Abstract
Recent proposals suggest that some interventions designed to improve language skills might also target or train selective attention. The present study examined whether six weeks of high-intensity (100 min/day) training with a computerized intervention program designed to improve language skills would also influence neural mechanisms of selective auditory attention previously shown to be deficient in children with specific language impairment (SLI). Twenty children received computerized training, including 8 children diagnosed with SLI and 12 children with typically developing language. An additional 13 children with typically developing language received no specialized training (NoTx control group) but were tested and retested after a comparable time period to control for maturational and test-retest effects. Before and after training (or a comparable delay period for the NoTx control group), children completed standardized language assessments and an event-related brain potential (ERP) measure of selective auditory attention. Relative to the NoTx control group, children receiving training showed increases in standardized measures of receptive language. In addition, children receiving training showed larger increases in the effects of attention on neural processing following training relative to the NoTx control group. The enhanced effect of attention on neural processing represented a large effect size (Cohen’s d = 0.8), and was specific to changes in signal enhancement of attended stimuli. These findings indicate that the neural mechanisms of selective auditory attention, previously shown to be deficient in children with SLI, can be remediated through training and can accompany improvements on standardized measures of language.
Section
Cognitive and Behavioral Neuroscience
Keywords: Attention, Selective attention, Event-related potentials, Language impairment, Attention training
1. Introduction
For some children, the development of oral language skills does not proceed as expected. Despite normal intelligence, this small but significant minority of otherwise typically developing children—roughly 7% of kindergarteners– experiences considerable difficulty with language acquisition [32,59]. The particular areas of linguistic weakness vary from one child to the next, and many of these children also experience subtle deficits in nonlinguistic aspects of sensory processing [for a review, see 32]. Given the heterogeneity of deficits in children with specific language impairment (SLI), a major challenge for intervention research is to identify what skills should be targeted by language training programs and what benefits can be expected to result from such training.
Recent proposals suggest that some interventions that have been developed to improve language skills might also train, or improve language in part by training, selective attention [19–21,24,52]. However, to date no studies have examined the effects of putative language training programs on measures of selective attention. The overarching goal of the present study was to examine whether intensive training with a computerized intervention program designed to improve language skills also leads to changes in the neural mechanisms of selective auditory attention previously shown to be deficient in children with SLI [51].
1.1 Attention Deficits in SLI
Current hypotheses about the underlying causes of SLI differ in the relative emphasis placed on linguistic versus sensory/cognitive mechanisms. For example, several researchers have proposed that SLI is characterized by deficits in aspects of syntax and morphology [13,38,39,61]. Although different researchers emphasize different linguistic processes, these theories hold in common the claim that SLI, at least in some children, is fundamentally a language-specific disorder; that is, a disorder specific to the linguistic system. In contrast, other researchers have proposed that SLI emerges from more domain-general deficits in sensory/cognitive mechanisms, such as reduced speed of processing or difficulty integrating rapidly presented auditory stimuli [6,32,35,55]. Such domain-general deficits are proposed to have particularly profound consequences for those aspects of language that place heavy processing demands on less perceptually salient phonemes and morphemes [32,33].
Consistent with the hypothesis that domain-general deficits are characteristic of SLI, several recent behavioral studies have reported deficits in selective attention in children with disorders of language, including SLI and dyslexia [1–3,12,29,49]. These studies indicate that children with disorders of language have particular difficulty selectively attending to task-relevant stimuli when co-present task-irrelevant stimuli must be actively filtered. Importantly, the observed attention deficits generalize across both linguistic and nonlinguistic contexts in both the auditory and visual modalities, suggesting that the attention deficit implicated in children with disorders of language is both domain-general and multi-sensory.
1.2 Postulated Role of Attention in Interventions for Children with SLI
The range of deficits proposed to underlie SLI has given rise to interventions focusing on different aspects of linguistic or sensory/cognitive systems including grammar, phonological awareness, and rapid auditory processing. Although purely attention-based interventions have not been used with children with SLI, it has been proposed that some interventions for children with SLI may also influence selective attention [19–21,24,52]. This proposal has been most prominent in discussions of the Fast ForWord intervention products. The Fast ForWord – Language program, hereafter referred to simply as FFW, targets oral language skills through intensive, computer-based activities with acoustically modified speech and nonspeech sounds [53]. The program is based on the theory that language deficits arise from more basic perceptual deficits in processing, and specifically in processing sounds that are separated by brief durations or are themselves short in duration [53,54]. Children receiving the FFW intervention typically train with the software for 100 minutes per day, five days per week, for four to six weeks. To date, over half a million children in thousands of public schools across the United States have used the Fast ForWord programs [47].
Although FFW is typically considered an intervention targeting rapid auditory processing, the creators of FFW contend that the program also influences the domain-general systems of memory and attention.1 Other researchers have also suggested that when FFW is effective, it may work in part by training attention or other generalized mechanisms [19,24,52]. When FFW is implemented as intended, children spend sustained amounts of time (100 min/day) engaging with and attending to auditory stimuli. Thus, it is reasonable to assume, and some clinicians have speculated [19], that FFW training improves attention skills in children.
Recent reviews indicate that the research base for FFW remains controversial [e.g., see 19,21,60]. Studies of individual children receiving FFW training with one-on-one coaching indicate that gains in standardized assessments of language can approach or exceed one standard deviation in magnitude [18,34]. However, the particular areas of language improvement are inconsistent across children, and there is little evidence that language gains are related to performance measures on FFW tasks [7,16,18,34]. When FFW has been directly compared to other intervention programs (e.g., Lindamood Phoneme Sequencing Program, Laureate Learning Systems, Earobics), the gains from different interventions are generally indistinguishable [16,20,37,52]. Although there is great variability in the presence and magnitude of standardized test gains depending upon the study and particular implementation procedure, it is remarkable that training effects are seldom treatment-specific. It has been suggested [20,21,52] that the similar gains observed across FFW and other computer-based [16,37,52] and interpersonally-delivered [20,37] interventions might reflect the common influence of these interventions on selective attention.
A recent neuroimaging study provides preliminary support for the hypothesis that FFW may influence attentional systems [58]. In this study, twenty adolescents with dyslexia received Fast ForWord training through a private clinic. Before and after training, children completed a phonological processing task during functional magnetic resonance imaging (fMRI) scanning. Following training, the children with dyslexia showed increased recruitment of brain regions associated with phonological processing, as well as increased activation in the anterior cingulate, an area associated with attentional control [10]. The same changes were not observed in a typically developing, no-treatment control group retested after a comparable period of time. As the authors noted, the observed increased activation in the anterior cingulate could be an indication of changes in attentional skills related to FFW training. However, to our knowledge no studies have directly examined whether training with FFW influences the mechanisms of selective attention.
1.3 Electrophysiology of Selective Auditory Attention
Event-related brain potentials (ERPs) are changes in the electrical activity of the brain in response to specific events. ERPs can be recorded with surface electrodes placed on the scalp and, for over 30 years, have been used as a noninvasive method to examine the mechanisms of selective auditory attention in adult humans. In a classic selective auditory attention ERP paradigm, separate streams of auditory stimuli (e.g., tone pips) are presented to each ear [26]. Participants attend to one of the two streams to detect rare target events, and ERPs are recorded to standard (non-target) stimuli in the attended and ignored stream. A comparison of the ERPs elicited by stimuli in the attended and ignored streams reveals the effects of selective attention on sensorineural processing: A number of studies have reported that approximately 100 msec after sounds are presented, the first negative wave (N1) of the ERP is amplified (i.e., more negative) in response to stimuli in the attended relative to the ignored stream [26,27,62]. This early amplification likely results from the joint processes of signal enhancement of the attended stimuli and suppression of the competing stimuli presented in the ignored channel. Several studies have indicated that these early ERP attention effects have a spatial gradient [56], can select for non-spatial auditory dimensions [63], and are apparent in the visual modality, as well [25]. This ERP attention effect is associated with improved behavioral performance on detection tasks, as measured by response accuracy, reaction time, and d-prime [36,40,50,56,57].
We have recently conducted studies using similar ERP paradigms with children [14,46]. In these studies, separate narrative stories are presented to each ear, and ERPs are recorded to probe stimuli superimposed on the attended and ignored story. Although children show a broad positivity (rather than an N1) in response to probe stimuli approximately 100 msec after stimuli are presented, the broad positivity is amplified (i.e., more positive) with attention in children as young as three years of age [46]. Interestingly, unlike typically developing children, children with SLI do not show evidence of early attentional modulation in this paradigm, even when performing the task as directed [51]. Further, the deficits in SLI are linked specifically to reduced amplification of the neural response to probes in the attended channel (i.e., signal enhancement) rather than difficulties in suppression of responses to probe stimuli in the ignored channel (i.e., distracter suppression) [51].
Given the evidence that attention skills are compromised in children with SLI [1–3,12,29,49,51], any effect of FFW training on attention skills may be particularly beneficial to these children. The electrophysiological data reviewed above suggest that SLI is associated with specific deficits in the neural mechanism of early auditory signal enhancement. Thus, if FFW does influence the mechanisms of selective attention, these changes might be evident in electrophysiological indices of selective auditory attention.
1.4 Overview of the Present Study
The present study examined whether training with Fast ForWord, when delivered in a controlled laboratory setting by trained FFW coaches, would influence the neural mechanisms of selective auditory attention previously shown to be deficient in children with SLI. Before and after receiving FFW training, children completed an electrophysiological assessment of selective auditory attention. Children were cued to attend to one of two simultaneously presented children’s stories differing in content, location (a speaker located to their right or to their left), and narrator voice (male or female). ERPs were recorded to probe stimuli embedded in the attended and unattended stories. The difference in mean amplitude response to probe stimuli when attended versus unattended was compared from pre- to post-intervention. Children also completed the receptive and expressive subscales of the standardized Clinical Evaluation of Language Fundamentals -3 [CELF-3, 48] assessment before and after training.
We predicted that training with FFW would increase the effects of attention on early (100–200 msec post-stimulus onset) neural processing, and that these increases would exceed any changes observed in a no-contact control group retested after a comparable period of time. Further, changes were expected to result from increases in the amplitude of response to attended stimuli (i.e., improvements in signal enhancement), as this has been identified as the specific locus of deficits in attentional modulation in children with SLI [51]. We also predicted that FFW training would lead to gains in standardized measures of receptive and expressive language.
Two groups of children received FFW training: a group diagnosed with SLI and presenting with poor receptive language skills (n=8) and a group of typically developing children (n=12). The inclusion of the latter group provided a strong test of the hypothesis that training with FFW would also confer advantages to children without poor language or selective attention skills. A no-treatment control group of typically developing children (n=13) was also tested twice, at comparable time points, to control for any maturational or test-retest effects.
2. Results
Three dependent measures were examined: CELF-3 receptive language composite scores, CELF-3 expressive language composite scores, and the ERP index of selective auditory attention. Standard scores, which control for participants’ age, were used in all analyses of standardized test data. A schematic of the ERP experimental paradigm is provided in Figure 1. Table 1 presents demographic characteristics of the three groups of children (FFW-LI, FFW-TD, NoTx), separately for children with data available for the behavioral and ERP analyses. Changes in each dependent measure from pre- to post-testing were assessed by comparing the gain scores using a one-way ANOVA with three levels of the between-subject factor of Group (FFW-LI, FFW-TD, NoTx). Follow-up t-tests and contrasts were used to examine the nature of any differences among groups, as indicated below.
Figure 1.
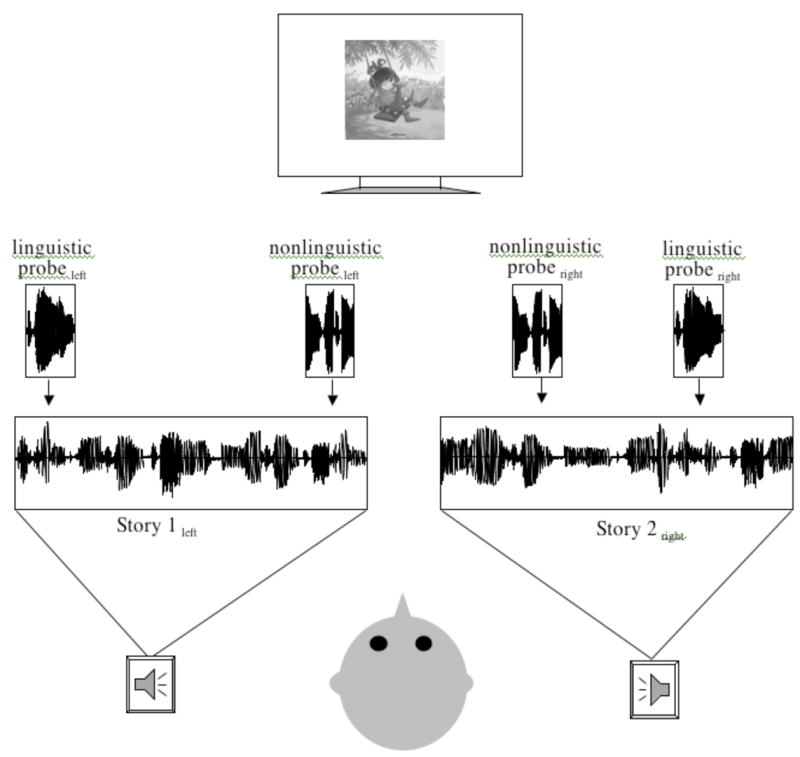
Schematic representation of the ERP selective auditory attention paradigm.
Table 1.
Demographic characteristics of participants. Standard deviation in parentheses.
| (a) Participants with standardized test data available
| ||||
|---|---|---|---|---|
| FFW-LI | FFW-TD | NoTx Control | P | |
| N | 7 | 9 | 13 | - |
| # Male / # Female | 5 / 2 | 2 / 7 | 6 / 7 | - |
| Age | 7.5 (.7) | 7.6 (.7) | 7.7 (.7) | ns |
| Maternal Educationa | 4.7 (.5) | 5.0 (1.2) | 5.3 (.9) | <.01c |
| Familial SESb | 35 (13) | 42 (9) | 44 (12) | ns |
| (b) Participants with electrophysiological data available
| ||||
| FFW-LI | FFW-TD | NoTx Control | P | |
|
| ||||
| N | 7 | 9 | 11 | - |
| # Male / # Female | 5 / 2 | 4 / 5 | 5 / 6 | - |
| Age (in years) | 7.2 (.9) | 7.6 (.8) | 7.8 (.8) | ns |
| Maternal Educationa | 5.1 (.9) | 5.0 (.7) | 5.7 (.8) | ns |
| Familial SESb | 39 (16) | 42 (10) | 42 (12) | ns |
Level of maternal education measured using categories from the Hollingshead Index of Social Status (Hollingshead, 1975). Values range from 1 (less than 7th grade) to 7 (graduate professional training). A score of 4 indicates high school graduation, and a score of 5 represents partial college.
Familial socio-economic status (SES) measured using the Hollingshead Index of Social Status. Scores on the index represent one of five social strata corresponding to upper (55–66), upper-middle (40–54), middle (30–39), lower-middle (20–29), or lower (8–19) class.
Post-hoc Tukey’s tests indicated that maternal education in the FFW-LI group was significantly lower than the NoTx control group (P < .01). No other between-group differences were statistically significant (all P > .1)
2.1 Progression Through FFW Activities
Despite the high level of attendance and multiple methods for progress monitoring, children’s actual performance on the FFW games was relatively poor. On average, the FFW-LI group achieved a 41% rate of completion across the FFW exercises, and the FFW-TD group a 55% rate of completion. This difference was not statistically significant. At the end of the training period, only one child in the FFW-LI group and two children in the FFW-TD group had achieved the program-set performance criterion of 80% completion on five of seven exercises.
2.2 CELF-3 Receptive Language Scores
Receptive language scores from pre- and post-testing for the three groups are presented in Table 2 and Figure 2. Critical to the hypothesis that FFW training would improve receptive language scores, results indicated differential change from pre- to post-testing across the three groups (main effect of Group, F(2,26) = 5.01, P < .05). Both FFW groups showed evidence of increased receptive language scores following training, with the FFW-LI group increasing +14.2 SS points (t(6) = −5.36, P < .01), and the FFW-TD group showing a similar trend for improvement with a +5.6 SS point increase (t(8) = −2.00, P < .1). In contrast, the NoTx control group showed a non-significant +0.8 SS change (t(12) = −0.30, P = .77). The combined FFW group (FFW-LI and FFW-TD) was directly compared to the NoTx control group using a contrast analysis. The FFW-LI and FFW-TD groups were each assigned weights of 0.5, and the NoTx control group was assigned a weight of −1.0. This contrast indicated that the combined FFW group’s receptive language scores improved significantly relative to the NoTx control group (t(26) = 4.32, P < .001). This change represented a Cohen’s d effect size of 0.91 relative to changes in the NoTx control group (based on a pooled standard deviation change score of 9.38 SS across the sample of 27 participants).
Table 2.
Standardized test and ERP selective attention scores at pre- and post-test for the FFW-LI, FFW-TD, and NoTx control groups. Standard test scores presented as standard scores. ERP selective attention scores presented as mean amplitude of the difference wave (Attended – Unattended) from 100–200 msec post-stimulus onset, indicating the effect of selective attention on sensorineural processing. Standard errors in parentheses.
| FFW-LI | FFW-TD | NoTx Control | |
|---|---|---|---|
| Receptive Language (SS) | |||
| PRE | 78.1 (3.4) | 107.8 (3.3) | 110.4 (3.0) |
| POST | 92.3 (3.8) | 113.4 (3.8) | 111.2 (2.9) |
| Change score | +14.2 | +5.6 | +0.8 |
| Expressive Language (SS) | |||
| PRE | 78.0 (4.1) | 104.1 (4.6) | 106.8 (4.3) |
| POST | 86.4 (4.4) | 106.1 (4.3) | 109.8 (3.2) |
| Change score | +8.4 | +2.0 | +3.0 |
| Selective attention (mean amp, μV) | |||
| PRE | 0.07 (0.2) | 0.63 (.4) | 0.52 (.3) |
| POST | 1.43 (0.4) | 1.36 (.5) | 0.54 (.3) |
| Change score | +1.4 | +0.7 | +0.0 |
Figure 2.
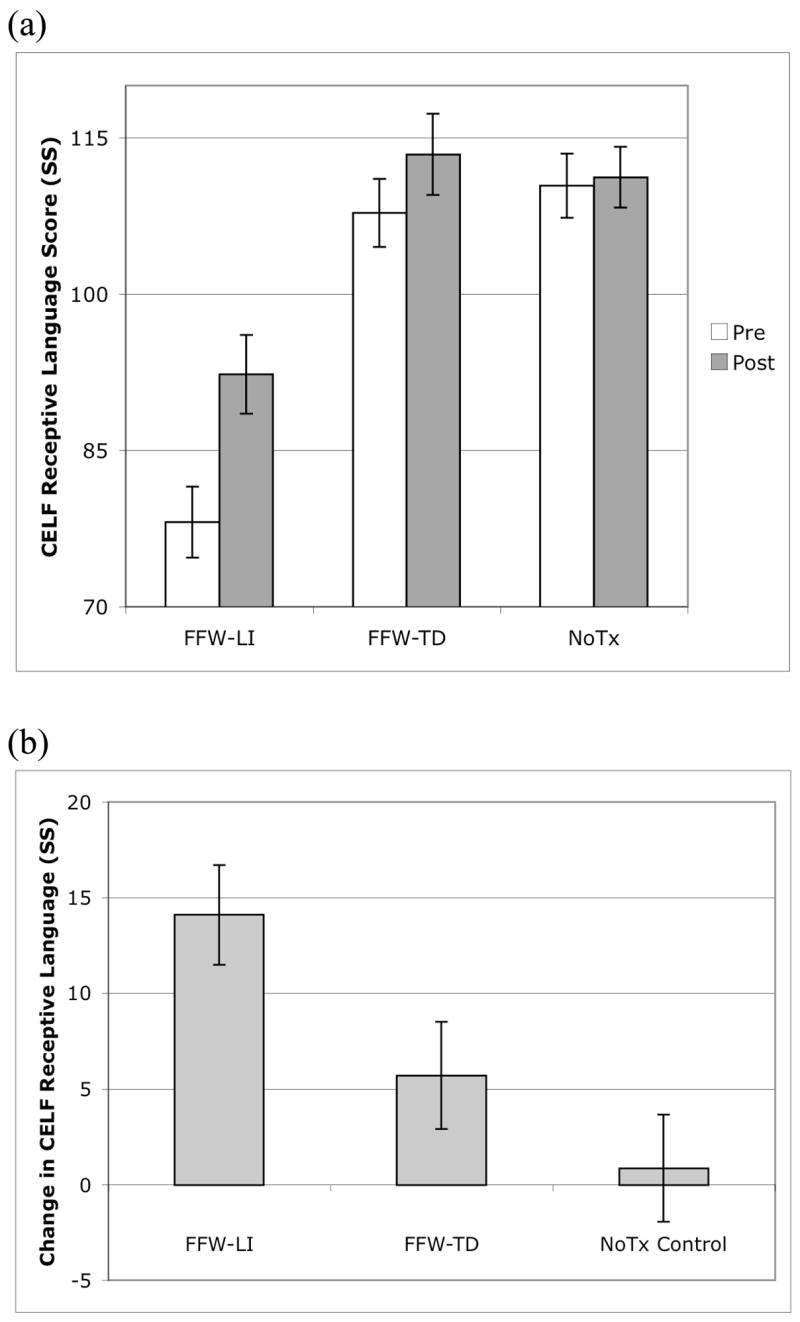
Scores on the CELF receptive language composite. All values are represented in standard score units. Error bars represent standard error of the mean. (a) Receptive language composite scores for the FFW-LI, FFW-TD, and NoTx control groups, separately for pre- and post-testing. (b) Change in receptive language composite score from pre- to post-testing for the FFW-LI, FFW-TD, and NoTx control groups. The FFW-LI group showed significant increases from pre- to post-testing, with the FFW-TD group showing a similar trend. The NoTx control group did not show changes from pre- to post-testing.
2.3 CELF-3 Expressive Language Scores
To examine whether the gains observed in receptive language generalized to expressive language measures, a comparable analysis was conducted on children’s scores on the CELF-3 expressive language composite. These data are presented in Table 2 and Figure 3. Contrary to the hypothesis that FFW training would improve expressive language scores, there was no evidence for differential change from pre- to post-test across the three groups (main effect of Group, F(2,26) = 1.05, P = .36). Although there was no evidence for differential change in expressive language scores as a function of training, across all children there was an overall +4.3 SS increase in expressive language scores from pre- to post-testing (t(28) = −2.56, P < .05).2
Figure 3.
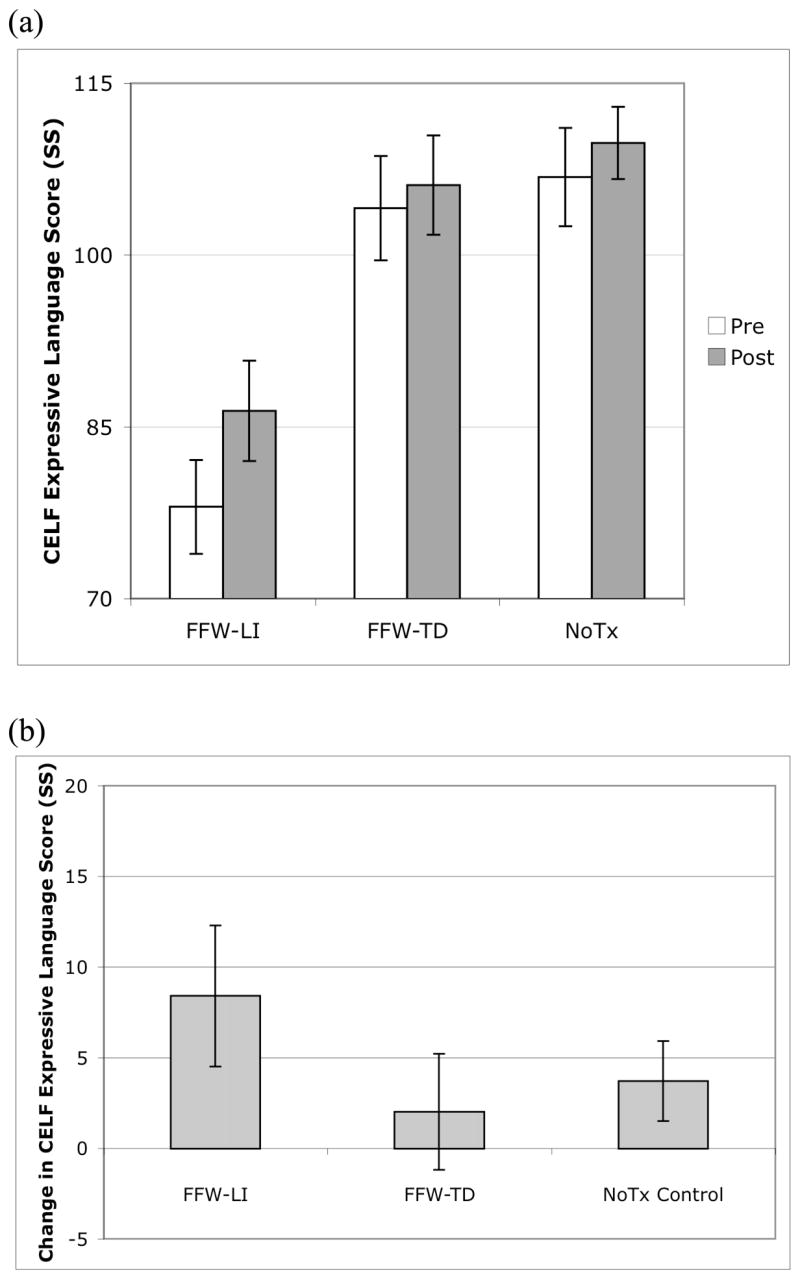
Scores on the CELF-3 expressive language composite. All values are represented in standard score units. Error bars represent standard error of the mean. (a) Expressive language composite scores for the FFW-LI, FFW-TD, and NoTx control groups, separately for pre- and post-testing. (b) Change in expressive language composite score from pre- to post-testing for the FFW-LI, FFW-TD, and NoTx control group. Gains were not significantly different across groups.
2.4 Electrophysiology of Selective Attention
Results from the standardized language tests suggested that FFW training leads to increases in receptive language scores in 6- to 8-year-olds. To assess whether changes in the neural mechanisms of selective auditory attention might accompany these changes in receptive language scores, children were also assessed using the ERP measure of selective auditory attention described above.
Grand average ERP waveforms for the FFW-LI, FFW-TD, and NoTx control groups are presented in Figure 4 for both pre- and post-intervention (or comparable time points for the NoTx control group) at representative electrode sites FC5 and FC6. These data are quantified in Table 2 and Figures 5 and 6. These figures and the analyses below collapse across the two probe types (linguistic and nonlinguistic) as preliminary analyses indicated that none of the results were influenced by this factor.
Figure 4.
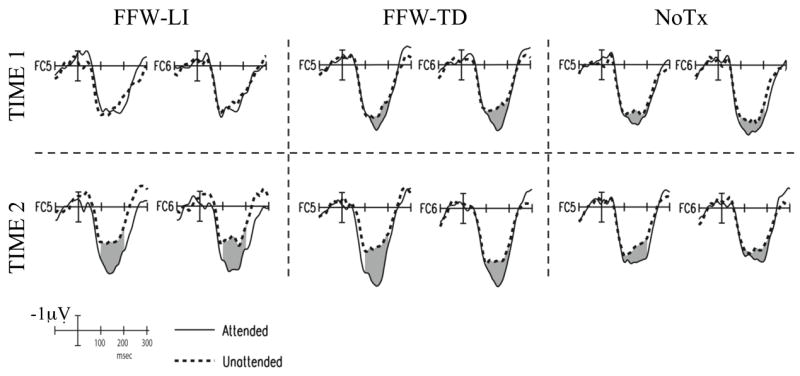
Grand average ERP waveforms from the selective auditory attention paradigm show the effects of attention on sensorineural processing. Top row shows data from time 1 (pre-intervention) and bottom row shows data from time 2 (post-intervention or, for no treatment controls, at retest following a comparable period of time) for each of the three groups: FFW-LI, FFW-TD, and NoTx control. ERPs to probes in the attended channel are shown in solid lines and ERPs to probes in the unattended channel are shown in dashed lines. Shading indicates the attention effect. Negative is plotted up.
Figure 5.
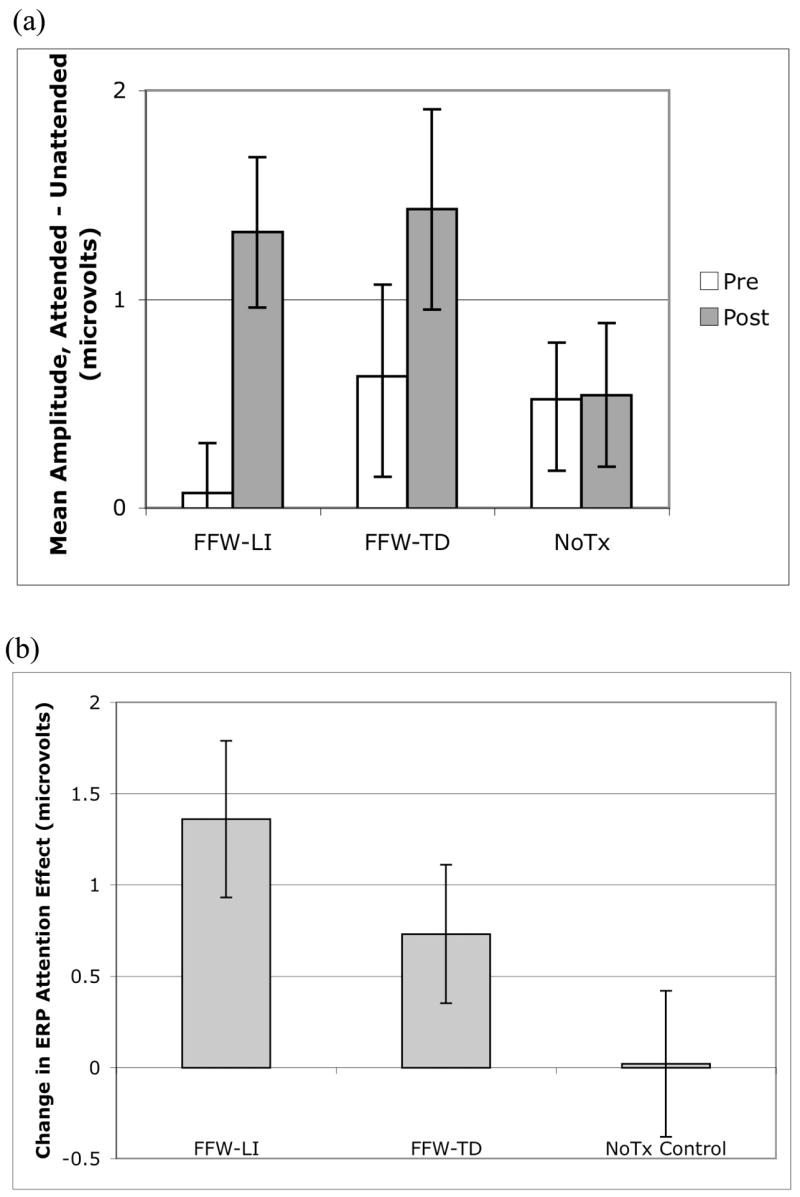
Magnitude of the ERP attention effect at pre- and post-testing for FFW-LI, FFW-TD, and NoTx control groups. The attention effect was measured as mean amplitude from 100–200 msec of the attended minus unattended channel. Error bars represent standard error of the mean. (a) ERP attention effect for the FFW-LI, FFW-TD, and NoTx control groups, separately for pre- and post-testing. (b) Change in attention effect from pre- to post-testing for the combined FFW-LI, FFW-TD, and NoTx control group. The FFW-LI group showed significant increases from pre- to post-testing, with the FFW-TD group showing a similar trend. The NoTx control group did not show changes from pre- to post-testing.
Figure 6.
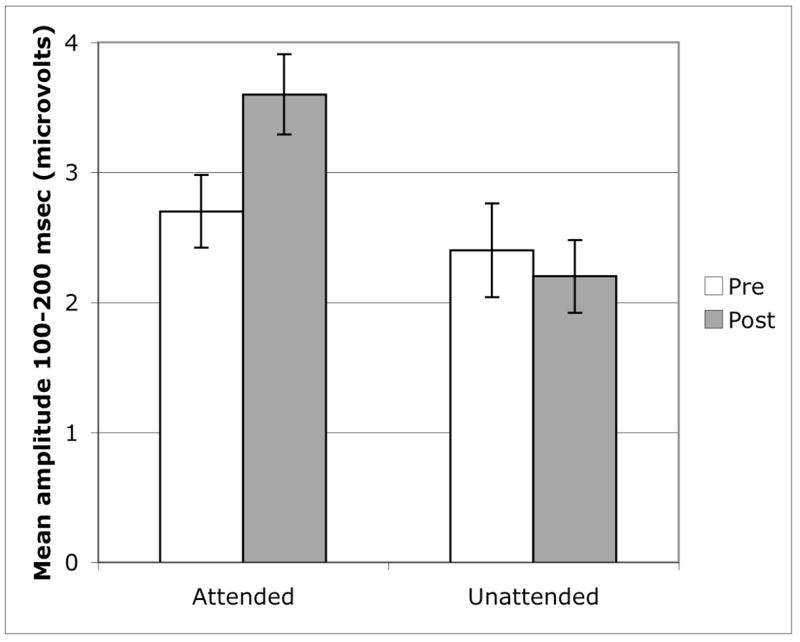
Change in mean amplitude of ERP response (100–200 msec) to attended and unattended probes in children receiving FFW training (combined group of children with SLI and typically developing children, FFW-LI and FFW-TD). Only changes in the response to attended stimuli were significant from pre- to post-testing.
The ERP data were first examined visually to assess qualitative patterns of change. As seen in Figure 4, at pre-testing the FFW-LI group exhibited similar responses to attended and unattended probes within the 100–200 msec time window, whereas the FFW-TD and NoTx control group showed larger responses to attended than unattended probes during this same epoch. At post-testing, the effects of attention on sensorineural processing (i.e., the difference in response to attended and unattended probes) appeared larger than at pre-testing from 100–200 msec in the groups receiving FFW training. In contrast, the data for the NoTx control group looked similar at pre- and post-test time points. Quantitative analyses, described below, confirmed these observations.
Critical to the hypothesis of the study, there was evidence for differential change in the effect of selective attention on early sensorineural processing across the three groups (main effect of Group, F(2, 24) = 2.66, P < .1). Both FFW groups showed evidence of increased effects of attention on sensorineural processing following training, with the FFW-LI group showing a +1.4 μV increase in the attention effect (t(6) = −3.16, P < .05), and the FFW-TD group showing a trend toward a significant +0.7 μV increase in the attention effect (t(8) = −1.91, P < .1). In contrast, the NoTx control group showed no change in the magnitude of the attention effect from pre- to post-testing (t(10) = −0.06, P = .96, mean increase +0.02 μV). The combined FFW group (FFW-LI and FFW-TD) was directly compared to the NoTx control group using a contrast analysis. The FFW-LI and FFW-TD groups were each assigned weights of 0.5, and the NoTx control group was assigned a weight of −1.0. This contrast indicated that the combined FFW group showed a significantly larger change in the magnitude of the attention effect from pre- to post-testing relative to the NoTx control group (t(24) = 3.37, P < .01). This change represented a Cohen’s d effect size of 0.81 relative to changes in the NoTx control group (based on a pooled standard deviation of change scores of 1.22 μV across the full sample of 27 participants).
Follow-up paired t-tests were used to assess whether the changes following training in the combined FFW group resulted from an increased response to attended stimuli (i.e., improved signal enhancement) or a decreased response to unattended stimuli (i.e., improved distractor suppression) or both. Responses to unattended stimuli did not change from pre- to post-FFW training (t(15) = 0.93, P = .37). In contrast, the neural response to attended stimuli did increase following FFW training (t(15) = −2.40, P < .05; see Figure 6), suggesting that improvement in signal enhancement is a specific mechanism underlying changes in selective auditory attention following FFW training.
Changes in the number of correctly answered comprehension questions about the attended story did not show differences among the FFW-LI, FFW-TD, and NoTx groups (main effect of Group, F(2, 24) = 0.50, P = .61). Across all three groups, there was a trend toward an increase in the number of attention questions correctly answered (t(26) = −1.78, P < .1, mean correct pre 88%, mean correct post 93%).
3. Discussion
The present study provides the first direct assessment of changes in the neural mechanisms of selective auditory attention following training with the Fast Forward – Language program. Following FFW training, children with SLI showed evidence for increased effects of selective auditory attention on neural processing during a language listening task, and typically developing children showed a similar trend. These increases were nearly one standard deviation in magnitude and were not observed in a no-treatment control group retested after a comparable period of time. Receptive language scores also showed increases following FFW training in children with SLI, with a similar trend for typically developing children, while such gains were not observed in the no-treatment control group. Taken together, these data suggest that intensive computerized training with FFW, when administered in a laboratory setting with high levels of monitoring by adult coaches, can increase the effects of selective auditory attention on neural processing during language listening tasks and improve receptive language skills.
3.1 Changes in Attention Following Training
Several previous behavioral studies have reported attention deficits in both linguistic and nonlinguistic tasks in children with language or reading impairments [1–3,12,29,49]. Our previous electrophysiological study traced this deficit to the early stages of auditory sensory processing that are typically modulated by attention and, more specifically, to the mechanism of signal enhancement [51]. The present study, using the same electrophysiological selective auditory attention paradigm, demonstrated that children with SLI showed increased effects of attention on early sensorineural processing during a language listening task following FFW training. When the mechanisms of signal enhancement and distractor suppression were examined separately, these changes were localized to improved signal enhancement. These data indicate that deficits in the early effects of selective auditory attention, at least within a language listening context, can be habilitated following FFW training. Further, this training appears to target the specific mechanism (i.e., signal enhancement) previously shown to be impaired in children with SLI.
The early ERP amplitude changes following FFW training observed in the present study are consistent with results from an fMRI study of children completing FFW training [58]. As mentioned above, this previous study reported increased activation of the anterior cingulate during a pseudoword reading task in readers with dyslexia following FFW training. The authors speculated that the increased anterior cingulate activation might be related to changes in aspects of attentional processing. The anterior cingulate has been associated with attentional control, particularly when task demands produce response conflict (e.g., during Stroop or flanker tasks) [8,10]. In contrast, the changes observed in the present paradigm, which specifically manipulated selective auditory attention, provide direct evidence for changes in selective attention following this computerized training.
Although marked changes in neural processing related to selective auditory attention following FFW training were evident in the present study, no behavioral measures of attention were included. Despite this, it seems reasonable to assume that the enhanced effects of attention on sensorineural processing reflect improved attention abilities, as several studies have indicated that an increased effect of attention on sensorineural processing is associated with improved performance on behavioral measures of attention [36,40,50,56,57]. However, to address this question directly, it will be important for future studies to examine the effects of FFW training on behavioral as well as neural indicators of attention.
It may seem surprising that changes in the mechanisms of selective auditory attention were observed following use of an intervention program designed to target language and auditory processing. However, several authors have proposed that attention may be trained by FFW [19–21,24,52]. Indeed, the very nature of FFW training, which involves large amounts of time spent attending to auditory stimuli, might be expected to improve selective auditory attention. The present results indicated increased effects of attention on sensorineural processing following FFW training, and these improvements generalized across both linguistic and nonlinguistic probe stimuli. This suggests that the changes in attention following FFW training may generalize beyond auditory linguistic stimuli. However, it is also true that the selective auditory attention paradigm used in the current study was embedded in a linguistic context (i.e., listening to an auditorily presented story). Thus, we cannot determine from these data alone whether the attentional enhancement observed can be generalized to nonlinguistic or visual domains. Despite this, the strong conclusions that can be drawn from the electrophysiological findings are that FFW training can influence selective attention in an auditory linguistic context, and that these changes are evident at the earliest stages of auditory processing previously shown to be modulated by selective attention in both typically developing children and adults [14,46].
These ERP data add to the growing body of evidence indicating considerable variability and modifiability in the neural mechanisms of selective attention. While impairments have been found in individuals with clinical disorders including SLI [51] and autism [5,57], enhancements in the effects of attention on sensorineural processing have been observed among individuals born congenitally deaf [4,36] or blind [40]. Recent studies also indicate behavioral improvements in attention following video game training in adults [22,23] and children [43]. The present data suggest that training programs can be effective at normalizing the brain systems important for the earliest effects of selective attention on sensorineural processing.
3.2 Changes in Language Following Training
Children receiving FFW training showed improvements on a standardized language assessment. Relative to the no-treatment control group, children receiving FFW training made large gains of 0.9 standard deviations on the standardized measure of receptive language. On the measure of expressive language, both the FFW and control groups made gains from pre- to post-test that were statistically indistinguishable. However, it should be noted that the mean gains in expressive language were two to four times larger in the FFW-LI group than in the other two groups of children.
The increase in receptive language scores following FFW training for children with SLI is consistent with results from previous case studies of children receiving individualized FFW instruction [18,20,34]. However, the gains in the current study are considerably larger than those reported in recent studies implementing FFW in small groups [37], under parental supervision [16], or in school-based randomized field trials [7,42]. Results of some of these latter studies have indicated no or minimal improvements in receptive language scores with FFW training. One critical difference among these studies may be in the implementation of FFW training. In the current study, the coach-to-student ratio was maintained from 1:1 to 1:2.5. An elaborate token economy system was implemented as coaches indicated that following the first few days of FFW, motivating children to continue was very difficult. Thus, the implementation of FFW in the present study may have provided a level of monitoring and encouragement needed to keep children engaged and on task.
Previous studies have also found no relationship between language outcomes and FFW performance measures including number of days attending training, number of minutes played per day, or percent completion on the activities [7,15]. In the present study, children did not achieve high levels of percent completion on the FFW games but still showed large gains in receptive language scores. This pattern of findings suggests that something other than progression through the FFW exercises may be responsible for the receptive language gains observed, for example, the daily interactions with the FFW coaches or improved attention (discussed below).
3.3 Relationship Between Improvements in Language and Attention
As noted above, the auditory attention measure used in the current study was embedded in a linguistic context (i.e., attending to an auditory story). As such, the present data provide strong evidence that selective auditory attention in a linguistic context is enhanced following computerized training, but the data cannot speak directly to whether aspects of domain general attention are improved.
With this caveat in mind, given the evidence for nonlinguistic attentional deficits in children with language and reading impairments, as well as the present results, it is worth considering whether FFW might improve language in part by training selective auditory attention. At a surface level, improved selective attention could enable a child to perform better under standardized testing conditions, without actually changing a child’s linguistic competence. However, improved selective attention might have more direct impacts on language. It has been noted that children with SLI have the most difficulty with linguistic forms that are less perceptually salient, such as the past tense –ed inflection, possessive s, or articles [32,33]. Improvements in the early neural mechanisms of selective attention may facilitate the perception and processing of these more vulnerable linguistic forms. Temporally selective attention, which allows for the preferential processing of information presented at specific time points in rapidly changing streams, has also been shown to modulate early auditory evoked potentials [30,31]. Skilled listeners may apply temporally selective attention during speech perception to help them process only the most relevant of the rapid acoustic changes. Indeed, recent electrophysiological studies indicate that speech segmentation is associated with early neural modulation similar to the effects of selective attention on auditory processing [44,45].
If FFW activities are training auditory attention, and if it is this attention training that mediates language improvement, it might be beneficial to design activities for children with SLI that target attention explicitly. For example, a recent study of attention training for typically developing preschoolers showed that short-duration attention training (five one-hour sessions) led to improvements in behavioral and neural measures of attention that also generalized to measures of non-verbal intelligence [43]. This suggests that training that targets attention explicitly might be an efficient means of improving not only attention, but also generalized intelligence and learning in domain-specific contexts. Consistent with this proposal, a recent study reported that adolescents with dyslexia showed greater gains following a 10 week writing intervention if they first received 10 weeks of attention skills training (as opposed to 10 weeks of reading fluency training) [11]. This suggests that prior training in attention might help children with language deficits benefit more from targeted instruction in an academic domain.
3.4 Limitations and Future Directions
As noted, the largest limitation in the present study was the absence of a nonlinguistic measure of selective attention. The electrophysiological improvements in selective attention generalized across both linguistic and nonlinguistic probe types, but the overarching context of the paradigm—listening to an auditory story—was undeniably linguistic. If the ERP attention changes observed in the current study were limited to linguistic contexts, then we would predict that following FFW training, gains would not be observed in nonlinguistic selective auditory attention tasks. We are currently developing a nonlinguistic selective auditory attention ERP paradigm that would be useful in testing this hypothesis directly.
Another limitation is that the control group in the present study differed in many ways from the FFW treatment groups. Whereas children in the control group received no intervention of any kind, children in the treatment group received exposure to the FFW materials, experience in a highly structured classroom setting, large amounts of attention from adults, and incentives for maintaining attention and engagement with the computerized activities. It could be a combination of these factors, or any factor in isolation, that accounts for the changes observed.
Although a typically developing, no-treatment control group was included in this study, difficulties with subject recruitment precluded the inclusion of a no-treatment control group of children with SLI. However, we do not expect that test-retest effects would be any larger or different in children with SLI than those observed in typically developing children. Although children with low initial scores on assessments are more likely to show increases on subsequent testing due to regression toward the mean, standardized language scores among children with SLI are known to be consistently low, even with repeated testing [32]. Further, estimates of CELF-3 test-retest effects for children with SLI can be gleaned from a recent controlled intervention study that did include an SLI no-treatment control group [16]. Twenty-seven children with SLI, whose age and language profiles were similar to children in the current study, received no intervention but were retested after nine weeks. On average, the children with SLI showed language changes of +3.8 and −0.1 SS for the receptive and expressive CELF composites, respectively [16]. These changes are similar to those observed in the typically developing children in the no-treatment control group in the present study and suggest that the large receptive language gains for the children with SLI in the present study cannot be explained by test-retest or regression to the mean effects.
Although children receiving FFW training in the present study showed gains in receptive language scores and electrophysiological measures of selective auditory attention immediately following FFW training, it is unclear whether either the attention or receptive language gains persisted after the intervention ended, as there was no longitudinal follow-up. One longitudinal study that followed children with dyslexia for two years after the end of FFW training reported that standard scores on language assessments regressed back toward pre-intervention levels by the end of the second follow-up year [28]. Another study reported that some, but not all, standardized test gains following FFW training had remitted three months after the end of intervention [34]. These findings suggest that the language gains observed in the present study might not be maintained over time, but the impacts of FFW training specifically on attention have not been studied long-term.
To our knowledge these are the first data showing that abnormalities in the mechanisms of selective auditory attention, as observed in the children with SLI, can be remediated with training. However, recent research indicates that a sensitive period might constrain modifiability of the earliest effects of attention on sensorineural processing [17]. Specifically, whereas congenitally blind adults show changes in early N1 attention effects [40], adults blinded later in life show changes only in later attention effects, several hundred milliseconds after the N1 peak [17]. In contrast, regardless of the age at which they became blind, blind adults show similar behavioral improvements in spatial selective attention [17]. In addition, training studies in adults indicate that behavioral measures of attention can show improvement, even in adults [22,23]. This pattern of results suggests that whereas certain behavioral indices of attention show change throughout life, these changes may be mediated by different neural mechanisms at different ages. Thus, it will be important for future studies to examine whether modifiability in the early neural mechanisms of selective auditory attention can be induced in adolescents or adults.
3.5 Conclusions
The results of the present study suggest that, when administered in small groups with a high coach-to-student ratio, training with FFW can be effective at improving the neural mechanisms of selective auditory attention during language listening tasks in children with SLI and, to some extent, in typically developing children. These changes in selective auditory attention occur alongside improvements in standardized measures of receptive language. These findings indicate that the neural mechanisms of early selective auditory atte tion in linguistic contexts are malleable in children and can be habilitated through training, offering a promising direction for future intervention research.
4. Experimental Procedures
4.1 Participants
A total of 33 children participated in this study, either as participants in FFW training or as members of a no-treatment control group. Twenty children received FFW training, including 8 children diagnosed with specific language impairment (FFW-LI) and 12 children with typically developing language (FFW-TD). An additional 13 children with typically developing language received no specialized training (NoTx control group) but were tested and retested at time points comparable to the FFW intervention groups.
All children were 6- to 8-year-old (average age 7.6 years) monolingual native English speakers with normal hearing (20dB at 500, 1000, and 4000 mHz) and normal or corrected-to-normal vision (Kindergarten Snellen). All children had non-verbal IQ scores above 80 SS as assessed by the Stanford-Binet 5 [41] or Columbia Mental Maturity Scale [9]. Children with known neurological disorders, taking psychoactive medications, or diagnosed with AD/HD were excluded from the study.
Potential participants were recruited from local schools and advertisements placed in the community. Children in the FFW-LI group were previously diagnosed as language impaired by private clinicians or through their schools and had current CELF-3 receptive language composites less than or equal to 88 SS (21st percentile). On average, the FFW-LI group had both receptive and expressive language scores in the 5th percentile (76 and 75 SS, respectively). Children in the FFW-TD and NoTx control groups had no history of language difficulties and CELF-3 receptive language composites greater than or equal to 94 SS (34th percentile). On average, the typically developing children had receptive and expressive language scores in the 73rd and 61st percentiles, respectively (109 and 104 SS, respectively).
Descriptive data for the three groups are shown in Table 1, separately for children with data available for analyses of standardized test and electrophysiological data.
4.2 FFW Administration
FFW training took place during the summer or, for a minority of students (n = 4), after school during the regular academic year. Participating children completed FFW training in small group settings (1–5 students) at university- or school-based computer laboratories. Trained FFW coaches monitored children’s progress and implemented a daily schedule and token economy system to encourage children’s engagement with the FFW activities. The coach-to-student ratio ranged from 1:1 to 1:2.5, depending upon the needs of the child. All coaches completed the online FFW training program and received certification as coaches. In addition, lead coaches received a half-day, in person training from a FFW representative. A behavior specialist provided further training to coaches across three half-day sessions.
Children trained with the Fast ForWord – Language commercial software. Each intervention session lasted for two hours, with 100 minutes dedicated to FFW activities. The remaining time was used for transition between activities, including snack and bathroom breaks. During the first week of FFW training, the initial 5–10 minutes of each session was used for a short “circle time” in which classroom expectations were reinforced. For staying on-task during the FFW sessions, children received “brain bucks” that could be used once per week to purchase small toys from a treasure box. Brain bucks were awarded at the discretion of FFW coaches as children continued or finished FFW activities, but were not related to FFW performance. Instead, brain bucks were used to encourage children to stay on task and focused on the computer exercises. At the completion of each FFW activity, children recorded the number of points earned on a personal progress chart, which each child took home at the end of each week. Each day that a child attended the FFW camp, his or her name was also entered into a drawing, held once per week, for a small prize.
Coaches monitored children’s performance using the FFW progress monitoring tools. In addition, all children’s computer stations were equipped with bifurcated headphones. Using these headphones, coaches could monitor children’s responses to the auditory stimuli and assist each child as needed in understanding the FFW games.
Children in the current study completed six weeks (30 training days) of FFW. FFW days missed due to child illness or family vacation were made up prior to post-testing. Although this was not possible in all cases, every child in the FFW intervention received at least 21 days of FFW training (mean = 29 days, range 21–30 days). Previous studies have used similar periods of training for FFW assessments [20,37].
4.3 Assessment Protocol
All children in both the FFW and NoTx control groups completed the same behavioral and electrophysiological assessment battery. The behavioral battery included the standardized CELF-3 receptive and expressive language composites [48]. The electrophysiological battery included a measure of selective auditory attention on which children with SLI have previously shown deficits [51]. Behavioral and electrophysiological assessments took place on separate days, with the standardized language assessments spread across one or two days depending upon the needs of the child. Pre-testing occurred within 30 days of the start of the intervention.3 Post-testing occurred within 30 days of completion of FFW training. Pre- and post-testing dates for the NoTx control group were selected to ensure similar pre- to post-test dates to the intervention groups, after controlling for FFW absences. On average, 85 days elapsed between pre- and post-testing. The number of days between pre- and post-testing did not differ across the three groups, either for electrophysiological (F(2, 24) = 0.24, P = .79) or standardized language (F(2, 26) = 1.02, P = .38) assessments.
All study procedures were approved by the University of Oregon Institutional Review Board. Informed consent was obtained from parents or legal guardians of participating children, and all children gave assent for participation.
4.4 Behavioral Test Administration
Standardized assessments were conducted at the University of Oregon or in a quiet room at the student’s school. As part of this assessment, both the receptive and expressive language composite scores were calculated for each child using relevant subtests from the CELF-3 [48]. The following subtests comprised each composite score:
Receptive Language Composite
Sentence Structure
The child chooses which of four visually presented pictures best represents a sentence provided orally by the examiner (e.g. The boy is being followed by the dog. / The boy who is sitting under the big tree is eating a banana.). Grammatical structures assessed include: negation, modification, prepositional phrase, indirect object, infinitive, verb phrase, relative clause, subordinate clause, interrogative, passive, indirect request, and compound.
Concepts & Directions
The child points to black and white shapes according to instructions by the experimenter (e.g., Point to the circle that is farthest from the big square. / Point to the big circle, the little square, and the black triangle. / Point to the big circle and the black triangle, but not the big or little squares.) Grammatical concepts assessed include: inclusion/exclusion, coordination, location, sequence, condition, and temporal order.
Word Classes
The child identifies which words go together out of a set of three or four words presented by the examiner (e.g. fish, frog, bird, horse, in which case the correct answer would be fish and frog, because these are both animals that can live in water). Semantic categories assessed include: part/whole, semantic class, synonym, and antonym.
Expressive Language Composite
Formulating Sentences
The child views a picture of a scene and must formulate a sentence using a target word provided by the experimenter (e.g., providing the word while, unless, or although). Grammatical structures assessed include: noun, verb, adjective, adverb, conjunction (coordinating, subordinating, correlative), conjunctive adverb, and phrases.
Word Structure
The child inserts proper words as prompted by the experimenter according to visually presented pictures (e.g., the experimenter says Here is one book. Here are two _____ (books) / Here is one foot. Here are two ____ (feet) / The children are playing a game. This is the game the children _____(played) / The boy is drawing a cat. This is the cat the boy _____ (drew)). Grammatical structures assessed include: phonological conditioning (regular plural, possessive noun, third person singular, regular past tense, future tense), irregular forms (irregular plural, irregular past tense), derivational forms (derivation of nouns, derivation of adjectives, comparative and superlative derivations), verb complex (auxillary+ing), and pronominalization (objective, possessive, demonstrative, subjective, and reflexive).
Recalling Sentences
The child repeats sentences presented orally by the examiner of increasing length and grammatical complexity (e.g. Did the girl catch the baseball? / The catcher caught the ball and the crowd cheered loudly. / The teacher in the room next door promised to water the plants during our summer vacation.) The grammatical structures assessed include: active declarative (with conjunction deletion, coordination, noun modification, subordinate clause, or relative clause), active interrogative, active negative interrogative, passive declarative (negative, with coordination, and with subordinate clause), and passive interrogative.
All standardized assessments were administered by either a certified speech language pathologist (SLP) or graduate students trained and supervised by a certified SLP. This training involved a 4-week, 50-hour comprehensive program in which students learned the assessment protocols, observed experienced testers, and completed practice test sessions with non-study adult and child volunteers. Prior to independent testing, graduate students demonstrated test administration accuracy. Continued reliability was monitored by a supervising SLP who observed multiple testing sessions in parallel through a one-way mirror and headphones connected to a suite of testing rooms. Offline, two independent coders scored all assessment protocols, and any discrepancies between coders were resolved through discussion with the supervising SLP. The standardized tests were administered by individuals uninvolved with FFW coaching and implementation.4 Standardized test data were available from 29 children, including 7 in the FFW-LI group, 9 in the FFW-TD group, and 13 of the NoTx controls.
4.5 Electrophysiological Testing
Electrophysiological testing was conducted at the University of Oregon. The ERP stimuli, tasks, and procedures were the same as those used in our previous studies of selective auditory attention in typically developing children and children with language impairment5 [46,51].
Participants were instructed to attend selectively to one of two simultaneously presented recordings of children’s narrative stories. The stories differed in location (left/right speaker), narration voice (male/female), and content. Small images from the attended story were presented on a central monitor (see Figure 1). Each participant attended to a total of four 2.5–3.5 minute stories (two from each speaker location). ERPs were recorded to linguistic and nonlinguistic probe stimuli (100 msec duration) embedded in the attended and unattended stories. The linguistic probe was the syllable /ba/, spoken by a female speaker (different from the female narrators) and then digitized and edited to 100 msec duration. The nonlinguistic probe was created by scrambling 4–6 msec segments of the /ba/ stimulus. This resulted in a broad-spectrum “buzz” that, while sounding nonlinguistic, preserved many of the acoustic properties of the linguistic probe. The two stories were played at 60 dB SPL (A-weighted), and the probe stimuli were played at 70 dB. The interstimulus interval (ISI) between probes was 200, 500, or 1000 msec, with an equal number of probes presented at each ISI. Across the stories, 201–252 trials of each of the four probe types (linguistic/nonlinguistic x attended/unattended) were presented. The number of children attending/not attending to each story set was roughly balanced across groups (FFW-LI, FFW-TD, and NoTx control) and testing sessions (pre- and post-testing).
During ERP testing, an adult experimenter sat next to the child at all times to administer instructions and monitor the child’s behavior. To encourage the child to pay attention, following each story the experimenter asked the child three basic two-alternative comprehension questions about the attended story (an answer of “I don’t know” was counted as an incorrect response). These questions were not designed as a sensitive assay of children’s language or attention abilities, but were instead included to reinforce to the child the goal of paying careful attention to a single story. After answering the three questions, the child heard another story concerning the same characters and read in the same voice. This procedure was repeated four times until the child had listened to four stories (attending twice to the left speaker and twice to the right speaker) and answered twelve comprehension questions.
ERP data were unavailable from six children for the following reasons: failure to visit the electrophysiology laboratory (one child in the FFW-LI group and one in the FFW-TD group), experimenter error during either pre- or post-test recording (one child in the FFW-TD and one NoTx control), or poor ERP data quality at either pre- or post-test (one child in the FFW-TD group and one NoTx control). Thus, the final ERP data set was comprised of 27 children, including 7 in the FFW-LI group, 9 in the FFW-TD group, and 11 NoTx controls.
4.6 Electrophysiological Recording Conventions
The electroencephalogram (EEG) was recorded from 29 tin electrodes mounted in an elastic cap (Electro-Cap International, Eaton, OH). Recording sites included: FP1/2, F7/8, FT7/8, F3/4, FC5/6, C3/4, C5/6, T3/4, CT5/6, P3/4, T5/6, TO1/2, O1/2, Fz, Cz, and Pz. Additional electrodes were placed at the outer canthus of each eye and on the cheek beneath the right eye to monitor eye movements and blinks, respectively. On-line, electrodes were referenced to the right mastoid. Off-line, electrodes were re-referenced to the average of the left and right mastoid. Electrode impedances were kept below 10KΩ for eye electrodes, 5KΩ for scalp electrodes, and 3KΩ for mastoid electrodes. The EEG was amplified 10,000 times using Grass 7P511 amplifiers (bandpass .01 to 100 Hz) and digitized online (250 Hz sampling rate). To reduce electrical noise in the data, a 60 Hz digital filter was applied off-line.
To remove artifacts due to blinks, muscle movement, or eye movement, individual artifact rejection parameters were selected for each participant. Parameters were selected based on inspection of the raw data to identify the smallest change in amplitude observed during a blink (based on shape of traces recorded from the eye electrodes and reversal in polarity above and below the eye) or eye movement (based on shape and distribution). Muscle movement was assessed based on channel blocking. Trials thus determined to be contaminated by eye or muscle movements were not included in further analyses. Following artifact rejection, there was an average of 149 trials in each of the four conditions (attend/unattend x linguistic/nonlinguistic probe types) at each time point. On average, the FFW-LI, FFW-TD, and NoTx control groups had 146, 150, and 152 trials per condition, which was not a significant difference (F(2, 24) = 0.06, P = .94). The number of available trials also did not differ by probe type, attention condition, or time of testing (smallest P = .53).
Separate ERPs were averaged to the probe stimuli in the attended and unattended channels. Mean amplitude measurements were taken from 100–200 msec post-stimulus onset, using the 100 msec immediately prior to probe stimulus presentation as a baseline. Measurements were taken for attended and unattended probes separately, as well as for the difference wave (Attended – Unattended), which more directly indexes the effect of attention on sensorineural processing. Analyses were conducted on these mean amplitude measurements averaged over 16 electrodes comprising the four most anterior rows of the electrode montage (F7/8, FT7/8, F3/4, FC5/6, C3/4, C5/6, CT5/6, T3/4). Analyses with the 100–200 msec time window over this set of electrodes have been used in our previous research with this paradigm and show reliable effects of selective attention in typically developing 6- to 8-year-old children [46,51].
Acknowledgments
We are grateful to the school districts, parents, and children who so generously gave of their time to participate in the study. This research was supported by NIH/NIDCD grant DC00481 to HJN. CS was supported by an NSF Graduate Research Fellowship and DC by NIH/NICHD NRSA Postdoctoral Fellowship (HD08598). The Fast ForWord licenses were provided free of charge by the Scientific Learning Corporation for the purposes of this independent research study. Paul Compton and Ray Vukcevich provided technical assistance during all aspects of the project. We also wish to thank the members of the Brain Development Lab who assisted with data collection and administrative aspects of different phases of the study, including Olivia Bagdad, Jeff Currin, Linda Heidenreich, Linda Hesketh, Petya Ilcheva, Brittni Lauinger, Nicole Makarenco, David Paulsen, Wendy Skendzel, David Paulsen, Lisa Stewart, Libbey White, and Brad Wible. Additionally, Mike Posner provided valuable feedback on a preliminary version of this manuscript.
Footnotes
For a description, see the Scientific Learning Corporation web site, http://www.scilearn.com/prod2/ffwd/main=home/hp#maps.
An anonymous reviewer noted that, although the ANOVA did not show a significant effect of group, the expressive language gains in the FFW-LI group were several times larger than those in the FFW-TD and NoTx control groups. Additional analyses examining each group separately showed the following results for change in expressive language standard scores from pre- to post-testing: FFW-LI group (t(6) = 2.15, P < .1), FFW-TD group (t(8) = −0.63 P = .55), and NoTx control group (t(12) = −1.69, P = .12).
For one child in the FFW-LI group, pre-testing occurred more than 30 days prior to the start of the intervention due to scheduling difficulties at the school’s computer laboratory.
Data from four children post-tested by examiners who were also involved in FFW coaching were removed prior to analysis. Gain scores for these children were three times as large as gain scores from children post-tested by examiners blind to experimental condition.
Two children in the FFW-LI group were tested using the auditory attention paradigm reported in Coch et al., 2005. This paradigm used the same structure and probe stimuli as the current study, but with different children’s stories and images. We have previously shown that the two paradigms produce the same pattern of ERP results in typically developing children (Coch et al., 2005; Sanders et al., 2006). Supplemental analyses conducted excluding these two children resulted in no changes in the main pattern of results reported in the text.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asbjørnsen AE, Bryden MP. Auditory attentional shifts in reading-disabled students: Quantification of attentional effectiveness by the Attentional Shift Index. Neuropsychologia. 1998;36:143–148. doi: 10.1016/s0028-3932(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 2.Asbjørnsen AE, Helland T, Obrzut J, Boliek C. The role of dichotic listening performance and tasks of executive functions in reading impairment: A discriminant function analysis. Child Neuropsychology. 2003;9:277–288. doi: 10.1076/chin.9.4.277.23521. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson J. Review of human visual development: Crowding and dyslexia. In: Cronly-Dillon J, Stein J, editors. Vision & visual dysfunction. Vol. 13. Vision & Visual Dyslexia; 1991. pp. 44–57. [Google Scholar]
- 4.Bavelier D, Tomann A, Hutton C, Mitchell T, Corina D, Liu G, Neville HJ. Visual attention to the periphery is enhanced in congenitally deaf individuals. The Journal of Neuroscience. 2000;20:1–6. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Bishop D, McArthur G. Individual differences in auditory processing in specific langyage impairment: A follow-up study using event-related potentials and behavioural thresholds. Cortex. 2005;41:327–341. doi: 10.1016/s0010-9452(08)70270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borman G, Benson J. A randomized field trial of the Fast ForWord Language computer-based training program. Madison: University of Wisconsin-Madison, Wisconsin Center for Education Research; 2006. Can brain research and computers improve literacy? (WCER Working Paper No. 2006–05) Retrieved October 9, 2006 from www.wcer.wisc.edu/publications/workingPapers/papers.php. [Google Scholar]
- 8.Botvinick M, Cohen J, Carter C. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Burgemeister B, Blum L, Lorge I. Columbia Mental Maturity Scale (CMMS), Harcourt Assessment. San Antonio; Texas: 1972. [Google Scholar]
- 10.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 11.Chenault B, Thomson J, Abbott RD, Berninger VW. Effects of prior attention training on child dyslexics’ response to composition instruction. Developmental Neuropsychology. 2006;29:243–260. doi: 10.1207/s15326942dn2901_12. [DOI] [PubMed] [Google Scholar]
- 12.Cherry R, Kruger B. Selective auditory attention abilities of learning disabled and normal achieving children. Journal of Learning Disabilities. 1983;16:202–205. doi: 10.1177/002221948301600405. [DOI] [PubMed] [Google Scholar]
- 13.Chiat S, Hirson A. From conceptual intention to utterance: A study of impaired output in a child with developmental dysphasia. British Journal of Disorders of Communication. 1987;22:37–64. doi: 10.3109/13682828709088687. [DOI] [PubMed] [Google Scholar]
- 14.Coch D, Sanders L, Neville H. An event-related potential study of selective auditory attention in children and adults. Journal of Cognitive Neuroscience. 2005;17:605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- 15.Cohen D, Ball DL. Instruction, capacity, and improvement (CPRE Research Report Series RR-43) Consortium for Policy Research in Education; Philadelphia: 1999. pp. 1–41. [Google Scholar]
- 16.Cohen W, Hodson A, O’Hare A, Boyle J, Durrani T, McCartney E, Mattey M, Naftalin L, Watson J. Effects of computer-based intervention through acoustically modified speech (Fast ForWord) in severe mixed receptive-expressive language impairment: Outcomes from a randomized field trial. Journal of Speech, Language, and Hearing Research. 2005;48:715–729. doi: 10.1044/1092-4388(2005/049). [DOI] [PubMed] [Google Scholar]
- 17.Fieger A, Roder B, Teder-Salejarvi W, Hillyard S, Neville H. Auditory spatial tuning in late-onset blindness in humans. Journal of Cognitive Neuroscience. 2006;18:149–157. doi: 10.1162/089892906775783697. [DOI] [PubMed] [Google Scholar]
- 18.Friel-Patti S, DesBarres K, Thibodeau L. Case studies of children using Fast ForWord. American Journal of Speech-Language Pathology. 2001;10:203–215. [Google Scholar]
- 19.Gillam R. Computer assisted language intervention using Fast ForWord: Theoretical and empirical considerations for clinical decision-making, Language, Speech. and Hearing Services in Schools. 1999;30:363–370. doi: 10.1044/0161-1461.3004.363. [DOI] [PubMed] [Google Scholar]
- 20.Gillam R, Crofford J, Gale M, Hoffman L. Language change following computer-assisted language instruction with Fast ForWrod or Laureate Learning Systems software. American Journal of Speech-Language Pathology. 2001;10:231–247. [Google Scholar]
- 21.Gillam R, Loeb D, Friel-Patti S. Looking back: A summary of five exploratory studies of Fast ForWord. American Journal of Speech-Language Pathology. 2001;10:269–273. [Google Scholar]
- 22.Green C, Bavelier D. Action video game modifies visual attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- 23.Green C, Bavelier D. Effects of action video games on the spatial distribution of visuospatial attention. Journal of Experimental Psychology Human Perception and Performance. 2006;32:1465–1478. doi: 10.1037/0096-1523.32.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends in Cognitive Sciences. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- 25.Hillyard S, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Science. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillyard SA, Hink R, Schwent V, Picton T. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- 27.Hillyard SA, Woldoff M, Mangun G, Hansen J. Mechanisms of early selective attention in auditory and visual modalities. The London Symposia, EEG supplement. 1987;39:317–324. [PubMed] [Google Scholar]
- 28.Hook P, Macaruso P, Jones S. Efficacy of Fast ForWord training on facilitating acquisition of reading skills by children with reading difficulties- A longitudinal study. Annals of Dyslexia. 2001;51:75–96. [Google Scholar]
- 29.Klein R, D’Entremont B. In: Filtering performance by good and poor readers. Everatt J, editor. Attention, Reading, and Dyslexia, Routtledge; London: 1999. [Google Scholar]
- 30.Lange K, Röder B. Orienting attention to points in time improves stimulus processing both within and across modalities. Journal of Cognitive Neuroscience. 2006;18:715–729. doi: 10.1162/jocn.2006.18.5.715. [DOI] [PubMed] [Google Scholar]
- 31.Lange K, Rösler F, Röder B. Early processing stages are modulated when auditory stimuli are presented at an attended moment in time: An event-related potential study. Psychophysiology. 2003;40:806–817. doi: 10.1111/1469-8986.00081. [DOI] [PubMed] [Google Scholar]
- 32.Leonard L. Children with specific language impairment. Massachusetts Institute of Technology; Cambridge, MA: 1998. [Google Scholar]
- 33.Leonard L. Language learnability and specific language impairment in children. Applied Psycholinguistics. 1989;10:179–202. [Google Scholar]
- 34.Loeb D, Stoke C, Fey M. Language changes associated with Fast ForWord-Language; Evidence from case studies. American Journal of Speech-Language Pathology. 2001;10:216–230. [Google Scholar]
- 35.McArthur G, Bishop D. Speech and non-speech processing in people with specific language impairment: A behavioural and electrophysiological study. Brain and Language. 2005 doi: 10.1016/j.bandl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Neville H, Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study. II. Congenitally deaf adults. Brain Research. 1987;405:268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- 37.Pokorni J, Worthington C, Jamison P. Phonological awareness intervention: Comparison of Fast ForWord, Earobics, and LiPS. The Journal of Education Research. 2004;97:147–157. [Google Scholar]
- 38.Rice M. Specific language impairments: In search of diagnostic markers and genetic contributions. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3:350–357. [Google Scholar]
- 39.Rice M, Wexler K, Hershberger S. Tense over time: The longitudinal course of tense acquisition in children with specific language impairment. Journal of Speech & Hearing Research. 1998;41:1412–1431. doi: 10.1044/jslhr.4106.1412. [DOI] [PubMed] [Google Scholar]
- 40.Roder B, Teder-Salejarvi W, Sterr A, Rosler F, Hillyard SA, Neville H. Improved auditory spatial tuning in blind humans. Nature. 1999;400:162–166. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- 41.Roid GH, editor. Stanford-Binet intelligence scales (SB-5) 5. Riverside Publishing; Itasca, IL: 2003. [Google Scholar]
- 42.Rouse C, Krueger A. Putting computerized instruction to the test: A randomized evaluation of a “scientifically based” reading program. Economics of Education Review. 2004;23:323–338. [Google Scholar]
- 43.Rueda MR, Rothbart M, McCandliss B, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the USA. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders L, Neville H. An ERP study of continuous speech processing. I. Segmentation, semantics, and syntax in native speakers. Cognitive Brain Research. 2003;15:228–240. doi: 10.1016/s0926-6410(02)00195-7. [DOI] [PubMed] [Google Scholar]
- 45.Sanders L, Newport E, Neville H. Segmenting nonsense: An event-related potential index of perceived onsets in continuous speech. Nature Neuroscience. 2002;5:700–703. doi: 10.1038/nn873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders L, Stevens C, Coch D, Neville H. Selective auditory attention in 3- to 5-year-old children: An event-related potential study. Neuropsychologia. 2006;44:2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Scientific Learning Corporation, Vol. 2007, 2007.
- 48.Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals (CELF-3) 3. The Psychological Corporation: Harcourt Brace & Co; San Antonio: 1995. [Google Scholar]
- 49.Sperling AJ, Lu ZL, Manis F, Seidenberg M. Deficits in perceptual noise exclusion in developmental dyslexia. Nature Neuroscience. 2005;8:862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- 50.Squires K, Hillyard S, Lindsay P. Vertex potentials evoked during auditory signal detection: Relation to decision criteria. Perception and Psychophysics. 1973;14:265–272. [Google Scholar]
- 51.Stevens C, Sanders L, Neville H. Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Research. 2006;1111:143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- 52.Sundberg U, Lacerda F. Does training with manipulated stimuli improve auditory perception in non-typical language learning children?. Reports in Phonetics Umeå University, PHONUM 9, Proceedings från FONETIK 2003, Lövånger; June 2–4, 2003; Umeå University, Sweden. 2003. pp. 13–16. [Google Scholar]
- 53.Tallal P. Improving language and literacy is a matter of time. Nature Reviews Neuroscience. 2004;5:721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- 54.Tallal P, Merzenich M, Miller S, Jenkins W. Language learning impairments: Integrating basic science, technology, and remediation. Experimental Brain Research. 1998;123:210–219. doi: 10.1007/s002210050563. [DOI] [PubMed] [Google Scholar]
- 55.Tallal P, Miller S, Bedi G, Byma G, Wang X, Nagarajan S, Schreiner C, Jenkins W, Merzenich M. Language comprehension in language-learning impaired children improved with acoustically modified speech. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 56.Teder-Salejarvi W, Hillyard S. The gradient of spatial auditory attention in free field: An event-related potential study. Perception & Psychophysics. 1998;60:1228–1242. doi: 10.3758/bf03206172. [DOI] [PubMed] [Google Scholar]
- 57.Teder-Salejarvi W, Pierce K, Courchesne E, Hillyard S. Auditory spatial localization and attention deficits in autistic adults. Cognitive Brain Research. 2005;23:221–234. doi: 10.1016/j.cogbrainres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 58.Temple E, Deutsch G, Poldrack R, Miller S, Tallal P, Merzenich M, Gabrielli J. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Science of USA. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomblin JB, Records N, Buckwawlter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children, Journal of Speech. Language, and Hearing Research. 1997;35:832–843. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Troia G, Whitney S. A close look at the efficacy of Fast ForWord Langyage for children wtih academic weaknesses. Contemporary Educational Psychology. 2003;28:465–494. [Google Scholar]
- 61.van der Lely H, Rosen S, Adlard A. Grammatical language impairment and the specificity of cognitive domains: Relations between auditory and language abilities. Cognition. 2004;94:167–183. doi: 10.1016/j.cognition.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Woldoff M, Hillyard SA. Modulation of early auditory processing during selective listenting to rapidly presented tones. Electroencephalography and Clinical Neurophysiology. 1991;79:170–191. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
- 63.Woods D, Alho K, Algazi A. Brain potential signs of feature processing during auditory selective attention. Neuroreport. 1991;2:189–192. doi: 10.1097/00001756-199104000-00007. [DOI] [PubMed] [Google Scholar]


