Abstract
Background
We studied the efficacy and durability of recombinant adeno-associated virus serotype 9 (rAAV9) vector mediated gene transfer to the transplanted rat heart.
Methods
ArAAV9-CMV-lacZ vector diluted in cold (4°C)University of Wisconsin solution was used to perfuse the rat coronary vasculature for 20 minutes prior to syngenic heterotopic transplantation. Perfusion experiments (6 groups, n = 3 / group) were performed without rAAV9 and at four separate doses ranging from 2 × 109 to 2 × 1012 viral genomes/ml. The transplanted heart was recovered 10 days or 3 months after transplantation and expression of lacZ assessed by histology, ELISA and real time RT-PCR. In a final group (n = 3) rAAV9 was administered systemically to compare the cardiac transduction efficiency and viral distribution to other organs.
Results
Transduction efficiency of perfused virus correlated with vector dose (p < 0.0001) with myocardial transduction ranging up to 71.74% at the highest dose. Cardiac expression of lacZ was equivalent at 10 days and 3 months. There was no evidence of viral gene transfer to other organs after heart transplantation.
Conclusions
This data demonstrates efficient and durable rAAV9 mediated gene transfer to the transplanted heart after ex vivo perfusion and suggests that AAV9 is a promising vector for cardiac gene therapy.
Keywords: Adeno-Associated Virus 9 Vector, Cold Perfusion System, Gene Therapy, Heart Transplantation, Rat
Introduction
In transplantation the donor organ is uniquely available for genetic modification ex vivo prior to implantation into a recipient. Gene therapy offers a potential approach to modify the donor organ to modulate the effects of ischemic-reperfusion injury and acute or chronic immune rejection after organ transplantation. Various strategies including direct injection into the myocardium,(1,2) bolus injection into the coronary vasculature(3,4) and in situ warm perfusion,(5) have been used to deliver genes to the transplanted heart. We have developed a cold ex vivo perfusion gene delivery system using adenoviral vectors in both rat(6) and pig(7) heart transplantation models.
Although adenoviral vectors can efficiently transduce cardiomyocytes in the transplanted heart, these vectors remain of limited utility to their pathogenicity and immunogenicity. Immune responses to adenovirus vectors can cause severe side effects.(8) Additionally most of transgene expression was diminished after 30 days by immune attack on host cells expressing viral antigens, DNases and/or methylation.
Recombinant adeno-associated virus (rAAV) vectors have been reported as promising alternatives. These vectors are less pathogenic and capable of establishing long-term stable transgene expression. Among the various serotypes of AAV, rAAV vectors based on AAV serotype 2 has been most extensively investigated as gene delivery vectors. However, the transduction efficiency of rAAV2 mediated gene transfer to the heart in vivo remains unsatisfactory. Recently, rAAV8(9, 10) and 9 have been reported to display strong myocardium tropism. Intravenous infusion of rAAV8 and 9 vectors resulted in efficient transduction of the murine heart with somewhat higher efficiency using rAAV9.(11–13) There have been no reports of cardiac gene transfer with rAAV9 using procedures that are compatible with clinical cardiac transplantation.
In the present study, we performed experiments with rAAV9 vector delivered by cold ex vivo perfusion prior to heterotopic rat heart transplantation. We investigated the dose dependency of myocardial transduction and the duration of transgene expression.
Materials and Methods
Animals
Inbred male Lewis rats (Harlan, Madison, WI), weighing 250–330 g, were used as donors and recipients for syngeneic heart transplantation. Procedures and handling of animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic and Foundation and as described in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1996).
Recombinant adeno-associated virus vector
AAV9-CMV-lacZ is a pseudo-serotyped rAAV vector that carries AAV serotype 2 vector genome expressing human cytomegalovirus immediate early gene enhancer-promoter-driven bacterial lacZ gene and is packaged with AAV serotype 9 caspids. The construction, production and tittering of AAV9-CMV-lacZ was described elsewhere.(11)
Heart transplantation and vector delivery
Hearts were harvested and perfused ex vivo using techniques described previously.(6) In brief, AAV9-CMV-LacZ was diluted in 5mL of cold UW solution and circulated through the coronary vasculature of the donor heart for 20 minutes by means of a peristaltic pump (Rainin, Emeryville, CA). The flow rate was 0.75mL/min, which generated a pressure of 40 to 50 mmHg. During the perfusion, the container with the heart and the vial with the vector solution were kept on ice, to avoid the temperatures of both containers exceeding 4°C. To study the effects of viral dose on transduction efficiency 5 groups of perfusion studies were performed (n = 3 in each) with viral doses of 0, 2 × 109, 2 × 1010, 2 × 1011, and 2 × 1012 viral genomes/ml. After perfusion, heterotopic abdominal heart transplantation was performed using standard microsurgical techniques.(14) Function of the graft was checked daily by palpation of the beating transplanted heart. Hearts were collected 10 days after transplant. To assess long term viral mediated gene expression an additional set of perfusions (n = 3) at a viral does of 2 × 1011 vg/ml was performed and the heart collected 3 months after transplants. The rAAV9 vector (1 × 1012 / rat) was also introduced into the rat jugular vein without transplantation (n=3) to investigate the transduction to the native heart and the viral distribution to the other organs in vivo.
Assessment of transgene expression
Ten days or 3 months after procedure, the animals were anesthetized by an intraperitoneal injection of pentobarbital (70 mg/kg). The transplanted hearts were removed and flushed with saline solution. Midventricular cross-sections were cut and embedded in OCT compound (Tissue-Tek; Sakura Finetek Inc., Torrance, CA) and frozen in a liquid nitrogen-cooled isopentane bath for histologic evaluation and 5-bromo-4-chloro-3-indolylphosphate (X-Gal) staining. For quantification of mRNA level of LacZ gene and β-galactosidase expression, the remaining apical ventricular segments were snap-frozen in liquid nitrogen and stored at −80°C.
Histological analysis
For histochemical detection of β-galactosidase expression, we performed X-Gal staining of the transplanted heart. We also performed X-gal staining of the thyroid gland, native heart, lung, liver, kidney, testis and skeletal muscle (quadriceps femoris). Five 10-µm-thick cryostat sections were prepared with 25-µm intervals for each portion, fixed with 1.25% glutaraldehyde and stained with X-gal using X-gal staining kit (Novagen, Madison, WI), and counterstained with hematoxylin. To quantify the transduction efficiency, we analyzed cells over 2000 nuclei in the heart, liver and kidney, and over 1000 skeletal myofibers in the quadriceps femoris for β-galactosidase expression in each animal.
Enzyme-linked immunosorbent assays
Expression levels of β-galactosidase in transplanted heart were determined by ELISA using a β-Gal ELISA kit (Roche Molecular Biochemicals, Indianapolis, IN). We normalized β-galactosidase levels with the total protein concentration determined by the BCA protein assay kit (Pierce, Rockford, IL), and described the values as picograms of β-galactosidase per milligram of protein.
Quantitative real-time reverse transcription-polymerase chain reaction analysis
Real-time PCR was performed to evaluate the expression level of LacZ mRNA. Total RNA was extracted with RNeasy mini kit (Qiagen Inc., Valencia, CA) and treated with RNase-free DNase 1 (Quiagen). 52.5ng total RNA was subjected to real-time PCR reaction with 1xTaqMan probe and primer sets (LacZ: Customer design probe primer set; GAPDH: Rn99999916-s1) and with one-step RT-PCR master mix reagents (Applied Biosystems, Foster, CA). The real-time RT-PCR reactions were carried out in ABI PRISM9700HT Sequence Detection System (Applied Biosystems) with a reverse transcription step at 48°C for 30 minutes and denaturing step at 95°C for 10 minutes followed by 40 cycles of amplification at 95°C for 10 seconds and 60°C for 60 seconds. Relative quantification (Δ Δ Ct) method was used to analyze gene expression levels. The target genes expression was normalized to GAPDH as an endogenous control and relative values were demonstrated.(15).
Statistical analysis
Data are presented as arithmetic mean ± standard deviation. Rank sum test was used to assess differences between groups. In a vector dose response study, the Spearman correlation coefficient was used to investigate the correlation. P-values of 0.05 or less were considered statistically significant.
Results
For syngeneic rat heart transplantation all hearts were rapidly stopped by perfusion with cold UW solution. Post transplantation all hearts showed early spontaneous recovery of sinus rhythm (within 1 to 5 minutes) after reperfusion. All transplanted hearts showed good contractility at the time of excision.
AAV9 showed vector dose response in transduction and expression
We investigated transduction efficiency after transplantation following ex vivo cold perfusion of AAV9-CMV-lacZ at four different doses and one control group (n=3 in each of the 5 groups). The frequency of X-gal-stained cells was 0.13 ± 0.06%, 2.43 ± 2.66%, 15.60 ± 7.50% and 55.52 ± 15.84% at 2×109, 2×1010, 2×1011 and 2×1012 vector genomes (vg)/ml of viral doses, respectively (Figure 1A, B). A maximum of 71.74% transduction in cardiomyocytes was achieved in one animal at the highest dose. In the control group, in which the transplanted heart was perfused without virus, no stained cells were seen. A strong positive correlation was observed between vector dose and transduction efficiency in this system; the higher the dose level, the higher the rate of transduced cells (the Spearman Correlation Coefficient = 0.9864, p-value <0.0001). The similar positive correlation was observed between vector dose and β-galactosidase expression (Figure 1C) (the Spearman Correlation Coefficient = 0.9493, p-value <0.0001). The β-galactosidase expression was 0.386 ± 0.332, 5.686 ± 4.059, 12.428 ± 6.993 and 32.778 ± 11.736ng/mg protein at 2×109, 2×1010, 2×1011 and 2×1012 vg/ml of viral doses, respectively.
Figure 1.
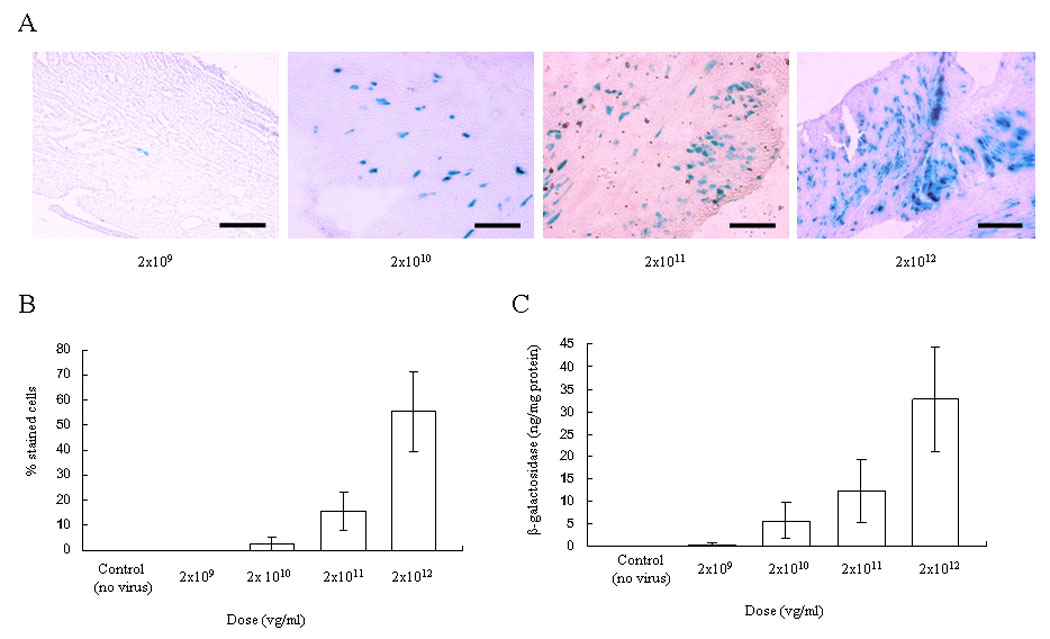
Vector dose-response in AAV9-CMV-lacZ transduction to the transplanted heart using ex vivo perfusion (0,2×109, 2×1010, 2×1011 and 2×1012 vg/ml). The transplanted heart was explanted 10 days after procedure and stained with X-gal and light hematoxylin. (A) A representative result is shown. Scale bar represents 100um. (B) The percentage of transduced cardiomyocytes. A strong positive correlation was observed between transduction efficiency and the vector dose (the Spearman Correlation Coefficient = 0.9864, p-value <.0001). (C) Total β-galactosidase level. The similar positive correlation was observed between β-galactosidase expression and vector dose (the Spea man Correlation Coefficient = 0.9493, p-value <.0001). Values are means ± standard deviation.
AAV9 perfusion vs intravenous injection
To compare the myocardial transduction efficiency after organ perfusion to intravenous injection and to define the tissue tropism of rAAV9 in rat, we injected 1×1012 vg/rat of AAV9-CMV-lacZ into the jugular vein of rats that did not receive transplants (iv injection group) and compared their level of myocardial transduction with syngeneic transplanted rat hearts after 20 minute perfusion with 1×1012 vg in 5ml of cold UW solution (ex vivo perfusion group). The level of myocardial transduction as determined by histological staining and β-galactosidase ELISA was determined after 10 days. The efficiency of transduction in the transplanted heart was 15.60 ± 7.50% in ex vivo perfusion group, compared to 12.21 ± 7.78% in the native heart in the iv injection group (p=0.62) (Figure 2A,B). The β-galactosidase expression detected by ELISA was 12.428 ± 6.993ng/ mg protein in the transplant group and 16.106 ± 9.024ng/ mg protein in the native heart in the iv injection group (p=0.70) (Figure 2C). There was no significant difference between the two groups in the percentage of X-gal stained cells or β-galactosidase expression. In the iv injection group, X-gal-stained cells were seen in liver (1.53 ± 0.24%), kidney (1.16 ± 0.74%), and skeletal muscle (0.03 ± 0.04%), but not in thyroid gland, lung or testis (Figure 2A). On the other hand, there was no evidence of transduction in the other organs in the transplanted group (Figure 2A).
Figure 2.
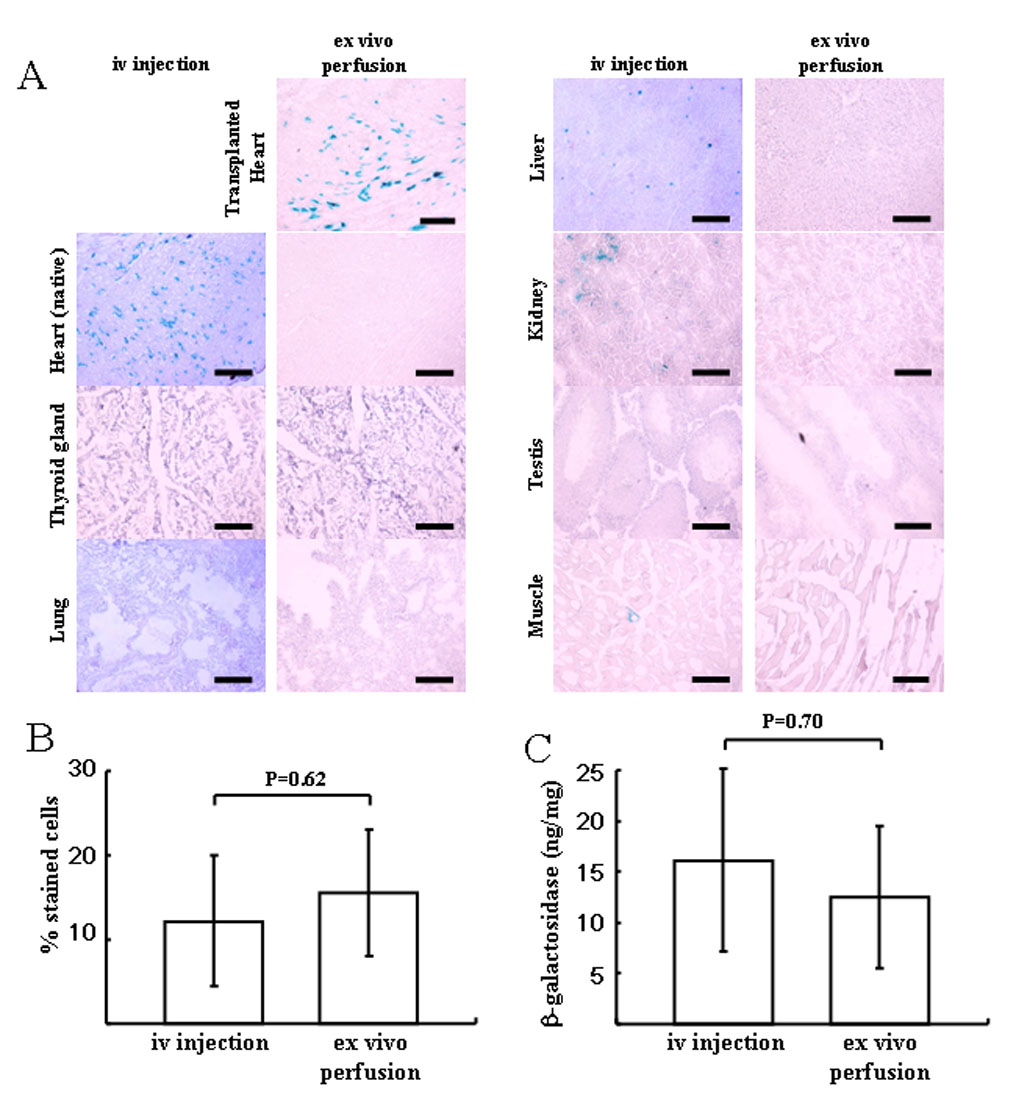
Tissue distribution of β-galactosidase-positive cells in the iv injection group and ex vivo perfusion group using AAV9-CMV-lacZ (1×1012 vg). (A) Representative microphotograph in each group. Each section was stained with X-gal and light hematoxylin. No positive cells were observed in thyroid gland, native heart, liver, kidney, skeletal muscle (quadriceps femoris) or testis of the recipient in ex vivo perfusion group. In the iv injection group, β-galactosidase positive cells were in heart>liver>kidney>muscle. Scale bar represents 100um. (B) Comparison of efficacy of lacZ gene transduction between the native heart in iv injection group and the transplanted heart in ex vivo perfusion group. (C) Total β-galactosidase antigen level. No significant difference was seen between two groups in the transduction efficiency. Values are means ± standard deviation.
AAV9 achieved the durable gene transfer for 3 months
In order to determine the durability of gene expression in this model, we examined hearts perfused with 2 × 1011 vg/mL at 10 days and 3 months after transplantation. We detected 15.60 ± 7.50% of X-gal stained cardiomyocytes at 10 days, and 20.23 ± 2.83% at 3 months (Figure 3A, B). The relative expression ratio of LacZ mRNA in comparison to GAPDH mRNA detected by real-time RT-PCR were 0.40 ± 0.24 at 10 days and 0.51 ± 0.23 at 3 months (p=0.40) (Figure 3C). The β-galactosidase expressions were 12.428 ± 6.993ng/ mg protein at 10 days and 65.504 ± 26.662ng/ mg protein at 3 months (p=0.08) (Figure 3D). There was no significant difference in the level of LacZ mRNA, efficiency of transduction and β-galactosidase expression between 10 days and 3 months after transplant.
Figure 3.
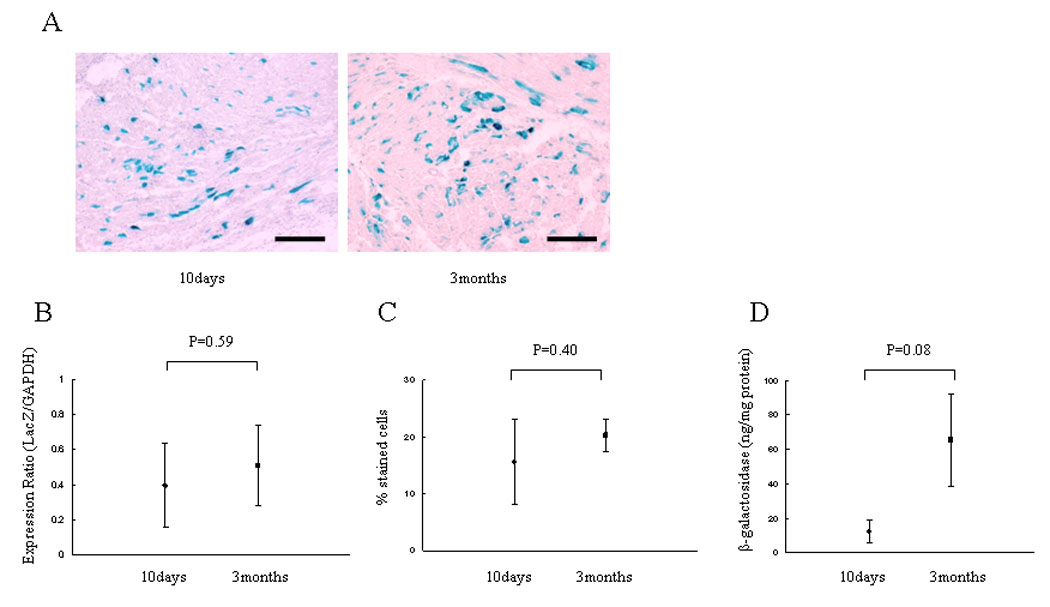
Durability of LacZ gene and β-galactosidase expression. 2×1011 vg/ml of AAV-CMV-lacZ was perfused into the donor heart and transplanted. Animals were sacrificed at 10 days and 3 months after the procedure and stained with X-gal and light hematoxylin. (A) Representative microphotographs at 10 days and 3 months. (B) The percentage of transduced cardiomyocytes. (C) LacZ mRNA level normalized to GAPDH as an endogenous control and relative values were demonstrated. (D) Total β-galactosidase antigen level. The level of transduction, transcription and expression are not different between 10 days and 3 months after transplantation.
Discussion
In the current study, we demonstrated the feasibility of rAAV9 vectors for gene transfer to the transplanted rat heart using a cold perfusion system. rAAV9 showed highly efficient (up to 71.74% of cardiomyocytes) and 3 months durable gene transfer to the transplanted heart. We also demonstrated a high level of cardiac tropism for rAAV9 after systemic administration. The affinity of rAAV9 to each organ in the rat was similar to that reported in the mouse.(11) This is the first demonstration of cardiac gene transduction of the donor heart using rAAV9. The final goal of this project is to develop a clinically relevant gene therapy approach for the treatment of ischemia/reperfusion injury, acute rejection and chronic allograft vasculopathy (CAV) after heart transplantation.
Heart transplantation is now widely accepted as a treatment for patients with end-stage heart failure, with a 1 and 5-year patient survival of 85 and 65%, respectively and a half-life of 9.6 years.(16) However, limitations such as acute and chronic rejection of allograft as well as ischemia/reperfusion injury associated with organ preservation still remain. Gene therapy approaches may be appropriate for these complications.(17) Successful experimental results of gene therapy have been obtained using adenoviral vectors encoding IL-10, TGF-β (18), CTLA4-Ig (19) or IL-17R-IgG (20) to modulate the graft in the setting of heart transplantation or with vascular endothelial growth factor (VEGF)-C to prevent ischemic injury.(21) However, inflammatory and immunogenic responses to adenoviral vectors occur. Gene expression with adenovirus vectors peaks within seven days and disappeared a few weeks after initial transduction. In contrast, AAV is a small, nonpathogenic, replication-defective parvovirus with a single-stranded DNA genome. AAV has shown less immunogenicity compared to adenovirus, and has shown long-term cardiac gene expression for up to 1 year.(22) Among the various serotypes of AAV, rAAV vectors based on serotype 2 (AAV2) have been most extensively investigated however rAAV2 is suboptimal in cardiac transduction efficiency. Recently, rAAV8 and 9 have been reported to show strong tropism to heart with rAAV9 showing superior cardiac transduction after systemic vector administration. Thus we selected rAAV9 as a vector in the rat heart transplant model using ex vivo cold perfusion system.
We investigated the in vivo biology of rAAV9 administrated systemically in the rat. The efficiency of transduction to the heart was 12.21 ± 7.78 % with 1.0×1012 vg/rat of rAAV9. While this is not as high as in the mouse we note that the viral dose/kg in the rat is lower then in the mouse and that the tissue tropism was similar in both animals. We also compared the delivery of virus by iv injection and ex vivo cold perfusion and found no significant difference in gene expression. While the transduction of rAAV9 by perfusion might be expected to be more efficient then systemic delivery, the efficiency of perfusion may be limited by the hypothermic conditions and other nonbiological circumstances.(23) The advantage of the perfusion system is that it avoids viral transduction of noncardiac tissue in the recipient (Figure 2). In addition, low temperature is safe for organ preservation, avoids anaerobic metabolism in the myocardium (24) and is consistent with current clinical techniques of cardiac preservation. Thus ex vivo perfusion appears to represent an ideal setting for administration of the vector via the blood vessels as the inevitable period of donor organ ischemia following harvesting allows a prolonged time to dwell within the target tissue.
Hypothermic conditions required for donor heart preservation may reduce gene transfer efficiency; however, the current study showed rAAV9 transduced up to 71.74% of the myocardium with a positive dose response. Compared with the reports in which various strategies of gene transduction to the donor heart using adenovirus and AAV (Table 1),(1,25,26) the efficiency of transduction in this study was excellent. rAAV9-mediated gene transfer to the transplanted heart using a cold perfusion system seems to be a clinically relevant gene therapy approach.
TABLE 1.
Comparison of the transduction efficiencies following ex vivo gene transfer to the transplanted heart
| Animal | Date after Tx | Virus | Titer | Method | Transduction Rate | Author |
|---|---|---|---|---|---|---|
| Rat | 7 days | Adenovirus5 | 109 pfu/0.2ml 5×109 pfu/ml | Direct injection Bolus into coronary artery | 30–40% | Wang |
| Rabbit | 4 days | Adenovirus5 | 1010 pfu/gram of heart | Bolus into coronary artery | Epi/Endo 90%/5–20% | Brauner |
| Rat | 4 days | Adenovirus5 | 1×109 pfu/ml 7×107 pfu/ml | Bolus Cold ex vivo perfusion | Epi/Endo 16/2.6% Epi/Endo 49/2.0% | Pellegrini |
| Mice | 4–5 weeks | AAV2 | 5×109 vg/ml | Bolus into coronary artery | 30–40% | Chen |
| Hamster | 2wks 1 yr | Adenovirus5 AAV2 | 5×109 pfu/ml 1×1011 vg/ml | Bolus | <10% 90% | Li |
| Pig | 7 days | Adenovirus5 | 1×108 pfu/ml 1×109 pfu/ml | Cold ex vivo perfusion | 0.04–0.07% 0.45–22.62% | Oi |
Epi: Epicardial site; Endo: Endocardial site; pfu: plaque-forming unit; vg: vector genomes
In this perfusion system, a perfusion pressure was used between 40 and 50 mmHg, based on previous results.(6) Su et al had reported the transduction efficiency by rAAV to the transplanted heart from antegrade (80–100mmHg) was lower than from retrograde (60–80mmHg).(27) Although the damage to the myocardium caused from high perfusion pressure or non-physiological route should be considered, it may be interesting to test other conditions in our gene delivery system.
rAAV9 showed durable gene transduction to the transplanted syngeneic heart for at least 3 months in this study. The LacZ mRNA, transduction efficiency and β-galactosidase expression at 3 months after transplantation remained at the same level as at 10 days. In addition, we did not see notable cardiomyocyte injury, myocarditis or lymphocytic infiltration in the transplanted heart at 10 days or 3 months post transplant. In a clinical trial of gene therapy for hemophilia using AAV2-Factor IX, the expression of factor IX was significantly diminished by a host immune response apparently to AAV2 capsid protein.(28) The current study indicates that rAAV9 can achieve long-term gene expression and offers an alternative approach.
In conclusion, this study demonstrates for the first time, the applicability of gene transfer by means of a cold perfusion system using rAAV9 vectors in a heterotopic rat heart transplant model. Efficient and durable myocardial transduction is achievable. Studies are required to further evaluate the potential of rAAV9 mediated gene transfer to modify cardiovascular disorders after heart transplantation using therapeutic genes.
Acknowledgments
The authors thank Guang-Ping Gao and James M. Wilson (University of Pennsylvania) for providing us with AAV9 helper plasmids, Scott Suddendorf, Paul Stalboerger, Junice Thompson and Merilyn Walters (Mayo Clinic) for technical help, Karen Schumacher (Mayo Clinic) for writing assistance and Makoto Sunamori (Tokyo Medical and Dental University) for broad support to the first author.
This research was supported by: National Institutes of Health Grant HL66958 P2, and Mayo Clinic and Foundation, Rochester, MN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang J, Ying M, Knechtle SJ. Adenovirus-mediated gene transfer into rat cardiac allografts: Comparison of direct injection and perfusion. Transplantation. 1996;61:1726–1729. doi: 10.1097/00007890-199606270-00011. [DOI] [PubMed] [Google Scholar]
- 2.Ardehali A, Fyfe A, Laks H, Drinkwater DC, Jr, Qiao JH, Lusis AJ. Direct gene transfer into donor hearts at the time of harvest. J Thorac Cardiovasc Surg. 1995;109:716–719. doi: 10.1016/S0022-5223(95)70353-5. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrini C, O'Brien T, Yap J, Jeppsson A, Tazelaar HD, McGregor CGA. Systematic evaluation of distribution of transgene expression after adenovirus-mediated gene transfer to the transplanted heart. Transpl Int. 1998;11:373–377. doi: 10.1007/s001470050160. [DOI] [PubMed] [Google Scholar]
- 4.Kypson AP, Peppel K, Akhter SA, et al. Ex vivo adenovirus-mediated gene transfer to the adult rat heart. J Thorac Cardiovasc Surg. 1998;115:623–630. doi: 10.1016/S0022-5223(98)70327-7. [DOI] [PubMed] [Google Scholar]
- 5.DeBruyne LA, Li K, Chan SY, Qin L, Bishop DK, Bromberg JS. Lipid-mediated gene transfer of viral IL-10 prolongs vascularized cardiac allograft survival by inhibiting donor-specific cellular and humoral immune responses. Gene Therapy. 1998;5:1079–1087. doi: 10.1038/sj.gt.3300694. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini C, Jeppsson A, Taner CB, et al. Highly efficient ex vivo gene transfer to the transplanted heart by means of hypothermic perfusion with a low dose of adenoviral vector. J Thorac Cardiovasc Surg. 2000;119:493–500. doi: 10.1016/s0022-5223(00)70128-0. [DOI] [PubMed] [Google Scholar]
- 7.Oi K, Davies WR, Tazelaar HD, et al. Ex vivo hypothermic recirculatory adenoviral gene transfer to the transplanted pig heart. J Gene Med. 2006;8:795–803. doi: 10.1002/jgm.913. [DOI] [PubMed] [Google Scholar]
- 8.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 10.Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkar R, Mucci M, Addya S, et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- 13.Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 14.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225–229. [PubMed] [Google Scholar]
- 15.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-second official adult heart transplant report-2005. J Heart Lung Transplant. 2005;24:945–955. doi: 10.1016/j.healun.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Isobe M, Kosuge H, Koga N, Futamatsu H, Suzuki J. Gene therapy for heart transplantation-associated acute rejection, ischemia/reperfusion injury and coronary arteriosclerosis. Curr Gene Ther. 2004;4:145–152. doi: 10.2174/1566523043346507. [DOI] [PubMed] [Google Scholar]
- 18.Brauner R, Nonoyama M, Laks H, et al. Intracoronary adenovirus-mediated transfer of immunosuppressive cytokine genes prolongs allograft survival. J Thorac Cardiovasc Surg. 1997;114:923–933. doi: 10.1016/S0022-5223(97)70006-0. [DOI] [PubMed] [Google Scholar]
- 19.Kita Y, Li XK, Ohba M, et al. Prolonged cardiac allograft survival in rats systemically injected adenoviral vectors containing CTLA4IG-Gene. Transplantation. 1999;68:758–766. doi: 10.1097/00007890-199909270-00007. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Simeoni E, Fleury S, et al. Gene transfer of soluble interleukin-17 receptor prolongs cardiac allograft survival in a rat model. Eur J Cardiothorac Surg. 2006;29:779–783. doi: 10.1016/j.ejcts.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Pätilä T, Ikonen T, Rutanen J, et al. Vascular endothelial growth factor C-induced collateral formation in a model of myocardial ischemia. J Heart Lung Transplant. 2006;25:206–213. doi: 10.1016/j.healun.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Woo YJ, Zhang JC, Taylor MD, Cohen JE, Hsu VM, Sweeney HL. One year transgene expression with adeno-associated virus cardiac gene transfer. Int J Cardiol. 2005;100:421–426. doi: 10.1016/j.ijcard.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini C, O'Brien T, Jeppsson A, et al. Influence of temperature on adenovirus-mediated gene transfer. Eur J Cardiothorac Surg. 1998;13:599–603. doi: 10.1016/s1010-7940(98)00064-5. [DOI] [PubMed] [Google Scholar]
- 24.McLaren AJ, Friend PJ. Trends in organ preservation. Transpl Int. 2003;16:701–708. doi: 10.1007/s00147-003-0659-2. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Lu L, Li J, Xiao X, Fung JJ, Qian S. Prolonged survival of heart allografts transduced with AAV-CTLA4Ig. Microsurgery. 2003;23:489–493. doi: 10.1002/micr.10181. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Wang D, Qian S, Chen Z, Zhu T, Xiao X. Efficient and long-term intracardiac gene transfer in delta-sarcoglycan-deficiency hamster by adeno-associated virus-2 vectors. Gene Therapy. 2003;10:1807–1813. doi: 10.1038/sj.gt.3302078. [DOI] [PubMed] [Google Scholar]
- 27.Su LT, Gopal K, Wang Z, et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112:1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- 28.Manno CS, Arruda VR, Pierce GF, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]


