Abstract
Mps1 is an upstream component of the spindle assembly checkpoint, which, in human cells, is required for checkpoint activation in response to spindle damage but not apparently during an unperturbed mitosis. Mps1 also recruits Mad1 and Mad2 to kinetochores. However, whether the enzymatic activity of Mps1 is required for these processes is unclear. To address this question, we established an RNA interference (RNAi) complementation assay. Repression of Mps1 triggers premature anaphase, often with unaligned or maloriented chromosomes. This phenotype is rescued by an RNAi-resistant wild-type Mps1 transgene but not by a catalytically inactive mutant. An analogue-sensitive allele, Mps1M602A, also rescues the RNAi-induced defect, but not when inhibited by the adenosine triphosphate analogue 1-NM-PP1. Thus, Mps1 activity does restrain anaphase during an unperturbed mitosis. Furthermore, although catalytically inactive Mps1 can restore kinetochore localization of Mad1, only the active kinase restores Mad2 localization. Thus, in human cells, Mps1 catalytic activity is required for spindle checkpoint function and recruitment of Mad2.
Introduction
The search and capture process by which kinetochores attach microtubules is stochastic; thus, the time taken from nuclear envelope breakdown (NEB) to alignment of the last kinetochore is highly variable (Rieder et al., 1994). However, sister separation at anaphase is a global event, triggered shortly after the anaphase-promoting complex (APC/C) initiates proteolysis of securin and cyclin B1 (Clute and Pines, 1999; Hagting et al., 2002). Consequently, maintaining accurate chromosome segregation requires that the APC/C is restrained until the last kinetochore stably attaches microtubules. This is achieved by an inhibitory network called the spindle assembly checkpoint (SAC; Musacchio and Salmon, 2007).
The SAC consists of kinetochore-bound sensors, including Bub1, Mad1, and Mps1; a signal transducer known as the mitotic checkpoint complex, comprising BubR1, Bub3, Mad2, and Cdc20; and an effector, namely the APC/C (Musacchio and Salmon, 2007). Bub1, BubR1, and Mps1 are protein kinases, and, although phosphorylation appears to be integral to the SAC (Nicklas et al., 1995), the specific roles of these kinases are far from clear. Indeed, kinase activity may not be required for checkpoint function itself; yeast strains harboring a Bub1 allele completely lacking the kinase domain are checkpoint proficient (Warren et al., 2002).
Mps1, originally identified as a regulator of spindle pole body duplication in Saccharomyces cerevisiae (Lauze et al., 1995), is also essential for SAC function (Weiss and Winey, 1996). Some studies have probed the requirement for Mps1 kinase activity in SAC signaling, but the emergent picture is complex. One study, which used kinase-dead and analogue-sensitive Mps1 alleles, showed that budding yeast Mps1 activity was required for SAC function when kinetochores fail to attach microtubules (Jones et al., 2005). However, a second study using the small-molecule inhibitor cincreasin demonstrated that Mps1 activity was required for checkpoint activation when attached kinetochores fail to come under tension (Dorer et al., 2005).
In vertebrates, the situation is equally complex. Consistent with a role in monitoring unattached kinetochores, reconstituting Xenopus laevis egg extracts with a kinase-dead Mps1 failed to restore the checkpoint (Abrieu et al., 2001). In this system, Mps1 is required for kinetochore recruitment of Bub1, Bub3, BubR1, Mad1, Mad2, and centromere protein E (Cenp-E), thus providing a possible mechanism for Mps1 function (Abrieu et al., 2001; Wong and Fang, 2006; Zhao and Chen, 2006). However, this is at odds with experiments in human tissue culture cells: although RNAi-mediated repression of Mps1 results in a failure to recruit Mad1 and Mad2, other checkpoint components such as Bub1, BubR1, and Cenp-E appeared unaffected (Liu et al., 2003).
Another surprising observation from RNAi studies is that Mps1 only appears to be required for SAC function after spindle damage and not during an unperturbed mitosis (Stucke et al., 2004; Schmidt et al., 2005). Similarly, inhibition of Mps1 with the nonspecific JNK inhibitor, SP600125, results in SAC override, but only in the presence of spindle poisons (Schmidt et al., 2005). In contrast, other vertebrate SAC components restrain mitosis in the absence of spindle damage (Musacchio and Salmon, 2007). Again, in contrast to the RNAi studies, SP600125 mislocalizes BubR1; its effect on Mad2 is unknown (Schmidt et al., 2005).
These observations are also complicated by the fact that Mps1 plays multiple roles. In addition to spindle pole body duplication (Fisk et al., 2004), Mps1 is required for kinetochore–microtubule interactions (Dorer et al., 2005; Jones et al., 2005), possibly via the phosphorylation of Dam1 (Shimogawa et al., 2006). In yeast, Mps1 promotes kinetochore biorientation by eliminating malorientations (Maure et al., 2007). Human Mps1 may serve a similar function by regulating the aurora B kinase via phosphorylation of the chromosome passenger Borealin (Jelluma et al., 2008).
In an attempt to reconcile these observations and dissect the role of Mps1 kinase activity in human cells, we established a complementation assay repressing endogenous Mps1 by RNAi followed by induction of RNAi-resistant Mps1 transgenes. Using time-lapse imaging of GFP-tagged chromosomes, we show that Mps1 kinase activity is required for SAC function during an unperturbed mitosis. Furthermore, we show that kinetochore localization of Mad2 but not Mad1 is acutely sensitive to the inhibition of Mps1 kinase activity.
Results and discussion
shRNA-mediated repression of Mps1
To define the role of Mps1 kinase activity in SAC function, we set up a complementation assay repressing endogenous Mps1 by RNAi while simultaneously expressing RNAi-resistant transgenes. To do this, we generated an anti-Mps1 antibody to monitor RNAi efficiency. This antibody detects a single protein of ∼95 kD and decorates prometaphase kinetochores, which is consistent with Mps1's known properties (Fig. S1, A and B; available at http://www.jcb.org/cgi/content/full/jcb.200712028/DC1). To inhibit Mps1, we screened a panel of short hairpin RNA (shRNA) vectors after transient transfection into HeLa cells. Immunoblotting and immunofluorescence identified one vector that efficiently repressed Mps1 (Fig. S1, C and D).
Quantitative immunoblotting indicated that Mps1 was repressed to <20% of its normal level (unpublished data). Because the transfections were typically ∼75% efficient, this residual protein is largely caused by the untransfected cells. Indeed, quantitation of pixel intensities in transfected cells reveals that kinetochore-bound Mps1 is reduced to <3% (Fig. S1 E). To determine whether this was sufficient to compromise Mps1 function, repressed HeLa cells were challenged with microtubule toxins for 18 h. While control cells arrested in mitosis, cells in the Mps1-RNAi population were in interphase, indicating SAC override (Fig. S1 F). Indeed, repression of Mps1 reduced the mitotic index twofold (Fig. S1 G), which is consistent with previous studies demonstrating that Mps1 is required for SAC function in response to spindle toxins (Stucke et al., 2002; Liu et al., 2003; Schmidt et al., 2005). Time-lapse microscopy confirmed that Mps1-deficient cells failed to arrest when challenged with the Eg5 kinesin inhibitor monastrol (Fig. S1 H). Despite extensive repression, we did not observe centrosome defects, allowing us to focus on Mps1's checkpoint functions.
Mps1 restrains anaphase during an unperturbed mitosis
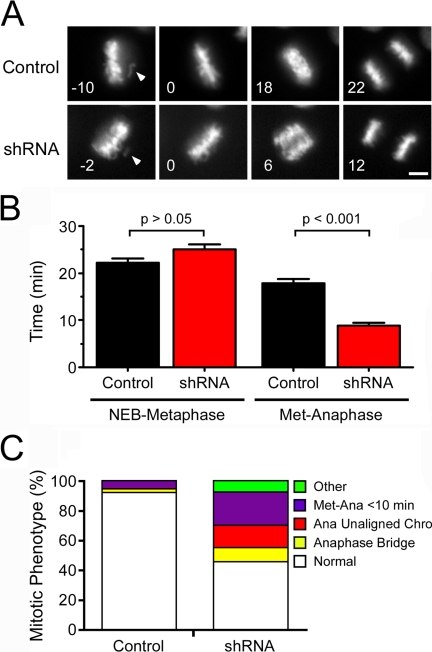
To study the role of Mps1 during an unperturbed mitosis, HeLa cells were cotransfected with shRNA vectors and a construct encoding GFP–histone H2B. Transfected cells were analyzed by time-lapse microscopy to determine (1) the time from NEB to alignment of the last chromosome (i.e., NEB to metaphase), (2) the time from alignment of the last chromosome to sister separation (i.e., metaphase to anaphase), and (3) the quality of the segregation process (i.e., scoring for signs of an abnormal mitosis such as anaphase with unaligned chromosomes or anaphase bridges). In controls, metaphase chromosome alignment took ∼22 min, and, after a delay of ∼18 min, anaphase initiated (Fig. 1, A and B). 92% of the controls completed mitosis normally, exhibiting a substantial delay (>10 min) after chromosome alignment (Fig. 1 C). In contrast, Mps1 repression affected both the timing and quality of chromosome segregation. Now, anaphase was initiated much quicker, occurring on average ∼9 min after metaphase (Fig. 1, A and B). Indeed, ∼23% of Mps1-deficient cells entered anaphase within 10 min of metaphase, whereas only 5% of controls did so (Fig. 1 C). Furthermore, whereas not a single control cell entered anaphase with an unaligned chromosome, ∼15% of the Mps1-deficient cells entered anaphase with one or more unaligned chromosomes. In addition, ∼9% exhibited anaphase bridges and ∼8% exhibited other mitotic defects. Indeed, only ∼45% of cells completed mitosis normally (Fig. 1 C). Thus, repression of Mps1 accelerates the onset of anaphase, often before all the chromosomes align at metaphase, indicating that Mps1 is required for SAC function during an unperturbed mitosis. The overall effect of Mps1 RNAi on mitotic timing was modest, reducing the mean time from NEB to anaphase from ∼41 to ∼36 min. In contrast, inhibiting mitotic checkpoint complex components dramatically accelerates mitosis, with NEB-anaphase taking <15 min (Meraldi et al., 2004). This suggests that Mps1 is not a component of the APC inhibitor but rather a kinetochore-based sensor responsible for generating the anaphase inhibitor.
Figure 1.
Mps1 is required for spindle checkpoint function during an unperturbed mitosis. HeLa cells cotransfected with control or Mps1 shRNA plus a GFP–histone H2B construct were analyzed by time-lapse microscopy. (A) Image sequences showing the time in minutes from when the last chromosome aligns (arrowheads) to anaphase onset. (B) Bar graph quantifying the time taken from NEB to metaphase and from metaphase to anaphase in control and Mps1-deficient cells. Values represent the mean and SEM (error bars) derived from at least 62 cells. (C) Bar graph quantifying mitotic abnormalities observed in control or Mps1-deficient cells. Bar, 5 μm.
Mps1 targets Mad1/Mad2 to kinetochores
Experiments with human cells indicate that Mps1 targets Mad1/Mad2 to kinetochores (Martin-Lluesma et al., 2002; Liu et al., 2003), whereas Xenopus egg extract data suggest that Mps1 also recruits Bub1, BubR1, and Cenp-E (Abrieu et al., 2001; Wong and Fang, 2006; Zhao and Chen, 2006). In our hands, in cells with virtually undetectable levels of Mps1 at kinetochores, localization of aurora B, Bub1, BubR1, Cenp-E, and Cenp-F appeared largely unaffected (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200712028/DC1; and not depicted). In contrast, kinetochore-bound Mad1 was significantly reduced, and Mad2 was largely absent (Fig. S2 A). To quantitate these effects, pixel intensities were normalized to the anticentromere antibody (ACA) signal and plotted as a percentage of the value derived from unrepressed cells (Fig. S2 B). Consistent with our qualitative assessment, Mps1 RNAi had marginal effects on aurora B, Bub1, BubR1, Cenp-E, and Cenp-F (Fig. S2 B). In contrast, the amount of Mad1 and Mad2 at Mps1-deficient kinetochores was reduced by ∼74% and ∼95%, respectively (Fig. S2 B). Thus, our shRNA-based observations are entirely consistent with previous siRNA observations and together indicate that Mps1 targets Mad1 and Mad2 to kinetochores (Martin-Lluesma et al., 2002; Liu et al., 2003).
Reconstitution of Mps1 function after shRNA-mediated repression
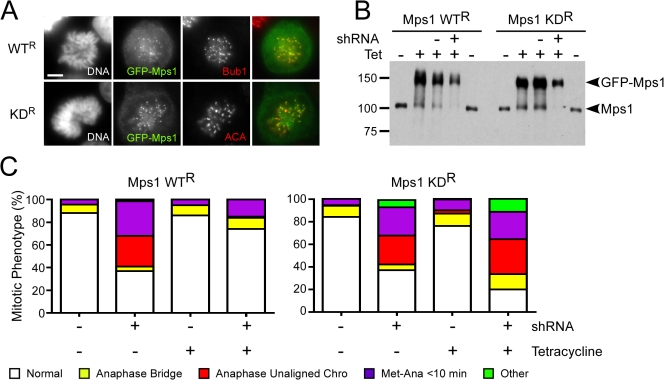
Next, we asked whether an RNAi-resistant Mps1 transgene could rescue the checkpoint defect observed after transfection of the shRNA vector. This would not only confirm that the SAC defect was caused by Mps1 repression, as opposed to an off-target effect, but it would also facilitate structure-function analyses. An Mps1 cDNA was rendered resistant to the shRNA sequence and cloned into an N-terminal GFP-tagged expression construct. Transfecting HeLa cells revealed GFP signal in the cytoplasm and at kinetochores (Fig. 2 A), which is consistent with Mps1's known localization. A stably transfected HeLa line was generated expressing the transgene under tetracycline control (Fig. 2 B). Transfection of the shRNA vector substantially reduced endogenous Mps1 levels, but the exogenous GFP-tagged protein was still abundant (Fig. 2 B). We then used time-lapse microscopy to determine the SAC status in Mps1-RNAi cells reconstituted with the wild-type transgene. As described above (Fig. 1), we scored as normal cells that delayed at metaphase for at least 10 min and then successfully completed chromosome segregation. Cells that initiated anaphase faster or with unaligned chromosomes, displayed lagging chromosomes in anaphase, or exhibited some other mitotic defect were considered abnormal. Although ∼90% of controls completed mitosis normally, only 37% of the Mps1-deficient cells did so (Fig. 2 C, left). Significantly, induction of the GFP-Mps1 transgene rescued the checkpoint defect, with 74% of the cells now completing a normal mitosis. Indeed, whereas 27% of the Mps1-deficient cells entered anaphase with one or more unaligned chromosomes (Fig. 2 C,red box), not one reconstituted cell entered anaphase with an unaligned chromosome. This demonstrates that the SAC defect is indeed caused by the repression of Mps1 as opposed to an off-target effect.
Figure 2.
Mps1 kinase activity is required for spindle checkpoint function. HeLa cells expressing tet-inducible RNAi-resistant GFP-tagged Mps1 fusions were transfected with shRNA vectors and analyzed by immunofluorescence microscopy, immunoblotting, and time-lapse microscopy. (A) Immunofluorescence images showing kinetochore localization of wild-type Mps1 (WTR) and the catalytically inactive D664A mutant (KDR). (B) Immunoblot showing repression of the endogenous Mps1 and induction of the RNAi-resistant GFP-tagged proteins. (C) Bar graph quantifying the mitotic phenotypes observed after repression of endogenous Mps1; whereas Mps1 WTR rescues the RNAi defect, Mps1 KDR exacerbates the phenotype. At least 132 cells were scored in each category. Bar, 5 μm.
Mps1 kinase activity is required for checkpoint function
To determine whether Mps1 activity is required for SAC function in human cells, we asked whether a catalytically inactive mutant could rescue the RNAi defect. We mutated the aspartic acid in subdomain VII of the catalytic domain to an arginine (Mps1 D664A; Hanks and Hunter, 1995), a mutation known to inactivate Mps1 (Lauze et al., 1995; Abrieu et al., 2001). When expressed as a GFP-tagged fusion, this mutant localized to the cytoplasm and kinetochores (Fig. 2 A). As described in the previous section, we generated stable HeLa cells where the kinase mutant was induced by tetracycline (Fig. 2 B). As before, these cells were transfected with shRNA constructs, the transgene was induced, and the cells were analyzed by time-lapse microscopy. Importantly, induction of the catalytic mutant failed to rescue the SAC defect (Fig. 2 C, right). Rather, the catalytic mutant exacerbated the defect, increasing the number of abnormal mitoses from 63 to 80%. Thus, cells in which the endogenous Mps1 has been replaced by a catalytically inactive mutant are checkpoint deficient, demonstrating that Mps1 kinase activity is required for SAC signaling in human cells.
Mps1 kinase activity is required for the kinetochore localization of Mad2
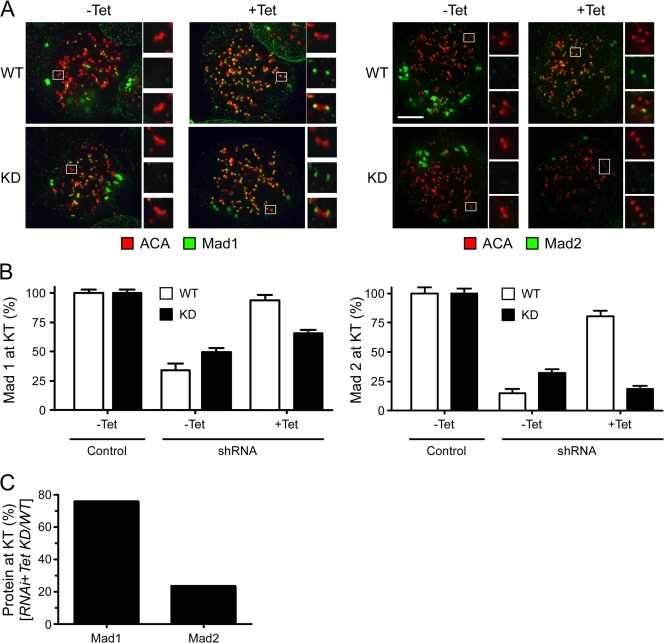
To further probe the role of Mps1, we asked whether the kinase mutant could restore Mad1/Mad2 localization. As described above (see section Mps1 targets Mad1/Mad2 to kinetochores), repression of Mps1 reduced kinetochore-bound Mad1 to ∼30% (unpublished data). Induction of wild-type Mps1 and the catalytic mutant restored Mad1 localization to ∼90% and ∼65%, respectively (Fig. 3, A and B). Thus, reconstitution of cells with a catalytically inactive Mps1 mutant results in substantial recruitment of Mad1 to kinetochores, suggesting that Mps1's role in Mad1 localization is only partially dependent on kinase activity. In contrast, kinetochore targeting of Mad2 was largely dependent on Mps1's catalytic activity; whereas the wild-type transgene restored kinetochore-bound Mad2 to ∼80%, the amount of kinetochore-bound Mad2 in cells expressing the catalytic mutant was only ∼19% (Fig. 3, A and B). To highlight this differential effect, we plotted the amount of kinetochore-bound protein restored by the catalytic mutant as a percentage of that restored by wild-type Mps1 (Fig. 3 C). Although the kinase mutant restored Mad1 levels to ∼75%, Mad2 levels were only restored to ∼25%. Thus, Mps1 kinase activity appears to play a key role in targeting Mad2 to kinetochores.
Figure 3.
Mps1 kinase activity is required for kinetochore localization of Mad2. HeLa cells expressing tet-inducible RNAi-resistant GFP-tagged Mps1 fusions were transfected with shRNA vectors, treated with nocodazole for 2 h, and analyzed to detect centromeres (ACA; red) and Mad1 or Mad2 (green). (A) Deconvolved image stacks showing that although Mps1 WTR restores Mad2 localization, Mps1 KDR does not. Enlargements of highlighted (boxed) kinetochores show the presence or absence of either Mad1 or Mad2. (B) Bar graph quantifying Mad1 and Mad2 pixel intensities at kinetochores normalized to the ACA signal. Values represent the mean and SEM (error bars) derived from at least 106 kinetochores from at least nine cells. (C) Bar graph plotting the amount of Mad1 and Mad2 at kinetochores in cells reconstituted with Mps1 KDR as a percentage of those reconstituted with Mps1 WTR. Bar, 5 μm.
Chemical genetic–mediated inhibition of Mps1 kinase activity
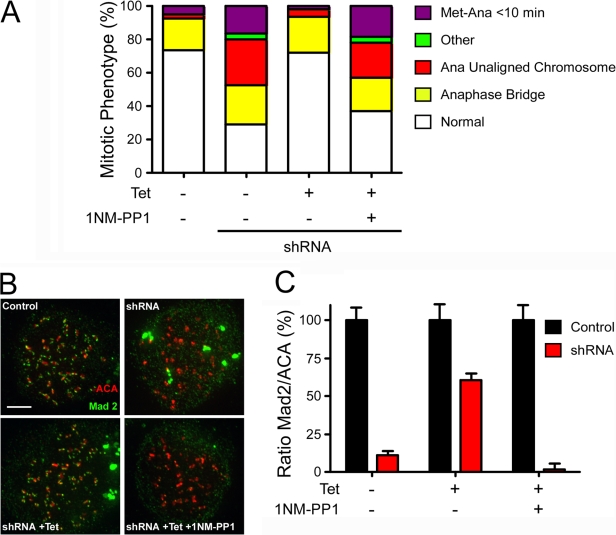
Although the aforementioned data are consistent with Mps1 kinase activity being required for Mad2 recruitment, we cannot rule out the possibility that the D664A mutation induces a structural effect that prevents Mad2 recruitment independent of any effect on Mps1's catalytic activity. Therefore, we turned to chemical genetics to selectively block Mps1 activity using an ATP competitive inhibitor. As described previously in budding yeast Mps1 (Jones et al., 2005), we mutated the gate-keeper residue, replacing methionine 602 with an alanine (Mps1M602A), thus rendering Mps1 sensitive to the ATP analogue 1-NM-PP1. We generated a stable cell line expressing GFP-tagged Mps1M602A under tetracycline control; cells were then cotransfected with vectors encoding Mps1 shRNA and GFP–histone H2B and analyzed by time-lapse microscopy to determine checkpoint status. Consistent with the aforementioned data, Mps1 repression inhibited the SAC, with only ∼30% of cells exhibiting a normal mitosis (Fig. 4 A). Induction of Mps1M602A rescued this defect, with ∼70% of cells completing mitosis normally, indicating that Mps1M602A possesses sufficient catalytic activity to sustain the checkpoint. Significantly, 1-NM-PP1 completely reversed the ability of Mps1M602A to rescue the checkpoint defect (Fig. 4 A). Furthermore, although Mps1M602A restored the kinetochore localization of Mad2, this was reversed by the addition of 1-NM-PP1 (Fig. 4, B and C). Thus, because Mps1M602A restores checkpoint function and Mad2 localization, the M602A mutation does not appear to induce a structural defect. However, because 1-NM-PP1 inhibits SAC function and Mad2 recruitment in cells reconstituted with Mps1M602A, the data demonstrate that Mps1's kinase activity is indeed essential for SAC signaling and Mad2 localization. Note that the kinetochore localization of Mad1 was restored in cells reconstituted with Mps1M602A despite exposure to 1-NM-PP1 (Fig. S3, A and B; available at http://www.jcb.org/cgi/content/full/jcb.200712028/DC1), again confirming that Mad1 recruitment is less dependent on Mps1 kinase activity.
Figure 4.
Chemical genetic inhibition of Mps1 kinase activity inhibits the checkpoint and Mad2 localization. HeLa cells expressing tet-inducible RNAi-resistant GFP-tagged Mps1 fusions were transfected with shRNA vectors and analyzed by time-lapse microscopy and immunofluorescence. (A) Bar graph quantifying the mitotic phenotypes observed after repression of endogenous Mps1; the ability of Mps1M602A to rescue the RNAi defect is reversed by 1-NM-PP1. (B) Projections of deconvolved image stacks of cells stained to detect centromeres (ACA; red) and Mad2 (green) showing that the ability of Mps1M602A to restore kinetochore localization of Mad2 is reversed by 1-NM-PP1. (C) Bar graph quantifying Mad2 pixel intensities at kinetochores normalized to the ACA signal. Values represent the mean and SEM (error bars) derived from at least 64 kinetochores from at least four cells. Bar, 5 μm.
Pharmacological inhibition of Mps1 kinase activity
The JNK inhibitor SP600125 overrides the SAC in human cells. SP600125 inhibits Mps1 in vitro, raising the possibility that the checkpoint override is induced by Mps1 inhibition (Schmidt et al., 2005). However, SP600125 inhibits kinetochore localization of BubR1, a phenomenon not phenocopied by Mps1 RNAi (Fig. S2; Martin-Lluesma et al., 2002; Liu et al., 2003). If SP600125 does indeed inhibit Mps1 activity in cells, our aforementioned observations predict that SP600125 should mislocalize Mad2. However, at concentrations at which SP600125 overrides the SAC (Fig. S3 C), we observed no obvious effect on the kinetochore recruitment of Mad2 (Fig. S3 D). Clarifying this discrepancy will require further analysis, but our data suggest that SAC override induced by SP600125 may not be the result of Mps1 inhibition.
Physiological role of Mps1 kinase activity
Using RNAi complementation and chemical genetics, we show that Mps1 kinase activity is required for SAC function during an unperturbed mitosis, and it is differentially required vis-à-vis kinetochore recruitment of Mad1 and Mad2. Because Mad1 is the kinetochore receptor for Mad2 (Musacchio and Salmon, 2007), Mps1 activity may be required for the Mad1–Mad2 interaction. However, this notion is at odds with experiments showing that the Mad1–Mad2 interaction can be recapitulated in vitro with recombinant proteins (i.e., in the complete absence of Mps1 activity) in a manner that reproduces Mad2's in vivo dynamics (Vink et al., 2006).
Although Mad1–Mad2 binding does not require Mps1 in vitro, it is conceivable that in cells, an Mps1-dependent phosphorylation event may enhance the Mad1-dependent recruitment of Mad2, possibly to amplify checkpoint signaling. Indeed, Mps1 can phosphorylate Mad1 in vitro (Hardwick et al., 1996). Mad2 has been shown to be phosphorylated (Wassmann et al., 2003), raising the possibility that it is a substrate of Mps1. We do not favor this possibility because phospho-Mad2 does not interact with Mad1 and is checkpoint incompetent, suggesting that Mad2 phosphorylation may be involved in checkpoint inactivation (Wassmann et al., 2003), which is inconsistent with the notion that Mps1 activity stimulates the SAC. The role for Mps1 activity may be indirect. Repression of two recently identified checkpoint components, Plk-interacting checkpoint helicase and Tao1 (a protein kinase), also dislodges Mad2 from kinetochores despite efficient Mad1 recruitment (Baumann et al., 2007; Draviam et al., 2007). Tao1 appears to be downstream of Mps1, as kinetochore localization of Mps1 does not require Tao1 (Draviam et al., 2007), so Mps1 may influence Mad2 recruitment via Tao1.
An alternative explanation may come from the fact that our observations are derived using an antibody against Mad2. Thus, it is conceivable that the effect observed in Mps1-deficient cells is not caused by the failure to recruit Mad2 but rather an inability to detect it. Indeed, Mad2 adopts either an open or closed conformation, O-Mad2 and C-Mad2, respectively, which are structurally very different (Musacchio and Salmon, 2007). When bound to Mad1, Mad2 adopts the closed conformation and serves as a template, recruiting O-Mad2 to first form a dimer. O-Mad2 is then handed over to Cdc20 and converted to the closed conformation. Therefore, a key question is whether any given anti-Mad2 antibody can recognize both Mad2 conformers. Interestingly, in interphase, Mad1 and Mad2 localize to nuclear pores (Campbell et al., 2001), as does Mps1 (Liu et al., 2003) and the Mad2 inhibitor p31comet (unpublished data). Because p31comet binds the Mad1–C-Mad2 core complex, thus preventing O-Mad2 recruitment (Mapelli et al., 2006), this suggests that the species present at the nuclear pore is a Mad1–C-Mad2/p31comet complex. Significantly, although we can detect Mad1 and p31comet at nuclear pores, we cannot detect Mad2 (unpublished data), suggesting that the anti-Mad2 antibody does not recognize C-Mad2. Thus, in cells lacking Mps1 kinase activity, C-Mad2 may well be bound to kinetochores but is simply not detectable by the antibody. Alternatively, because Mad2 oligomerizes when expressed in bacteria (Fang et al., 1998), the antibody, which was generated against bacterially expressed Mad2, may only recognize the Mad2 dimer. If the anti-Mad2 antibody recognizes only O-Mad2 or the Mad2 dimer, this, in turn, raises an interesting possibility: perhaps Mps1 kinase activity recruits O-Mad2 to the Mad1–C-Mad2 core complex, possibly by acting on p31comet as opposed to Mad1 and/or Mad2. Although we are currently testing this hypothesis, this issue highlights the need for tools that can distinguish the various conformers of Mad2 in a cell-based assay.
Materials and methods
Cell lines and drugs
hMps1 was PCR amplified from an expressed sequence tag (IMAGE No. 0511705), cloned into a pcDNA5/FRT/TO vector (Invitrogen) modified to contain an N-terminal GFP tag, and mutagenized (QuikChange; Stratagene) to create the D664A-, M602A-, and RNAi-resistant alleles (Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200712028/DC1). Vectors were then cotransfected into Flp-In TRex tetracycline transactivator HeLa cells with the Flp recombinase encoding plasmid pOG44 as described previously (Tighe et al., 2004). Hygromycin-resistant colonies were pooled and expanded, and transgene expression was induced with 100 ng/ml tetracycline (Sigma-Aldrich). Nocodazole and taxol (both obtained from Sigma-Aldrich) were used at final concentrations of 0.2 μg/ml and 10 μM, respectively. 1NM-PP1 and SP600125 (both purchased from EMD) were used at 10 μM. MG132 (EMD) was used at 20 μM.
shRNA
24 h before transfection, 4 × 104 cells were seeded into a 24-well plate. Vectors encoding shRNA sequences designed to target Mps1 (OriGene; Table S2, available at http://www.jcb.org/cgi/content/full/jcb.200712028/DC1) and a plasmid encoding a GFP–histone H2B fusion (Morrow et al., 2005) at a ratio of 9:1 were mixed with Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. DNA/lipid complexes were then added to the cells for 7 h. 24 h later, cells were replated onto coverslips, six-well plates, or chamber slides and analyzed 24 h later.
Molecular cell biology
The sheep polyclonal antibody SMP1.1 was raised and affinity purified against a GST fusion protein encoding amino acids 18–260 of Mps1 using methods described previously (Taylor et al., 2001). Immunoblotting was performed as described previously (Taylor et al., 2001; Tighe et al., 2004) using the antibodies shown in Table S3 (available at http://www.jcb.org/cgi/content/full/jcb.200712028/DC1). For immunofluorescence, cells grown on coverslips were washed in PBS, fixed in 1% formaldehyde for 5 min, quenched in glycine, pH 8.5, and then permeabilized with PBS plus 0.1% Triton X-100 (PBST). Incubation with primary antibodies (Table S3) for 30 min at room temperature was followed by washing with PBST. Incubation with secondary antibodies for 30 min at room temperature was followed by further washing, counterstaining with Hoechst 33358 (1 μg/ml in PBST), and mounting in 90% glycerol and 20 mM Tris-HCl, pH 8.0. Statistical analysis was performed using InStat version 3.0 (GraphPad Software, Inc.). For time-lapse imaging, cells were cultured in multiwell chamber slides (Laboratory Tek; Thermo Fisher Scientific) and analyzed as described previously (Morrow et al., 2005).
Cell cycle analysis
Mitotic index measurements were determined as described previously (Tighe et al., 2004). In brief, loosely attached and adherent cells were harvested and centrifuged onto microscope slides, fixed in 3% formaldehyde in PBS, stained with Hoechst, and mounted as described in the previous section. The state of chromosome condensation was used to score the cells as either mitotic or interphase.
Image acquisition and manipulation
Immunofluorescence images were acquired at room temperature on a restoration microscope (DeltaVision RT; Applied Precision) using a 100× NA 1.40 Plan Apo objective and the Sedat Quad filter set (Chroma Technology Corp.). The images were collected using a CCD camera (CoolSNAP HQ; Photometrics) with a Z-optical spacing of 0.2 μm. Raw images were then deconvolved using Softworx software (Applied Precision), and maximum intensity projections of these deconvolved images are shown in the results. Time-lapse microscopy was performed on a manual microscope (Axiovert 200; Carl Zeiss, Inc.) equipped with an automated stage (PZ-2000; Applied Scientific Instrumentation) and an environmental control chamber (Solent Scientific), which maintained the cells at 37°C in a humidified stream of 5% CO2. Imaging was performed using a 32× NA 0.40 LD A-Plan objective. Shutters, filter wheels, and point visiting were driven by MetaMorph software (MDS Analytical Technologies). Images were taken using a CoolSNAP HQ camera, whereas individual TIFF files were imported into Photoshop (Adobe) for printing or QuickTime (Apple) for videos.
Online supplemental material
Fig. S1 documents the Mps1 reagents used in this study, including the antibody, the GFP-tagged cDNA, and the shRNA constructs. Fig. S2 shows the effect Mps1 repression has on localization of various SAC components. Fig. S3 shows the effect 1-NM-PP1 has on Mad1 localization in cells reconstituted with the Mps1M602A allele. Tables S1, S2, and S3 present the PCR primers, shRNA sequences, and antibodies used in this study, respectively. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200712028/DC1.
Supplementary Material
Acknowledgments
We thank Pat Eyers for correcting the 5′ end of the Mps1 ORF, Bill Earnshaw for ACA antibodies, and Andrea Musacchio for helpful discussions.
A. Tighe was funded by AstraZeneca and a Wellcome Trust Value in People award, and S. Taylor is a Cancer Research UK Senior Fellow.
O. Staples' present address is Dept. of Surgery and Molecular Oncology, Ninewells Hospital, University of Dundee, Dundee DD1 9SY, Scotland, UK.
Abbreviations used in this paper: ACA, anticentromere antibody; APC/C, anaphase-promoting complex; Cenp, centromere protein; NEB, nuclear envelope breakdown; SAC, spindle assembly checkpoint; shRNA, short hairpin RNA.
References
- Abrieu, A., L. Magnaghi-Jaulin, J.A. Kahana, M. Peter, A. Castro, S. Vigneron, T. Lorca, D.W. Cleveland, and J.C. Labbe. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 106:83–93. [DOI] [PubMed] [Google Scholar]
- Baumann, C., R. Korner, K. Hofmann, and E.A. Nigg. 2007. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 128:101–114. [DOI] [PubMed] [Google Scholar]
- Campbell, M.S., G.K. Chan, and T.J. Yen. 2001. Mitotic checkpoint proteins HsMAD1 and HsMAD2 are associated with nuclear pore complexes in interphase. J. Cell Sci. 114:953–963. [DOI] [PubMed] [Google Scholar]
- Clute, P., and J. Pines. 1999. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1:82–87. [DOI] [PubMed] [Google Scholar]
- Dorer, R.K., S. Zhong, J.A. Tallarico, W.H. Wong, T.J. Mitchison, and A.W. Murray. 2005. A small-molecule inhibitor of Mps1 blocks the spindle-checkpoint response to a lack of tension on mitotic chromosomes. Curr. Biol. 15:1070–1076. [DOI] [PubMed] [Google Scholar]
- Draviam, V.M., F. Stegmeier, G. Nalepa, M.E. Sowa, J. Chen, A. Liang, G.J. Hannon, P.K. Sorger, J.W. Harper, and S.J. Elledge. 2007. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nat. Cell Biol. 9:556–564. [DOI] [PubMed] [Google Scholar]
- Fang, G., H. Yu, and M.W. Kirschner. 1998. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12:1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk, H.A., C.P. Mattison, and M. Winey. 2004. A field guide to the Mps1 family of protein kinases. Cell Cycle. 3:439–442. [PubMed] [Google Scholar]
- Hagting, A., N. Den Elzen, H.C. Vodermaier, I.C. Waizenegger, J.M. Peters, and J. Pines. 2002. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157:1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks, S.K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576–596. [PubMed] [Google Scholar]
- Hardwick, K.G., E. Weiss, F.C. Luca, M. Winey, and A.W. Murray. 1996. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 273:953–956. [DOI] [PubMed] [Google Scholar]
- Jelluma, N., A.B. Brenkman, N.J. van den Broek, C.W. Cruijsen, M.H. van Osch, S.M. Lens, R.H. Medema, and G.J. Kops. 2008. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 132:233–246. [DOI] [PubMed] [Google Scholar]
- Jones, M.H., B.J. Huneycutt, C.G. Pearson, C. Zhang, G. Morgan, K. Shokat, K. Bloom, and M. Winey. 2005. Chemical genetics reveals a role for Mps1 kinase in kinetochore attachment during mitosis. Curr. Biol. 15:160–165. [DOI] [PubMed] [Google Scholar]
- Lauze, E., B. Stoelcker, F.C. Luca, E. Weiss, A.R. Schutz, and M. Winey. 1995. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO J. 14:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S.T., G.K. Chan, J.C. Hittle, G. Fujii, E. Lees, and T.J. Yen. 2003. Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores. Mol. Biol. Cell. 14:1638–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli, M., F.V. Filipp, G. Rancati, L. Massimiliano, L. Nezi, G. Stier, R.S. Hagan, S. Confalonieri, S. Piatti, M. Sattler, and A. Musacchio. 2006. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 25:1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Lluesma, S., V.M. Stucke, and E.A. Nigg. 2002. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 297:2267–2270. [DOI] [PubMed] [Google Scholar]
- Maure, J.F., E. Kitamura, and T.U. Tanaka. 2007. Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr. Biol. 17:2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., V.M. Draviam, and P.K. Sorger. 2004. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 7:45–60. [DOI] [PubMed] [Google Scholar]
- Morrow, C.J., A. Tighe, V.L. Johnson, M.I. Scott, C. Ditchfield, and S.S. Taylor. 2005. Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J. Cell Sci. 118:3639–3652. [DOI] [PubMed] [Google Scholar]
- Musacchio, A., and E.D. Salmon. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393. [DOI] [PubMed] [Google Scholar]
- Nicklas, R.B., S.C. Ward, and G.J. Gorbsky. 1995. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol. 130:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., A. Schultz, R. Cole, and G. Sluder. 1994. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 127:1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M., Y. Budirahardja, R. Klompmaker, and R.H. Medema. 2005. Ablation of the spindle assembly checkpoint by a compound targeting Mps1. EMBO Rep. 6:866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa, M.M., B. Graczyk, M.K. Gardner, S.E. Francis, E.A. White, M. Ess, J.N. Molk, C. Ruse, S. Niessen, J.R. Yates III, et al. 2006. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr. Biol. 16:1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke, V.M., H.H. Sillje, L. Arnaud, and E.A. Nigg. 2002. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 21:1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke, V.M., C. Baumann, and E.A. Nigg. 2004. Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma. 113:1–15. [DOI] [PubMed] [Google Scholar]
- Taylor, S.S., D. Hussein, Y. Wang, S. Elderkin, and C.J. Morrow. 2001. Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J. Cell Sci. 114:4385–4395. [DOI] [PubMed] [Google Scholar]
- Tighe, A., V.L. Johnson, and S.S. Taylor. 2004. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J. Cell Sci. 117:6339–6353. [DOI] [PubMed] [Google Scholar]
- Vink, M., M. Simonetta, P. Transidico, K. Ferrari, M. Mapelli, A. De Antoni, L. Massimiliano, A. Ciliberto, M. Faretta, E.D. Salmon, and A. Musacchio. 2006. In vitro FRAP identifies the minimal requirements for Mad2 kinetochore dynamics. Curr. Biol. 16:755–766. [DOI] [PubMed] [Google Scholar]
- Warren, C.D., D.M. Brady, R.C. Johnston, J.S. Hanna, K.G. Hardwick, and F.A. Spencer. 2002. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell. 13:3029–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann, K., V. Liberal, and R. Benezra. 2003. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 22:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, E., and M. Winey. 1996. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, O.K., and G. Fang. 2006. Loading of the 3F3/2 antigen onto kinetochores is dependent on the ordered assembly of the spindle checkpoint proteins. Mol. Biol. Cell. 17:4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., and R.H. Chen. 2006. Mps1 phosphorylation by MAP kinase is required for kinetochore localization of spindle-checkpoint proteins. Curr. Biol. 16:1764–1769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.