to elevate calcium and activate calpains, they supported the hypothesis that retinal neuronal degeneration from hypoxic conditions found in man, such as in glaucoma, could be due to over-activation of calpains.
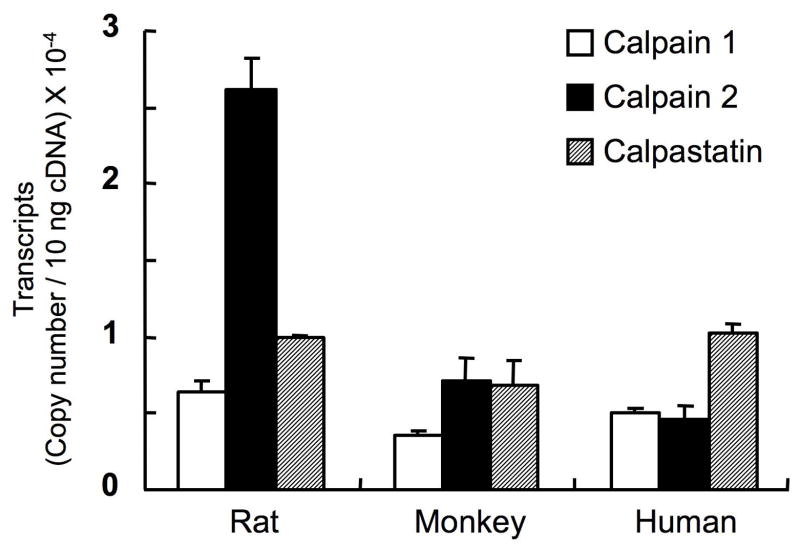
Fig. 7.
Copy numbers for members of the calpain system in the retinas from rats, monkey and man (modified with permission from Oka T et al36).
In Vivo Models
Rat Central Retinal Artery Occlusion
Several in vivo models also support that the idea that calpain isoforms play an important role in retinal ganglion cell death induced by hypoxia. These animal models also have the advantage of providing enough retinal samples for calcium analyses, and elevated calcium is, of course necessary for calpain activation.
An ischemia-reperfusion model was produced by occluding the central retinal artery in rats for one hour followed by reperfusion for seven days.45 This caused sloughing of the ganglion cell layer by 1 day, loss of cells in the inner plexiform layer at 3 days, and loss of cells in the inner nuclear layer (Fig. 8A). Calcium was significantly elevated in whole retinas by 12 hours, and they peaked at 3 days at an astounding level of 16 mM calcium (wet weight). Further, indicators of calpain activation in vivo were observed in the rat ischemia-reperfusion model. These included proteolysis of the calpain signature substrate α-spectrin to 150 and 145 kDa SBDPs and decreased caseinolytic activity for both calpains 1 & 2, indicating activation followed by autodegradation. Importantly for development of drugs against retinal ganglion cell degeneration, SJA6017 by intravenous injections caused a statistically significant, partial reduction in the number of cells lost from the ganglion cell layer due to ischemia-reperfusion injury (Fig. 8B). These data could be important for testing future glaucoma therapies.
Fig. 8.
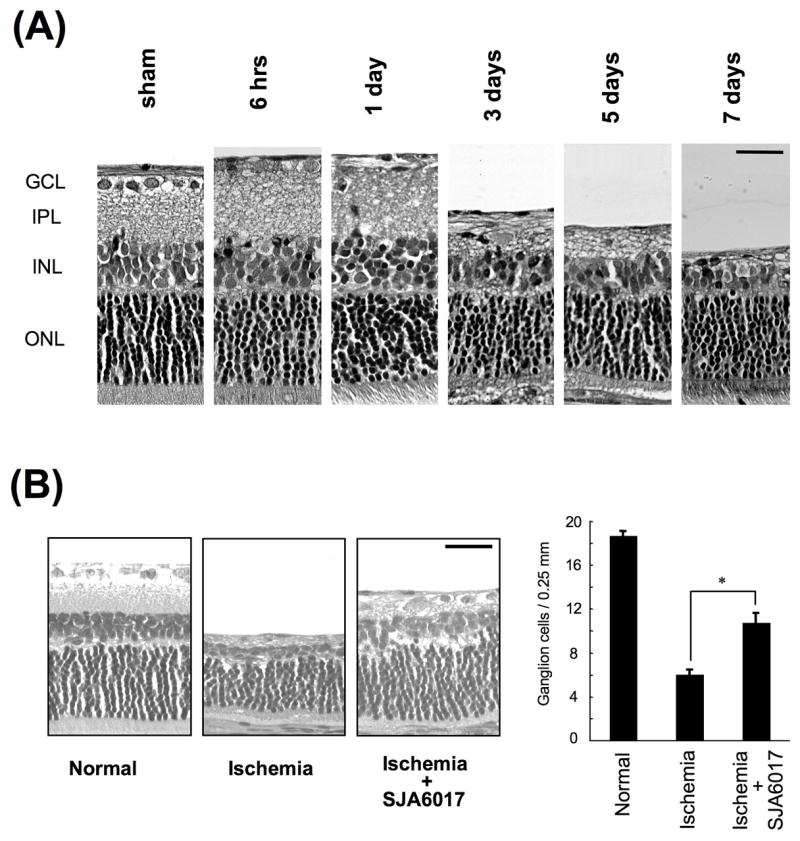
Histologic changes in rat retina from ischemia/reperfusion of the central retinal artery (A), and inhibition by SJA6017 on 7 days after ischemia/reperfusion (B) (modified with permission from Sakamoto YR et al45).
Elevated IOP/Hydrostatic Saline Model
The role of calpains in degeneration of the inner portion of the retina was also studied by elevating intraocular pressure (IOP) to 120 mm Hg pressure (normal = 17 mm) in live rats by cannulation of the anterior chamber and infusion of hydrostatic saline for one hour and then following the rat for 7 days.35 This procedure produced a significant decrease in ganglion cell numbers by day 1, and this decreased to 54% of normal by day 7. The inner nuclear and inner plexiform layers became thinner, but no changes were observed in the outer nuclear or outer segment layers. ERGs showed that the b-waves originating from the bipolar and Müller cells were completely lost, and calcium levels were significantly elevated to approximately 2.4 mM (wet weight) as early as 4 hours after the temporary elevation of IOP. Associated with the increased retinal calcium, direct and indirect evidence for activation of calpains 1 & 2 were present. Indirect evidence included: decrease of calpain 1 & 2 caseinolytic activities starting four hours after acute elevated IOP and production of 43 kDa autolysis fragment of calpain 2. Direct evidence included observation of calpain-specific, α-spectrin breakdown products at 145 and 150 kDa and complete loss of tau proteins, known substrates for calpains, at 4 hours.
Proteolysis of tau12 and hyperphosphorylation of tau30 have been proposed as neuortoxic. Conversion of the regulatory subunit p35 to p25 causes prolonged activation cyclin-dependent protein kinase (Cdk5), which phosphorylates tau.24 Hyperphosphorylated tau aggregates into insoluble tangled filaments, which cause regression of the neuronal processes and cell death. Acute ocular IOP caused significant elevation of p25 only at four hours. These data are consistent with the hypothesis that calpain initiates ganglion cell neurotoxicity by production of insoluble, hyperphosphorylated tau aggregates, in addition to proteolysis of critical retinal proteins such as α-spectrin (Fig. 6). Recent studies also indicate that activation of calpains occurs after oxidative stress to retinal microvascular endothelial cells42 and that calpain 1 may be involved in mediating NMDA-induced excitotoxicity in the rat retina.11
Follow-up studies showed that a more membrane permeable calpain inhibitor (SNJ-1945) was able to prevent some of the retinal degeneration and biochemical changes in the acute IOP rat model described above.36 Intraperitoneal injection of SNJ-1945 at 80 mg/kg and above completely prevented the loss of cells in the GCL. Further, even oral SNJ-1945 at 240 mg/kg provided 63% protection of GCL loss and inhibited production of the calpain-specific α-spectrin breakdown product at 145 kDa by 63 %.
Model of Elevated IOP in Monkey
If calcium is elevated and activates calpains in human glaucomatous patients, SNJ-1945 may be a candidate drug for testing to help ameliorate retinal degeneration in patients also receiving drugs to reduce IOP. Towards testing that hypothesis, chronic ocular hypertension was produced in monkeys using laser photocoagulation of the trabecular meshwork. At the end of 20 months, the optic nerve head showed disk cupping and the number of cells in the GCL were decreased (Fig. 9).
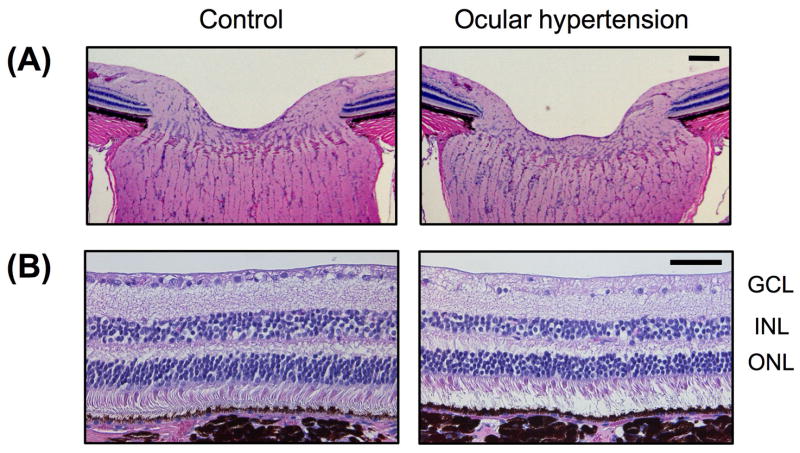
Fig. 9.
Photomicrographs of H & E stained retinal sections from monkey with induced chronic ocular hypertension, showing decrease in GLC cells and disc retraction in the optic nerve head (ONH). (A) is ONH. (B) is superior mid-peripheral retina. Scale bars are 100 μm in (A) and 50 μm in (B)
Similar signs were observed in human glaucoma (Fig. 10), and such data indicated that the monkey model could be useful for testing calpain inhibitors against glaucoma. Monkeys would be better models than rats since monkeys, like humans, have lower calpain transcript levels than rats, and monkey and human retinas have relatively more calpastatin than rats (Fig. 7). Despite these differences, exogenous calpain inhibitor SNJ-1945 was able to similarly inhibit calpain-specific proteolysis of α-spectrin after addition of calcium to homgenates of retinas from rats, monkeys and humans. Such data indicate that rat models are still useful for initial screening of calpain inhibitors as they relate to retinal damage in human glaucoma.
Fig. 10.
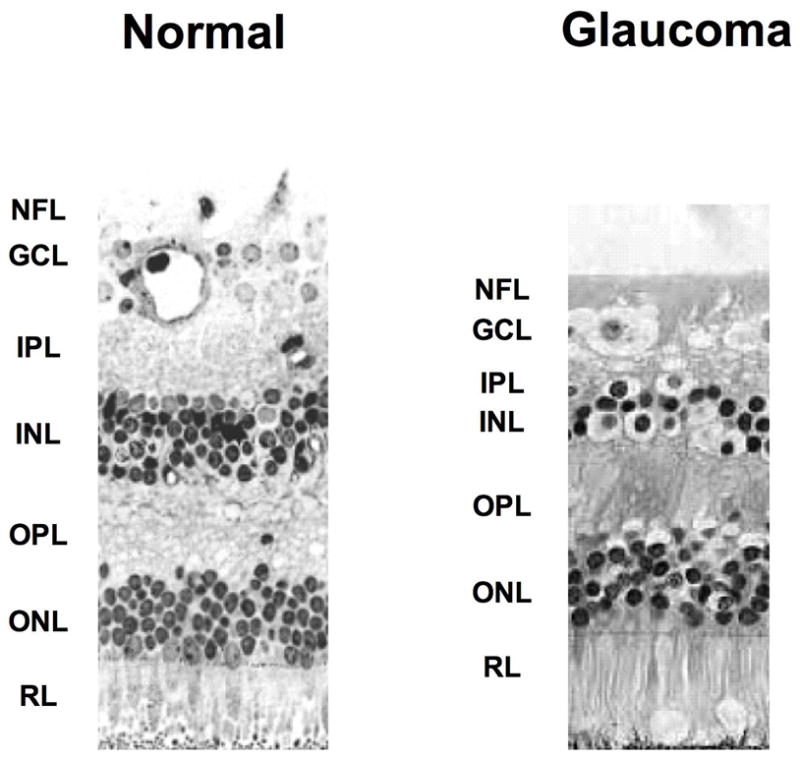
Histologic photograph of human glaucomatous retina showing loss of many ganglion cells. Arrows indicate the condensation of nucleus in the GCL. The NFL, GCL, and IPL were also involuted. The OPL, ONL, and photoreceptor layers were well maintained. NFL; Nerve Fiber Layer, GCL; Ganglion Cell Layer, IPL; Inner Plexiform Layer, INL; Inner Nuclear Layer, OPL; Outer Plexiform Layer, ONL; Outer Nuclear Layer, RL; Receptor Layer.
MODELS OF PHOTORECEPTOR CELL DEATH PERTINENT TO RETINITIS PIGMENTOSA (RP)
WBN/Kob Rat
Experiments using the 661W cell line suggested that calpain, cathepsin D, and caspases may be involved in photoreceptor (PR) cell degeneration during oxidative stress.46 A possible hereditary model of photoreceptor (PR) cell degeneration is the WBN/Kob rat, which starts to show significant decreased retinal thickness by 12 weeks of age.8 Almost total disappearance of photoreceptor cell layer occurred by 48 weeks with only a single layer of outer nuclear cells remaining. Both a- and b-waves of the ERG became non-recordable at 48 weeks of age. Total calcium levels were approximately 4 mM (wet weight). SBDPs at 145/150 kDa were observed, and, surprisingly, calpain 1 & 2 activity levels increased in the WBN/Kob rats. mRNA for retina-specific, splice variant calpain Rt88 was markedly down regulated. This may be significant because mutations in the parent calpain, p94, cause limb-girdle muscular dystrophy type 2A in humans.37 An interesting outcome from this paper, addressing the issue the histologic location of retinal calpains, was that in situ hybridization for calpain 2 mRNA showed staining throughout the retinal layers, especially the outer nuclear layer. Taken together, these data suggest that altered calpain activities (increases in calpains 1 & 2, decreases in Rt88) are associated with hereditary photoreceptor cell degeneration in rats.
rd Mice
Another animal model of PR degeneration showing involvement of calpains is the rd1 type mutant from the family of retinal degeneration (rd) mice.10 This model is pertinent to human retinitis pigmentosa because mutations in the β subunit of PR-specific phosphodiesterase-6 have been documented in both human autosomal recessive RP and rd mice. The mutation allows accumulation of cGMP in the PR, opens more of the cGMP-gated calcium channels, and allows influx of calcium. In the rd mouse, this is associated with rapid loss of rod cells between P8 and P15 followed by more gradual degeneration of the cones.38 Transcript levels for calpastatin (CS) and CREB-1, which regulates transcription of CS, decreased, while calpain activity levels increased 157 fold in the ONL of rd1 mice.39 Since this was associated with cells undergoing PR death, the data support the idea that calpain activation and hydrolysis of retinal proteins is one of the causes of PR death in rd1 mice. Furthermore, calpain inhibitors were able to reduce, but not totally prevent, calpain activation in the ONL in tissue sections. This is consistent with the idea that calpain inhibitors could be useful adjuncts for possible drug treatment of RP, if other pathways of PR degeneration are also controlled. For example, evidence has been presented that in addition to calpain, serine protease cathepsin D16 and caspase-349 may also be active in PR degeneration in cells from rd mice.
Royal College of Surgeons (RCS) Dystrophic Rats
RCS rats exhibit progressive photoreceptor loss due to a defect in phagocytosis in the retinal pigmented epithelium cells. This leads to the accumulation of cytotoxic debris in the subretinal space. Calpain activity was lower in the retina of the neonatal RCS rat. This occurred before the onset of any morphological deterioration, and it preceded any other detected abnormalities.5
Rat Model of Photoreceptor Cell Degeneration
Photoceptor cell degeneration also develops rapidly in rats after administration of N-methy-N-nitrosurea (MNU).34 MNU alters membrane fluidity and permeability. The outer nuclear layer and outer segment became thinner one day after MNU injection and these regions become more severely affected by 7 days (Fig. 11A), when whole retinal calcium levels were elevated to approximately 3 mM. Further, as early as 1 day after injection, cells in the ONL showed positive TUNNEL staining indicative of cell death. At 1 day after MNU, the calpain-specific SBDPs at 150 and 145 kDa were observed, caseinolytic activity for calpain 1 was almost totally lost by day 1, and the 43 kDa autolysis product indicative of calpain 2 activation appeared at 3 days. As in the rat model of acute IOP,35 MNU activation of calpains was also associated with conversion of p35 (Cdk5 regulator) to p25, suggesting that hyperphosphorylation of tau proteins may contribute photoreceptor cell degeneration in the MNU model. These data provide strong evidence that calpain was activated in a severe model of photoreceptor cell degradation.
Fig. 11.
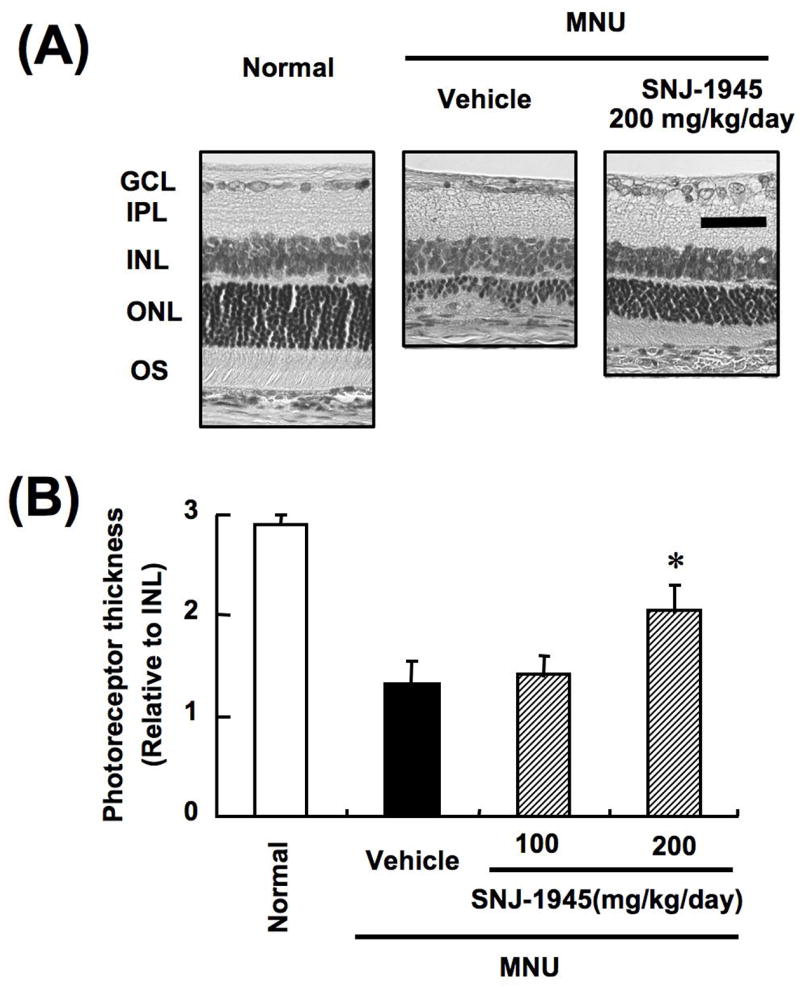
Histologic changes (A) and thickness of photoreceptor (B) in retinas from rats treated with MNU (modified with permission from Oka T et al34).
Calpain inhibitor SNJ-1945 was developed to provide oral availability, higher cell permeability, and longer stability in retina.52 Oral administration of 200 mg SNJ-1945/kg body weight to rats significantly inhibited the decrease in the thickness of the photoreceptor layer caused by MNU by 46% (Fig. 11). This was important since the MNU model is one of the most severe models of retinal degeneration. We hope that such compounds as SNJ-1945, and further derivatives,51 may hold promise for treatment of less severe and much more slowly developing forms of retinal degeneration such as RP.
Calpain Activation Mechanisms: Relevance to Use of Calpain Inhibitors as Drugs
MOLECULAR MECHANISM OF CALPAIN ACTIVATION
In the absence of calcium, the inactive calpain protein assumes a disc conformation due to interaction of an α-helix in domain I of the 80 kDa subunit anchored in a in cavity of the domain VI of the 30 kDa subunit (Fig. 12).22, 53, 54 The interaction between domains I and VI, and interaction between the acidic loop of domain IIb and domain III, helps maintain the active site cleft in an open state (>10 angstroms). Critical active site residue cysteine-105 in domain IIa is separated from active site residues asparagine-286 and histidine-262 in domain IIb in inactive calpain. Each subdomain IIa and IIb can bind one calcium ion20 in non-EF-hand sites, and domain II is highly homologous between various species. Domain III resembles other C2-like domains with an acidic loop for binding calcium and attaching to membranes. C-terminal domains IV and VI contain 5 EF-hand Ca2+ binding structures. The 5th C-terminal EF-hand structure in each of domains IV and VI do not bind calcium but interact with each other to cause dimerization with the 30 kDa regulatory subunit.
Fig. 12.
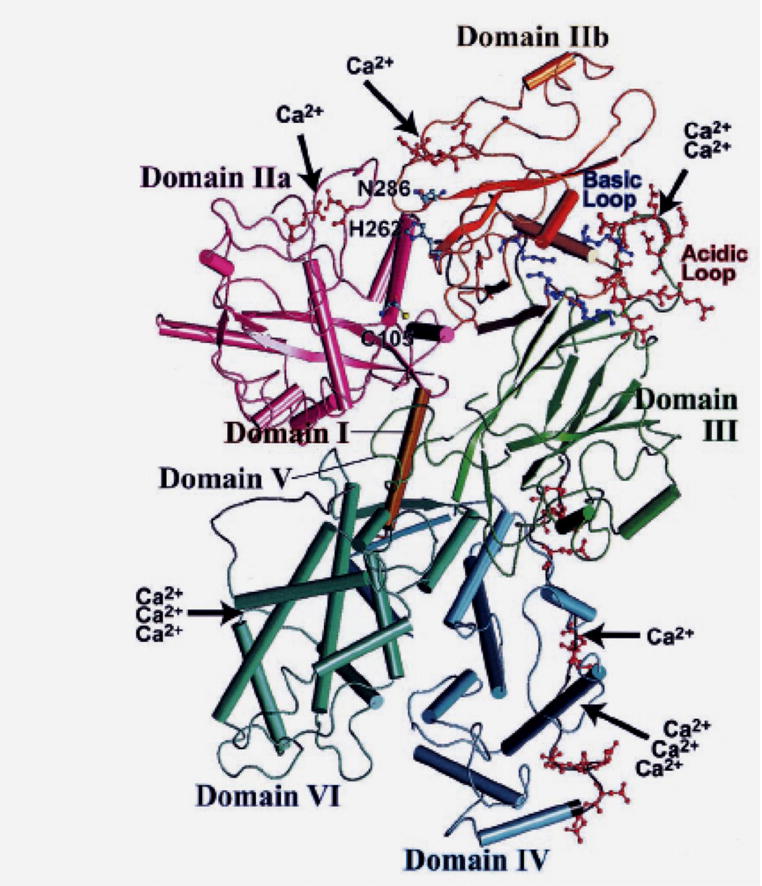
Three dimensional ribbon structure of calpain 2 without calcium (Reprinted with permission from Suzuki K et al54).
In addition to calcium, activity of calpains 1 & 2 is controlled in vivo by the endogenous tissue inhibitor calpastatin (CS), which does not inhibit cathepsin B or papain and is considered the most specific inhibitor of calpains 1 & 2. The complete form of CS contains four equivalent inhibitor domains each comprised of regions A, B, and C. A and C bind to regions IV and VI of the 80 and 30 kDa subunits while region B probably binds near the active site in a calcium-dependent manner. Addition of calcium causes dissociation of the 30 kDa subunit and therefore loss of CS binding. Calcium also promotes binding of the catalytic domain to phospholipids at membranes. Since binding to the 30 kDa domain is necessary for CS inhibition, the monomeric forms of calpains such as p94 are not inhibited by CS. A 27-residue peptide from region B, and a 12-residue middle conserved sequence, are also inhibitory and commercially available. Although very specific, neither CS nor its consensus inhibitory peptide is a useful drug because of ineffective membrane penetration.
During activation, the binding of calcium to the EF hand structures in domains IV and VI and to the acidic loop region in domain III cause loss of constraints on the conformation of the catalytic subunit. In a second step, binding of a calcium ion to each of the IIa and IIb subunits causes the critical active site residues cysteine-105 in domain IIa and asparagine-286 and histidine-262 in domain IIb to move closer together. The deep active site cleft is now hydrolytically active against substrates, as well as autolytic against the N-termini regions of domains I and V. Thus, calcium produces a functional active site, allows calpain to escape CS inhibition, promotes translocation to membranes, lowers the activation requirements by association with membrane phospholipids, and allows N-terminal truncation of the autolytic fragments on the 80- and 30 kDa subunits.
DOCKING OF CALPAIN INHIBITORS TO THE ACTIVE SITE OF CALPAIN
The calpain active site is also gated by two flexible loops on each of side of the active site cleft in sequences 69–82 (domain IIa) and loop 251–261 (in domain IIb).26 It is speculated that these loops mold around substrates and inhibitors and that sequence variations in the loops confer substrate specificity to different calpain isoforms. Interestingly, the loop in domain II of p94 is the unique insertion region known as ISI, which acts an internal propeptide and occludes the active site to both substrates and inhibitors. Addition of calcium promotes autolysis to remove the blocking peptide. The fragments of the core protein remain tightly associated, but the active site is now exposed.
Numerous calpain inhibitors have been developed over the last 35 years.15 Most alkylate the active site cyteine-105, either a reversibly (aldehydes) or irreversibly (epoxy groups). They include natural inhibitors such as leupeptin (Ac-Leu-Leu-Arg-H) and E-64 (trans epoxysuccinly-L-leucylamido-4-gaunidino-butane) and synthetic inhibitors such as calpeptin (benzyloxycarbonyl-Leu-nLeu-H) and SJA-6017 [N-(4-fluorophenylsulfonyl)-L-valyl-L-leucinal].18 Because of the similarity of the active site of calpain with other cysteine proteases, such as cathepsins B & L, no totally specific, cell permeable calpain inhibitor has yet been produced. An important method for development of inhibitors is to model docking of inhibitors to the known x-ray crystallography of the calpain active site (Fig. 13). The outcome of these studies is important for a virtual visualization of how inhibitors are bound to the active site, with the aim of producing biologically available, membrane permeable, specific calpain inhibitors that could be clinically useful. This method has been used to help develop SNJ-1945 [((1s)-1-((((1s)-1-benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-methylbutyl)carbamic acid 5-methoxy-3-oxapentyl ester],11, 42 containing a cyclic α-ketoamide warhead which is masked by a cyclopropane residue (Fig. 13). This masked warhead is more membrane soluble and less prone to react non-specifically with various biological amino and thiol groups, a problem with previous peptidyl inhibitors such as leupeptin, calpeptin, and SJA6017.
Fig. 13.
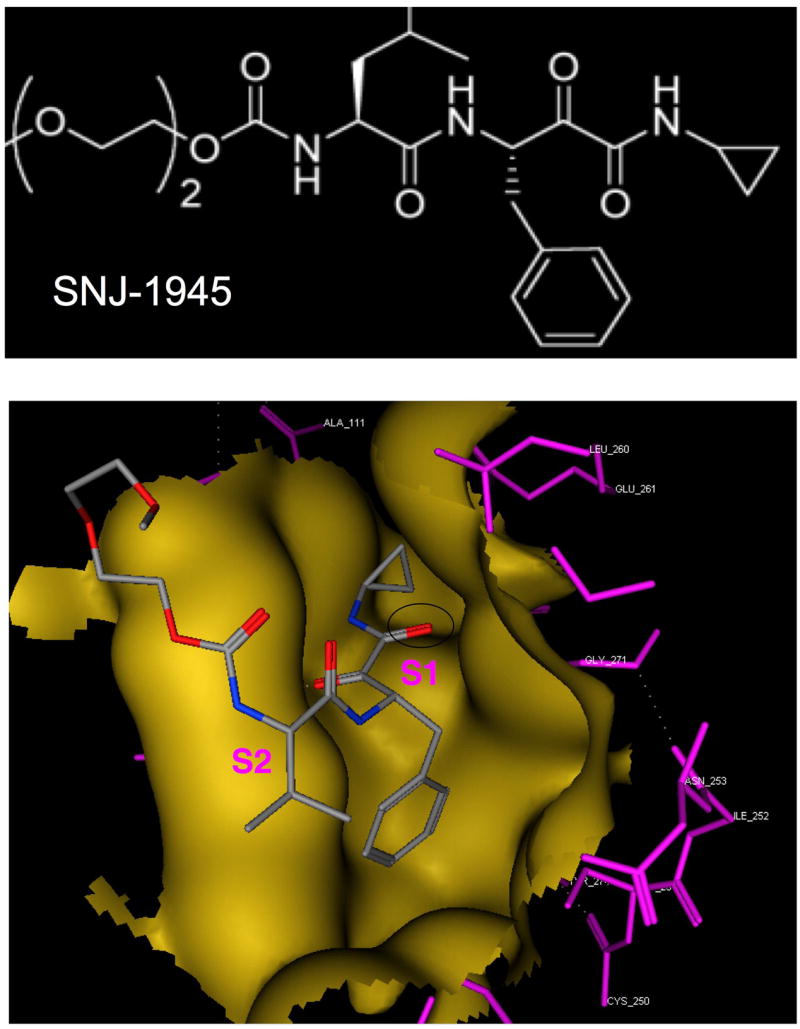
Molecular modeling of SNJ-1945 docking in the active site (gold) of calpain 1 (based on PDB#: 1TL9 - calpain 1 with calcium and leupeptin). Purple indicates the calpain 1 residues in the active site cleft; dotted lines are hydrogen bonds. In the SNJ molecule, the carbon atoms are colored gray, nitrogen atoms are blue, and oxygen atoms are red. The black oval indicates the ketoamide warhead in SNJ-1945, in relation to the S1 and S2 subsites.
Docking studies are supported by x-ray diffraction data using crystallized mini-calpains containing inhibitors in their active sites.13 Mini-calpains are recombinant polypeptides comprised of domains I and II. They are easier to produce by recombinate technology than full-length calpains, yet the active site conformations are similar when calcium is present. The crystal structure of SNJ-1945 bound in the active site pocket of mini-μcalpain I-II revealed these features: the activate site cysteine thiol reacting with the α-ketocarbonyl is stabilized by a strong hydrogen bond to His-272, the P3 diethylene glycol group shows weak electron density indicating high flexibility and minimal interactions with the binding site, hydrogen bonds connect the leucine P2 residue to Gly-271 and Gly-208 in the active site binding pocket, leucylyl side chains fit into S2 hydrophobic pocket, and the cyclopropylyl ring of SNJ-1945 extends into a sallow cleft on the S’ subsite. Interestingly, the gating loop in residues 251-261 of mini-calpain showed low electron density, indicating high flexibility of the loop and that cyclopropylyl group of SNJ-1945 displaces Glu-261 from the S’ site. Thus, the active site cleft is actually more open than expected.13 These studies have therapeutic potential because they show the pathway for development of inhibitors with S1’, S2’, and S3’ residues that more specifically fit the calpain active site cleft. With few exceptions, much of the previous work on calpain inhibitors has focused on P1 and P2 preferences, which are similar to those of other cysteine proteases such as cathepsins B and L. Such modeling studies have exciting potential for developing inhibitors that can very specifically inhibit over activation calpains in various retinal diseases.
Conclusion
This review has presented compelling evidence from the recent literature that activation of the calcium-activated proteases called calpains could be part of the mechanism for ganglion cell and photoreceptor cell death in retinas from experimental animals. The next important step is to test if calpain-induced proteolysis is also part of the mechanisms for human retinopathies, such as those associated with glaucoma and retinitis pigmentosa. Such work is in the initial stages using cultured cells and retinas from human and monkey eyes. Development of calpain-specific inhibitors is more advanced because of the detailed structures of calpain produced by x-ray crystallography and docking studies on the binding of inhibitors into the active site of calpain. The next challenges with inhibitors are to provide efficient drug delivery systems to retina and to provide clinical efficacy and safety studies. This last challenge is daunting and must be tested carefully because calpains are involved in a number of normal retina cell processes. It is hoped that these challenges can be resolved favorably and that drugs such as SNJ-1945 could help relieve some of the suffering due to retinopathies.
Acknowledgments
Partially funded by NIH grant No. EY05786. Dr. Shearer is a paid consultant for Senju Pharmaceutical Co., Ltd., a company that may have a commercial interest in the results of this research and technology. Dr. Azuma is an employee of Senju Pharmaceutical Co., Ltd. These potential conflicts of interest have been reviewed and managed by the OHSU. We thank Marjorie Shih and Dr. Larry David at OHSU for help in with mass spectroscopy, and Dr. Hong Ma for the pictures in Fig. 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10- a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159–71. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- 2.Artal-Sanz M, Tavernarakis N. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 2005;579:287–96. doi: 10.1016/j.febslet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Azarian SM, King AJ, Hallett MA, et al. Selective proteolysis of arrestin by calpain. Molecular characteristics and its effect on rhodopsin dephosphorylation. J Biol Chem. 1995;270:24375–84. doi: 10.1074/jbc.270.41.24375. [DOI] [PubMed] [Google Scholar]
- 4.Azarian SM, Schlamp CL, Williams DS. Characterization of calpain II in the retina and photoreceptor outer segments. J Cell Sci. 1993;105:787–98. doi: 10.1242/jcs.105.3.787. [DOI] [PubMed] [Google Scholar]
- 5.Azarian SM, Williams DS. Calpain activity in the retinas of normal and RCS rats. Curr Eye Res. 1995;14:731–5. doi: 10.3109/02713689508998502. [DOI] [PubMed] [Google Scholar]
- 6.Azuma M, Fukiage C, David LL, et al. Activation of calpain in lens: a review and proposed mechanism. Exp Eye Res. 1997;64:529–38. doi: 10.1006/exer.1996.0234. [DOI] [PubMed] [Google Scholar]
- 7.Azuma M, Fukiage C, Higashine M, et al. Identification and characterization of a retina-specific calpain (Rt88) from rat. Curr Eye Res. 2000;21:710–20. [PubMed] [Google Scholar]
- 8.Azuma M, Sakamoto-Mizutani K, Nakajima T, et al. Involvement of calpain isoforms in retinal degeneration in WBN/Kob rats. Comp Med. 2004;54:533–42. [PubMed] [Google Scholar]
- 9.Bartus RT, Dean RL, Mennerick S, et al. Temporal ordering of pathogenic events following transient global ischemia. Brain Res. 1998;790:1–13. doi: 10.1016/s0006-8993(97)01414-5. [DOI] [PubMed] [Google Scholar]
- 10.Chang B, Hawes NL, Hurd RE, et al. Retinal degeneration mutants in the mouse. Vis Res. 2002;42:517–25. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 11.Chiu K, Lam TT, Ying Li WW, et al. Calpain and N-methyl-d-aspartate (NMDA)-induced excitotoxicity in rat retinas. Brain Res. 2005;1046:207–15. doi: 10.1016/j.brainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Chung CW, Song YH, Kim IK, et al. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–72. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 13.Cuerrier D, Moldoveanu T, Inoue J, et al. Calpain inhibition by alpha-ketoamide and cyclic hemiacetal inhibitors revealed by X-ray crystallography. Biochem. 2006;45:7446–52. doi: 10.1021/bi060425j. [DOI] [PubMed] [Google Scholar]
- 14.Das A, Garner DP, Del Re AM, et al. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res. 2006;1084:146–57. doi: 10.1016/j.brainres.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Donkor IO. A survey of calpain inhibitors. Curr Med Chem. 2000;7:1171–88. doi: 10.2174/0929867003374129. [DOI] [PubMed] [Google Scholar]
- 16.Doonan F, Donovan M, Cotter TG. Activation of multiple pathways during photoreceptor apoptosis in the rd mouse. Invest Ophthalmol Vis Sci. 2005;46:3530–8. doi: 10.1167/iovs.05-0248. [DOI] [PubMed] [Google Scholar]
- 17.Ethen CM, Reilly C, Feng X, et al. The proteome of central and peripheral retina with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:2280–90. doi: 10.1167/iovs.05-1395. [DOI] [PubMed] [Google Scholar]
- 18.Fukiage C, Azuma M, Nakamura Y, et al. SJA6017, a newly synthesized peptide aldehyde inhibitor of calpain: amelioration of cataract in cultured rat lenses. Biochim Biophys Acta. 1997;1361:304–12. doi: 10.1016/s0925-4439(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda S, Harada K, Kunimatsu M, et al. Postischemic reperfusion induces alpha-fodrin proteolysis by m-calpain in the synaptosome and nucleus in rat brain. Neurochem. 1998;70:2526–32. doi: 10.1046/j.1471-4159.1998.70062526.x. [DOI] [PubMed] [Google Scholar]
- 20.Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 21.Inoue J, Nakamura M, Cui YS, et al. Structure-activity relationship study and drug profile of N-(4-fluorophenylsulfonyl)-L-valyl-L-leucinal (SJA6017) as a potent calpain inhibitor. J Med Chem. 2003;46:868–71. doi: 10.1021/jm0201924. [DOI] [PubMed] [Google Scholar]
- 22.Khorchid A, Ikura M. How is calpain activated by calcium. Nat Struct Biol. 2002;9:239–41. doi: 10.1038/nsb0402-239. [DOI] [PubMed] [Google Scholar]
- 23.Kupina NC, Nath R, Bernath EE, et al. The novel calpain inhibitor SJA6017 improves functional outcome after delayed administration in a mouse model of diffuse brain injury. J Neurotrauma. 2001;18:1229–40. doi: 10.1089/089771501317095269. [DOI] [PubMed] [Google Scholar]
- 24.Kusakawa G, Saito T, Onuki R, et al. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. Biol Chem. 2000;275:17166–72. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- 25.Ma H, Shih M, Hata I, et al. Protein for Lp82 calpain is expressed and enzymatically active in young rat lens. Exp Eye Res. 1998;67:221–9. doi: 10.1006/exer.1998.0515. [DOI] [PubMed] [Google Scholar]
- 26.Moldoveanu T, Campbell RL, Cuerrier D, et al. Crystal structures of calpain-E64 and -leupeptin inhibitor complexes reveal mobile loops gating the active site. J Mol Biol. 2004;343:1313–26. doi: 10.1016/j.jmb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima E, David LL, Bystrom C, et al. Calpain-specific proteolysis in primate retina: contribution of calpains in cell death. Invest Opthalmol Vis Sci. 2006;47:5469–75. doi: 10.1167/iovs.06-0567. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima E, Walkup RD, Ma H, et al. Low activity by the calpain system in primate lenses causes resistance to calcium-induced proteolysis. Exp Eye Res. 2006;83:593–601. doi: 10.1016/j.exer.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima T, Fukiage C, Azuma M, et al. Different expression patterns for ubiquitous calpains and Capn3 splice variants in monkey ocular tissues. Biochim Biophys Acta. 2001;1519:55–64. doi: 10.1016/s0167-4781(01)00212-3. [DOI] [PubMed] [Google Scholar]
- 30.Nath R, Davis M, Probert AW, et al. Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem Biophys Res Commun. 2000;274:16–21. doi: 10.1006/bbrc.2000.3070. [DOI] [PubMed] [Google Scholar]
- 31.Nath R, Raser KJ, Stafford D, et al. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319:683–90. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nixon RA. Fodrin degradation by calcium-activated neutral proteinase (CANP) in retinal ganglion cell neurons and optic glia: preferential localization of CANP activities in neurons. J Neurosci. 1986;6:1264–71. doi: 10.1523/JNEUROSCI.06-05-01264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nixon RA, Saito KI, Grynspan F, et al. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer's disease. Ann N Y Acad Sci. 1994;747:77–91. doi: 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- 34.Oka T, Nakajima T, Tamada Y, et al. Contribution of calpains to photoreceptor cell death in N-methyl-N-nitrosourea treated rats. Exp Neurol. 2007;204:39–48. doi: 10.1016/j.expneurol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Oka T, Tamada Y, Nakajima E, et al. Presence of calpain-induced proteolysis in retinal degeneration and dysfunction in rat acute hypertension model. J Neurosci Res. 2006;83:1342–51. doi: 10.1002/jnr.20827. [DOI] [PubMed] [Google Scholar]
- 36.Oka T, Walkup RD, Tamada Y, et al. Amelioration of retinal degeneration and proteolysis in acute ocular hypertensive rats by calpain inhibitor ((1s)-1-((((1s)-1-benzyl-3-cyclopropylamino-2,3-di-oxopropyl) amino)carbonyl)-3-methylbutyl)carbamic acid 5-methoxy-3-oxapentyl ester. J Neurosci. 2006;141:2139–45. doi: 10.1016/j.neuroscience.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 37.Ono Y, Shimada H, Sorimachi H, et al. Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A. J Biol Chem. 1998;273:17073–8. doi: 10.1074/jbc.273.27.17073. [DOI] [PubMed] [Google Scholar]
- 38.Pang J, Cheng M, Haire SE, et al. Efficiency of lentiviral transduction during development in normal and rd mice. Mol Vis. 2006;12:756–67. [PubMed] [Google Scholar]
- 39.Paquet-Durand F, Azadi S, Hauck SM, et al. Calpain is activated in degenerating photoreceptors in the rd1 mouse. J Neurochem. 2006;96:802–14. doi: 10.1111/j.1471-4159.2005.03628.x. [DOI] [PubMed] [Google Scholar]
- 40.Persson H, Kawashima S, Karlsson JO. Immunohistochemical localization of calpains and calpastatin in the rabbit eye. Brain Res. 1993;611:272–8. doi: 10.1016/0006-8993(93)90513-m. [DOI] [PubMed] [Google Scholar]
- 41.Quiniou C, Sennlaub F, Beauchamp MH, et al. Dominant role for calpain in thromboxane-induced neuromicrovascular endothelial cytotoxicity. J Pharmacol Exp Ther. 2006;316:618–27. doi: 10.1124/jpet.105.093898. [DOI] [PubMed] [Google Scholar]
- 42.Robertson LJ, Morton JD, Yamaguchi M, et al. Calpain may contribute to hereditary cataract formation in sheep. Invest Ophthalmol Vis Sci. 2005;46:4634–40. doi: 10.1167/iovs.04-1291. [DOI] [PubMed] [Google Scholar]
- 43.Saatman KE, Bozyczko-Coyne D, Marcy V. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996;55:850–60. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Saido TC, Yokota M, Nagao S, et al. Spatial resolution of fodrin proteolysis in postischemic brain. J Biol Chem. 1993;268:25239–43. [PubMed] [Google Scholar]
- 45.Sakamoto YR, Nakajima TR, Fukiage CR, et al. Involvement of calpain isoforms in ischemia-reperfusion injury in rat retina. Curr Eye Res. 2000;21:571–80. [PubMed] [Google Scholar]
- 46.Sanvicens N, Cotter TG. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem. 2006;98:1432–44. doi: 10.1111/j.1471-4159.2006.03977.x. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki M, Kunimatsu M, Ozaki Y, et al. Medical Aspects of Proteases and Protease Inhibitors. Burke, VA: IOS Press; 1997. pp. 43–57. [Google Scholar]
- 48.Schoenwaelder SM, Kulkarni S, Salem HH, et al. Distinct substrate specificities and functional roles for the 78- and 76-kDa forms of mu-calpain in human platelets. J Biol Chem. 1997;272:24876–84. doi: 10.1074/jbc.272.40.24876. [DOI] [PubMed] [Google Scholar]
- 49.Sharma AK, Rohrer B. Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem. 2004;279:35564–72. doi: 10.1074/jbc.M401037200. [DOI] [PubMed] [Google Scholar]
- 50.Shiraishi K, Naito K, Yoshida K. Inhibition of calpain but not caspase protects the testis against injury after experimental testicular torsion of rat. Biol Reprod. 2000;63:1538–48. doi: 10.1095/biolreprod63.5.1538. [DOI] [PubMed] [Google Scholar]
- 51.Shirasaki Y, Miyashita H, Yamaguchi M. Exploration of orally available calpain inhibitors. Part 3: Dipeptidyl alpha-ketoamide derivatives containing pyridine moiety. Bioorg Med Chem. 2006;14:5691–8. doi: 10.1016/j.bmc.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Shirasaki Y, Miyashita H, Yamaguchi M, et al. Exploration of orally available calpain inhibitors: peptidyl alpha-ketoamides containing an amphiphile at P3 site. Bioorg Med Chem. 2005;13:4473–84. doi: 10.1016/j.bmc.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 53.Sorimachi H, Suzuki K. The structure of calpain. J Biochem (Tokyo) 2001;129:653–64. doi: 10.1093/oxfordjournals.jbchem.a002903. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, Hata S, Kawabata Y, et al. Structure, activation, and biology of calpain. Diabetes. 2004;53(suppl):12–18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki T, Okumura-No K, Ogura A, et al. Calpain may produce a Ca2+-independent form of kinase c in long term potentiation. Biochem Biophys Res Comm. 1992;189:1515–20. doi: 10.1016/0006-291x(92)90247-i. [DOI] [PubMed] [Google Scholar]
- 56.Tamada Y, Fukiage C, Daibo S, et al. Involvement of calpain in hypoxia-induced damage in rat retina in vitro. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:221–5. doi: 10.1016/s1096-4959(01)00489-4. [DOI] [PubMed] [Google Scholar]
- 57.Tamada Y, Nakajima E, Nakajima T, et al. Proteolysis of neuronal cytoskeletal proteins by calpain contributes to rat retinal cell death induced by hypoxia. Brain Res. 2005;1050:148–55. doi: 10.1016/j.brainres.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 58.Tsung PK, Lombardini JB. Identification of low-Ca2+- and high-Ca2+-requiring neutral proteases in rat retina. Exp Eye Res. 1985;41:97–103. doi: 10.1016/0014-4835(85)90098-3. [DOI] [PubMed] [Google Scholar]
- 59.Ueda Y, Fukiage C, Shih M, et al. Mass measurements of C-terminally truncated alpha-crystallins from two-dimensional gels identify Lp82 as a major endopeptidase in rat lens. Mol Cell Proteomics. 2002;1:357–65. doi: 10.1074/mcp.m200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 60.Yokota M, Saido TC, Tani E, et al. Three distinct phases of fodrin proteolysis induced in postischemic hippocampus. Involvement of calpain and unidentified protease. Stroke. 1995;26:1901–7. doi: 10.1161/01.str.26.10.1901. [DOI] [PubMed] [Google Scholar]
- 61.Yoshimura N, Tsukahara I, Murachi T. Calpain and calpastatin in porcine retina. Identification and action on microtubule-associated proteins. Biochem J. 1984;223:47–51. doi: 10.1042/bj2230047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zalewska T, Zablocka B, Saido TC, et al. Response of calpain to rat brain postdecapitative ischemia. Mol Chem Neuropathol. 1998;33:185–97. doi: 10.1007/BF02815181. [DOI] [PubMed] [Google Scholar]