Summary
The gymnotiform electric fish Brachyhypopomus pinnicaudatus communicates with a sexually dimorphic electric waveform, the electric organ discharge (EOD). Males display pronounced circadian rhythms in the amplitude and duration of their EODs. Changes in the social environment influence the magnitudes of these circadian rhythms and also produce more transient responses in the EOD waveforms. Here we show that injections of serotonin produce quick, transient, dose-dependent enhancements of the male EOD characters similar to those induced by encounters with another male. The response to serotonin administered peripherally begins 5–10 min post injection and lasts approximately 3 h. The magnitude of the response to serotonin is tightly associated with the magnitude of the day-to-night swing of the circadian rhythm prior to injection. Taken together these findings suggest that the male’s social environment influences his response to serotonin by altering the function of some part of the downstream chain between the serotonin receptors and the ion channels involved in control of the EOD waveform. Although chronic activation of serotonin circuitry is widely known to elicit subordinate behavior, we find that 5-HT initially increases a dominance signal in these fish. These findings are consistent with the emerging view that serotonin facilitates different adaptive responses to acute and chronic social challenge and stress.
Keywords: Gymnotiformes, Hypopomidae, Brachyhypopomus pinnicaudatus, circadian rhythm, communication, electric organ discharge, 5-hydroxytryptamine
Introduction
Among its many roles in the body, serotonin (5-hydroxytryptamine, or 5-HT) mediates an animal’s behavioral response to social stress. In a wide variety of vertebrates, high serotonin turnover in the brainstem and telencephalon is associated with lowered aggression; manipulating central serotonergic circuits typically alters aggressive and subordinate behaviors in a manner consistent with this view (for a review, see Simon, 2002). Serotonin’s effect on behavior, however, may be broader than simply mediating subordinate behavior. Where the time course of serotonin release has been examined carefully, dominant animals show a short-term activation of serotonin circuits, followed by a long-term cessation, whereas activation in subordinates persists into the long-term (Overli et al., 1999; Summers et al., 1998). In crustaceans, such as lobsters and crayfish, serotonin has been shown to facilitate either aggressive or subordinate responses to agonistic encounters, depending on the animal’s prior social history (for reviews, see Edwards and Kravitz, 1997; Yeh et al., 1996, 1997). Serotonin’s effects during and following social conflict appear to be determined by the types of receptors involved, their locations in the nervous system, and their relative thresholds and time courses of activation (Summers, 2001; Summers et al., 1998; Teshiba et al., 2001).
Our interest in serotonin stems from its possible role in the regulation of communication behavior in gymnotiform electric fish. Weakly electric fish produce electric organ discharges (EODs) for electrolocation and communication. Most species in the genus Brachyhypopomus produce sexually dimorphic EODs consisting of discrete, biphasic pulses. The female EOD is a symmetric sinusoid. The male’s EOD differs from the female’s in two ways: its amplitude is greater and the repolarization time of the second phase (P2) is much longer (Hopkins et al., 1990; Stoddard et al., 1999). Extending the repolarization time of P2 boosts the energy in the sensory range of the ampullary electroreceptors approximately five times (Stoddard, 2002), of little consequence for electrolocation, but of great significance for communication (Naruse and Kawasaki, 1998; for a review, see Stoddard, 2002).
Males dynamically regulate the shape of their EOD waveforms with a circadian rhythm that maximizes their masculine signal traits (amplitude and repolarization time) in the early hours of the night, when social activities peak (Franchina and Stoddard, 1998; Hagedorn, 1995). Starting in the late afternoon, EOD amplitude increases in both phases, reaching a maximum shortly after dark when spawning is most likely to occur. Repolarization time of P2 increases, but begins its ascent later in the day than amplitude, but rising faster, to peak about the same time. Of key importance, males also alter these signal traits in response to social encounters. Typically the EOD amplitude rises and P2 repolarization time increases within a few minutes of the initial encounter. If the male loses the encounter, duration of P2 drops more than that of the victor, as seen by an increase in peak spectral frequency of the EOD (Hagedorn and Zelick, 1989).
We began screening neuroactive substances to find chemical modulators of the EOD waveform, initially focusing on those known to have peripheral as well as central effects. A prior study of gymnotiform electric fish demonstrated the expected central effect of serotonin on behavior (Maler and Ellis, 1987); serotonin inhibits the production of aggressive signaling, reducing how often the fish accelerates the EOD rate (chirp signals) in agonistic encounters. Based on the typical vertebrate pattern, one might expect serotonin to reduce the amplitude and repolarization time of the EOD, as both these EOD measures are associated with big, reproductive males (Curtis and Stoddard, in press; Hopkins et al., 1990), and both increase on social challenge (Franchina et al., 2001). However, our preliminary screen showed that serotonin injected peripherally caused a rapid and transient increase in amplitude and repolarization time of the EOD waveform. Here we characterize the response of the electric waveform to exogenous serotonin, making no assumptions or conclusions about the site of action.
Materials and methods
Subjects
We used 40 reproductively mature male electric fish, Brachyhypopomus pinnicaudatus (Hopkins, 1991) obtained from our captive breeding colony. Fish were kept outdoors under full sunlight in 450 liter pools containing mixed-sex groups of 10–30 individuals. The water surface was covered with water hyacinths, Eichhornia crassipes and water lettuce, Pistia stratiotes. Water conductivity was maintained at 100 μS cm−1. Fish were transported to the laboratory from the outdoor colony, weighed, measured and placed in their test tank at least a day in advance of pharmacological treatment. EOD data collection began immediately after each fish was placed in the tank and continued until it was removed.
Data collection: the EOD machine
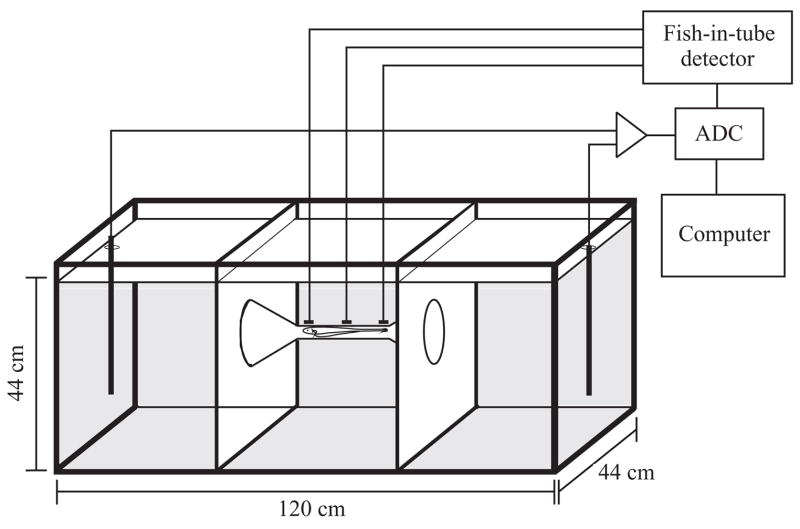
EODs were recorded in automated measurement tanks, 120 cm×44 cm×44 cm, shielded with grounded copper screen. Eight such tanks resided within a light- and temperature-controlled room on a 12 h:12 h L:D light cycle. Each tank was divided into thirds by plastic screening, with screen funnels directing fish through an unglazed ceramic tube that joined the outer two compartments (Fig. 1). Unlike plastic tubes, unglazed ceramic tubes interfere minimally with the electric fields. The fish rested in the tube during daylight hours and passed through frequently at night. Graphite electrodes on the ceramic tube registered the fish’s position in a dedicated analog circuit that compared rectified signal amplitudes against preset thresholds, customized for each fish. When this fish-in-tube detector registered an EOD from a fish centered in the tube, a TTL pulse was sent to a ring-buffered, analog-to-digital converter (ADC) (Tucker-Davis Technologies RP2, Gainesville, FL, USA) that digitized the entire EOD at approx. 50 kHz across a different pair of electrodes at either end of the tank. EODs were amplified 200× and lowpass filtered with an AC-coupled 8-pole Bessel filter set to a corner frequency of 10 kHz. Up to nine consecutive EODs were digitized, one series every 60 s if the fish was resting in the tube, or at most every 60 s if the fish was passing through on its own volition. The chamber was heated by an air pump and water temperature was maintained within half a degree of 27°C by a dedicated computer that monitored thermocouples and switched fans to exchange warm air for cool. Once a week the ADC and amplifier were calibrated to equivalent units of mV cm−1 at 10 cm (Franchina and Stoddard, 1998). Water conductivity was readjusted to 100 μS cm−1.
Fig. 1.
Experimental tank used to record calibrated EODs of free-swimming fish. When the fish passed through, or rested inside the ceramic tube, electrodes on the tube informed the fish-in-tube detector of the fish’s presence. Up to nine consecutive EODs were digitized from electrodes at the ends of the tanks while the fish-in-tube threshold criteria were met. ADC, analog-to-digital converter.
Online data analysis
EODs were analyzed on the fly, and made available to a custom-written data visualization program. For each EOD, the analysis program measured peak amplitudes for phase 1 (P1) and phase 2 (P2), durations at 10% of peak amplitude for each phase, and τ-P2, the time constant of the repolarization of phase 2 (Fig. 2). Measurements of amplitude peaks and durations were improved with cubic spline interpolation to enhance resolution over that obtainable with a sampling rate of 50 kHz. τ-P2 was calculated by fitting an inverse exponential curve to the repolarization segment of the second phase using successive approximation in the MATLAB function ‘fminsearch’ (Mathworks, Natick MA, USA). Median values were graphed as representative of each set of nine sequential EODs. We found that duration of the second phase measured 10% above baseline correlated strongly with the time constant τ-P2 of the repolarization of the second phase (r2=0.64; N=32). We chose τ-P2 as our measure of duration of the second phase of the EOD for several reasons: τ-P2 is more reliably measured, it applies only to the repolarization part of the waveform and is therefore less likely to be influenced by partial overlap with the first phase (Hopkins et al., 1990), and finally, τ-P2 is more easily related to underlying electrophysiological parameters such as ion-channel inactivation time (Ferrari et al., 1995; McAnelly and Zakon, 2000).
Fig. 2.
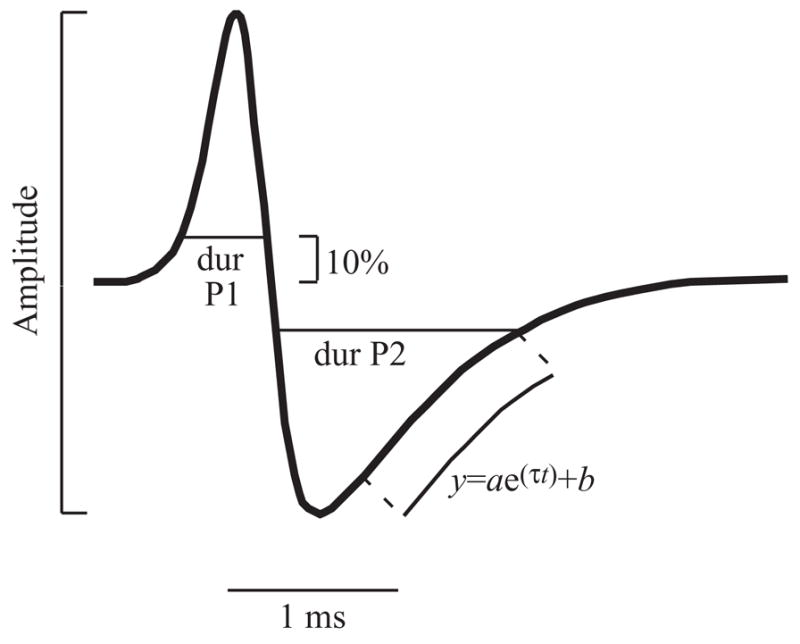
Parameters measured in each digitized EOD waveform. Amplitude is measured peak-to-peak using cubic spline interpolation to improve accuracy. The duration of each phase (dur) is calculated 10% of the distance from the zero-crossing to the peak. The time constant τ-P2 of the repolarization phase is estimated by fitting an inverse exponential to the middle 80% of the 2nd phase.
Offline data analysis
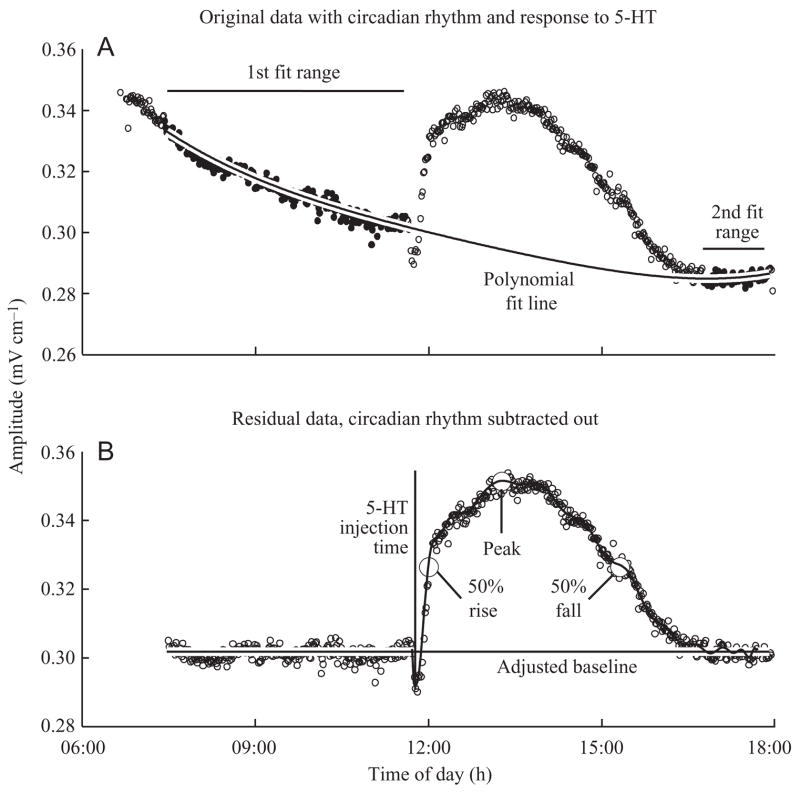
EOD waveform responses to pharmacological agents are superimposed on each fish’s circadian EOD rhythm (Franchina and Stoddard, 1998). While the acute responses to serotonin injections are clearly visible in the data, accurate measurements are confounded to various degrees by ongoing circadian oscillation in the baselines (Figs 3, 4). We wrote a graphical data analysis program in MATLAB to separate pharmacological effects from the circadian oscillations (Fig. 3). First, we performed a least-squares fit of a low-order (2–6) polynomial to the data segments prior to and following the pharmacological effect, omitting the time period from the injection to when the EOD parameter returned to its normal oscillating baseline. We subtracted this polynomial curve from the data to yield a set of residuals that included only the pharmacological effect. The order of polynomial was chosen to match the shape of the data from the preceding and following days when no pharmacological agents were given. When the pharmacological effect persisted for more than 3–4 h, a fit polynomial could have too much freedom to wander in the excluded time segment. In those cases, we superimposed the rhythm from the preceding or following day, adjusted it with linear regression to match the current day’s data, exclusive of the post-injection period, then fitted the polynomial to a combination of the best subsets of data excluding the pharmacologically affected data. The data residuals were fit with a high order (5–40) polynomial function using least-squares regression. The program analyzed the polynomial function to identify the time and amplitude of the data peaks. Three males produced amplitude data that were too noisy to analyze and we excluded these data from further analysis. One male produced a response in τ-P2 that was 6 standard deviations beyond the mean so we excluded these data as well. Further regression analyses and means tests were done with MATLAB.
Fig. 3.
Offline data analysis using polynomial regression to remove the circadian rhythm from the pharmacological response. (A) The raw data are shown with a fourth order polynomial least-squares regression fitted to segments of data before the injection and after the treatment had worn off. The polynomial is subtracted from the data to produce the residual data (B). A second, higher order polynomial is fitted to the residual data. The peak response and 50% peak response points are calculated for this polynomial.
Fig. 4.
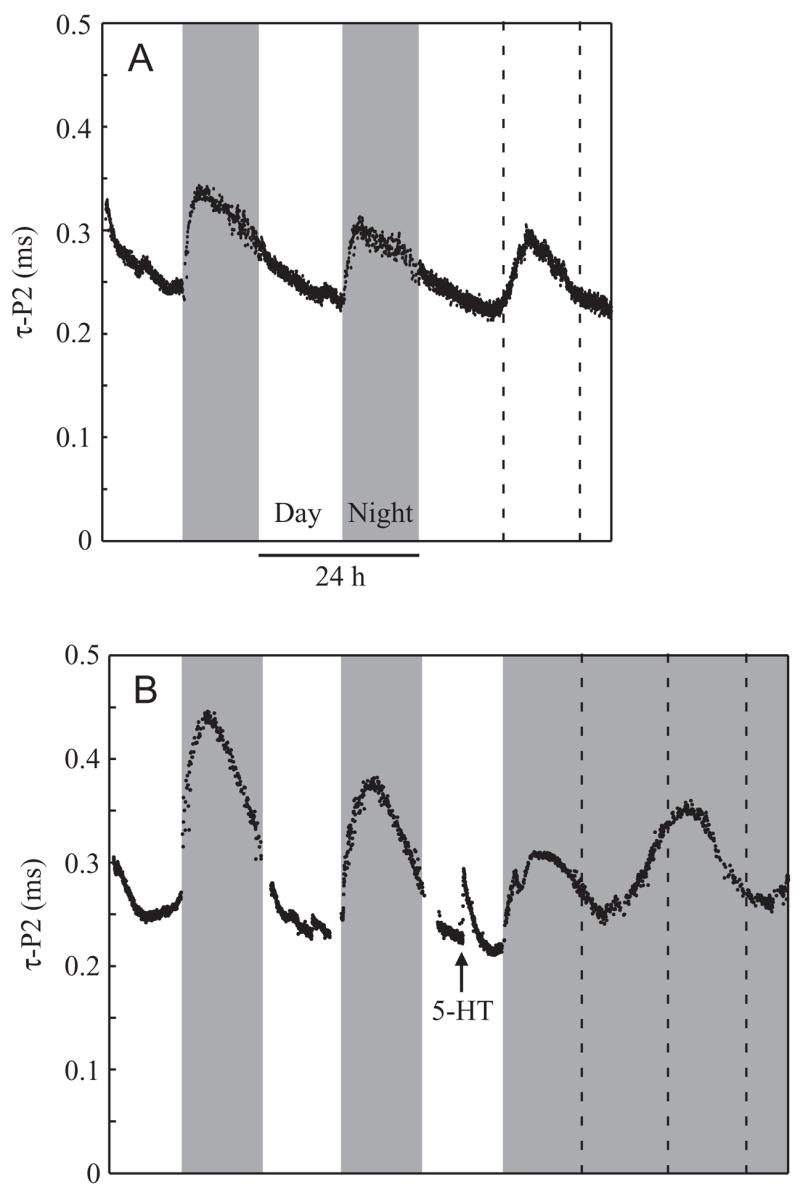
As a brief demonstration of the circadian rhythm, we left the lights on (A; male 029) or off (B; male 042) after completing an experiment. Under constant light or constant dark, EOD repolarization time (τ-P2) and amplitude (not shown) continue to oscillate on a shortened circadian rhythm, though the rise under free-running conditions is not as steep as occurs immediately after lights out in laboratory conditions. Vertical dashed lines indicate the previous times of lights on or off.
EOD amplitude and duration gradually diminish over 7–10 days when males are kept in social isolation, as they were in this experiment (Franchina et al., 2001). Thus, to identify the day–night swing for a given midday injection time, we found the day and night extremes both before and after the injection date and interpolated peak-to-peak and trough-to-trough lines to obtain an instantaneous measure of potential peak and trough values at the injection time.
Solutions and injection
Serotonin (5-hydroxytryptophan creatinine sulfate complex, Sigma) was dissolved in physiological saline: NaCl 114 mmol l−1, KCl 2 mmol l−1, CaCl2.2H2O 4 mmol l−1, MgCl2.6H2O 2 mmol l−1, Hepes 2 mmol l−1, pH adjusted to 7.2 with NaOH (modified from Ferrari and Zakon, 1993). We selected 25 nmol g−1 as our highest concentration of 5-HT because this dose had been shown to be active in other studies of fish (Khan and Thomas, 1992). At this dose, however, the fish emerged from their hiding tubes after injection and swam about the tanks disoriented and often upside down, very odd behavior for a gymnotiform fish at midday. We therefore tested fish on a variety of doses down to 0.025 nmol g−1 to generate a dose–response curve that could be used to establish the minimum effective dose. All solutions were prepared on the day of injection.
We allowed the fish to spend a day in the measurement tank before receiving an injection and an additional day after the injection as well. These extra data allowed us to better characterize the circadian changes so we could subtract these from the data trace to reveal the effect of the injection. We injected fish at midday, when visual inspection of plots of EOD repolarization time and amplitude showed these circadian rhythms to be at or approaching their daily minima. To inject a fish, we slid a plastic screen tube inside the ceramic tube where the fish was resting, pinched off the ends of the screen tube, and removed the fish from the tank. The fish was injected while confined to the screen tube and returned to the water within 30 s.
Experimental removal and handling without injection was found to have no measurable effect on the subsequent EODs, but injection of saline had a noticeable effect in about half the fish injected. For this reason, we use saline injections as our primary control treatment. Experimental and control solutions were injected at 1 μl g−1 intramuscularly (i.m.) or intraperitoneally (i.p.) using a Hamilton syringe fitted with a disposable 31.5 gauge needle. Male fish weighed 5–20 g so injection volumes were 5–20 μl. Since we found no difference in results between fish injected i.m. and i.p., we kept to i.m. injections, safer for this very wiggly animal, which has large epaxial muscles and a small peritoneum. We injected at a shallow angle to maximize needle penetration and reduce leakage.
Results
As shown previously (Franchina and Stoddard, 1998), EOD amplitude and duration varied consistently between day and night, being longer at night when the nocturnal fish are active. We confirmed Franchina’s finding (Franchina, 1997) that the cycles persist in isolated fish maintained in constant light or constant darkness for a couple of days, indicating that the cycles have a true circadian component (Fig. 4). The duration of the first phase (dur-P1) often varied slightly as well, but variation in dur-P1 was inconsistent and could be either longer or shorter by night than by day.
Compared to saline injections, injections of serotonin at doses of 2.5 nmol g−1 and higher produced greater increases in EOD amplitude, duration of the second phase (dur-P2), and repolarization time of the second phase (τ-P2, e.g. Fig. 5), but no significant difference in duration of the first phase (Fig. 6). The increases in τ-P2 were dose-dependent (r2=0.50; F=38; d.f.=1,41; P=0.000002), reaching an asymptote at doses of 2.5 nmol g−1 (Fig. 7). Amplitude also increased, but this response was more variable and the best fit dose-response curve (not shown) was not statistically significant. Effects of 5-HT on the duration of the first phase of the EOD were small and more variable yet (Fig. 8). In all cases, the response to serotonin was transitory (Fig. 8). Temporal distribution of peaks in τ-P2 were bimodal. Five individuals peaked 15–30 min after injection and the majority peaked later, 60–115 min after injection (Figs 5, 8). Amplitude response was unimodal, peaking 30 min after injection. The effective half-lives were 2–3 h for all parameters (Fig. 8). In some fish, injection of 5-HT increased both τ-P2 and amplitude, whereas in others only τ-P2 was affected (Figs 5, 9). In no fish did serotonin cause amplitude to increase while τ-P2 remained flat (Fig. 9).
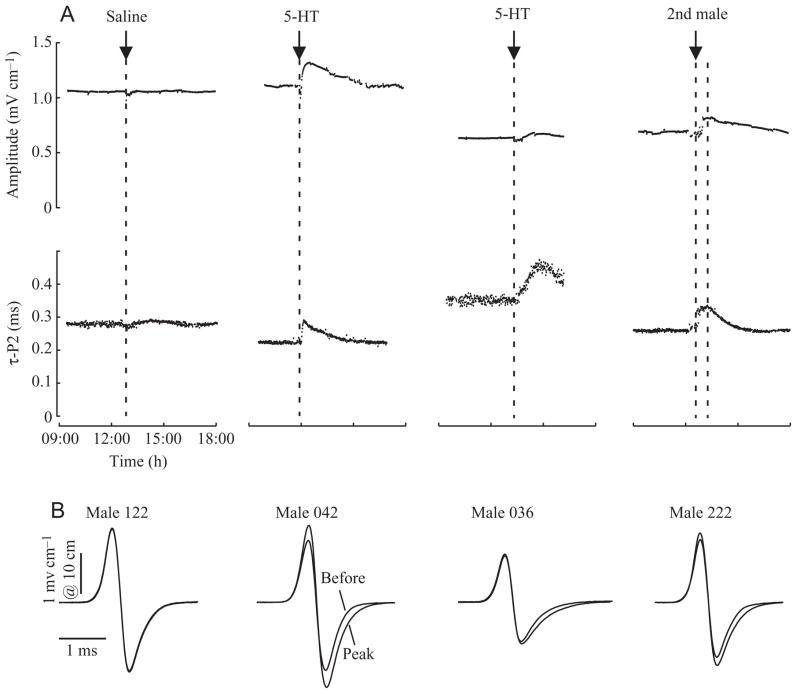
Fig. 5.
Effects of saline, serotonin, and male–male social interaction on the EOD waveform. (A) Effects on EOD amplitude and repolarization time (τ-P2). Changes due to circadian rhythm have been removed as described in Materials and methods (Fig. 3). (B) EOD waveforms sampled immediately before injection and at the time of peak effect. In the case of the saline injection (male 122) the two waveforms superimpose almost completely. Effects of serotonin varied between individuals. In some males (e.g. male 042), amplitude and τ-P2 both increased, whereas in others (e.g. male 036), repolarization time increased alone. Both males received saturating doses of 5-HT as indicated in Fig. 7 (male 042: 2.5 nmol g−1; male 036: 25 nmol g−1). Note the difference in the time courses of the τ-P2 response as well (see Fig. 8). As part of a different experiment, male 222 had a second male placed briefly in his hiding tube (first broken line). EOD amplitude and τ-P2 increased in a manner similar to EODs of fish injected with serotonin, then declined following the second male’s removal (second broken line).
Fig. 6.
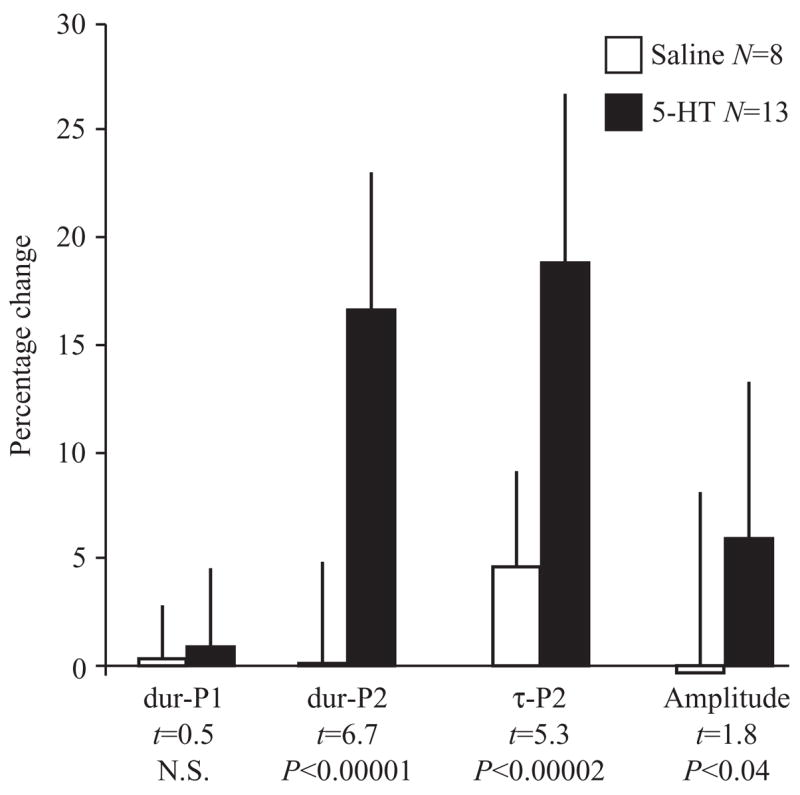
Percentage change (mean ± 1 S.D.) in responses of EOD waveform parameters to injection of serotonin at doses ≥2.5 nmol g−1, or to saline controls. EOD parameters were measured as in Fig. 2. Means were compared with two-sample t-tests; the P values shown are one-tailed. Compared to the controls, serotonin caused significant increases in amplitude, second phase duration (dur-P2), and second phase repolarization time (τ-P2), but not of first phase duration (dur-P1).
Fig. 7.
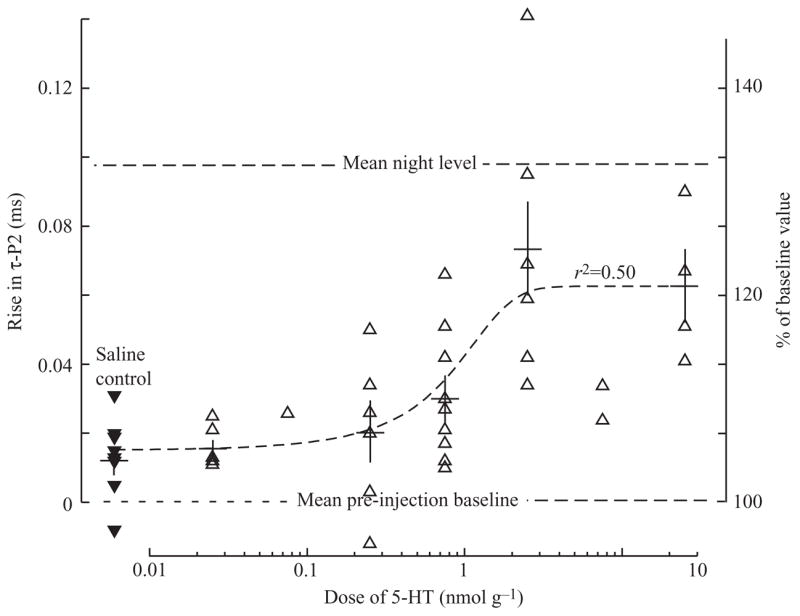
The serotonin dose–response curve for repolarization time (τ-P2) showing means (horizontal bars) and S.E.M. (vertical bars) for each dose, and a least-squares fit logistic function for all data. The response saturated at 2.5 μmol l−1 g−1, the dose we used for further experiments. The mean serotonin-induced rise at saturating doses is approx. 2/3 that of the circadian rhythm. The dose–response curve for amplitude was not significant and is not shown. Filled triangles, saline controls; open triangles, 5-HT-dosed.
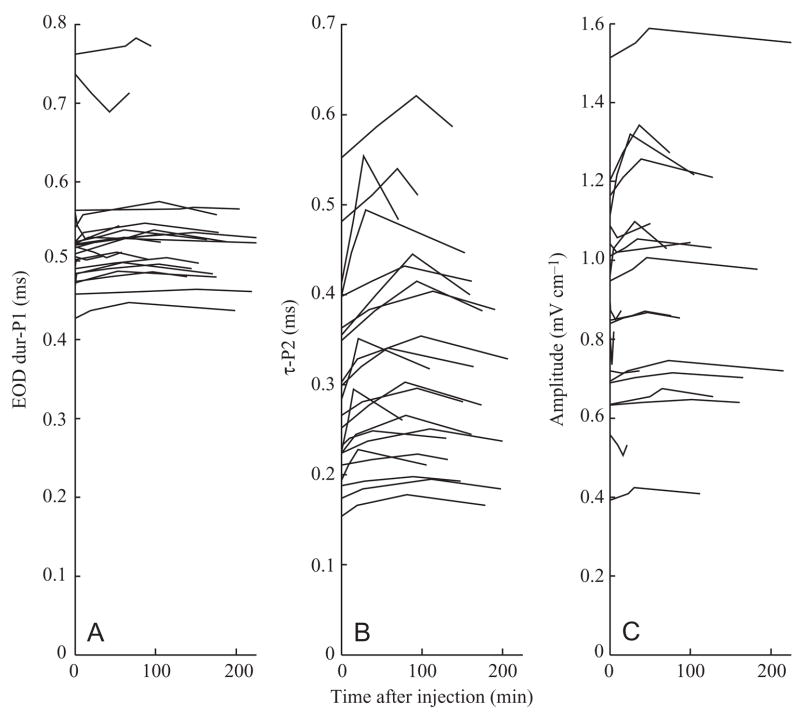
Fig. 8.
Time courses of EOD duration and amplitude responses for 5-HT doses of 0.75 nmol g−1 and higher. Points plotted are pre-injection baseline, 50% of peak, peak response, and response half-life. (A) Dur-P1 (duration of the first EOD phase) showed no clear response to 5-HT. (B) τ-P2, (repolarization time of the second phase) showed the clearest response, with a bimodal distribution of peaks occurring at approx. 25 and 90 min after injection. (C) Amplitude was variable; if it rose, it peaked by approx. 30 min.
Fig. 9.
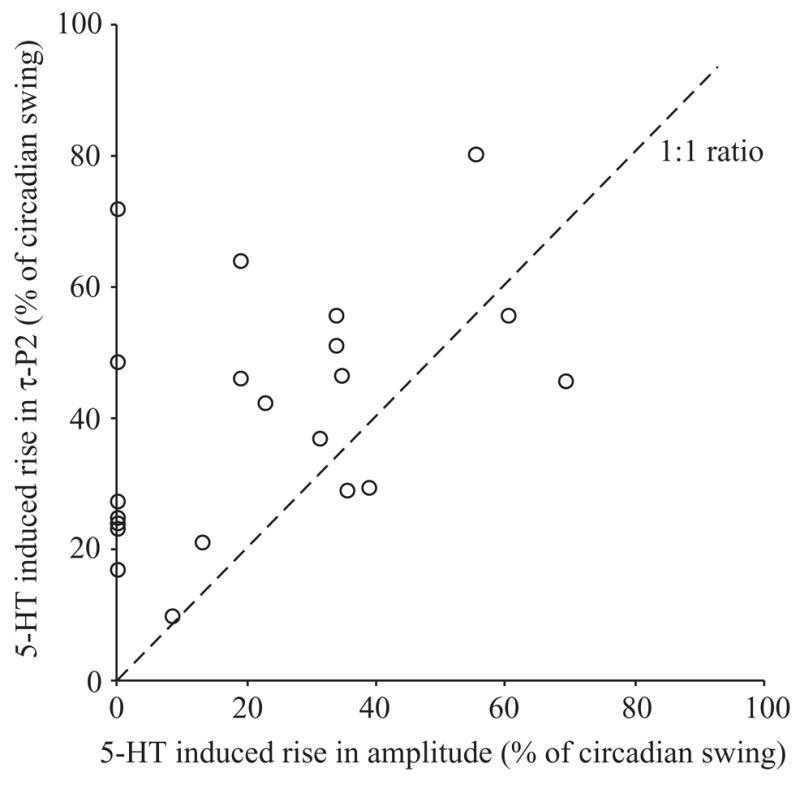
5-HT induced rise in EOD repolarization time (τ-P2) versus rise in amplitude, expressed as a percentage of the day–night swing, illustrates how the serotonin-induced changes in repolarization time and amplitude are semi-independent. Note that most of the data fall above the 1:1 ratio (broken) line, indicating that both variables may change or repolarization time may change alone, but amplitude never changes without a change in repolarization time. Values shown are for males injected with 5-HT doses 0.75 nmol g−1 and higher.
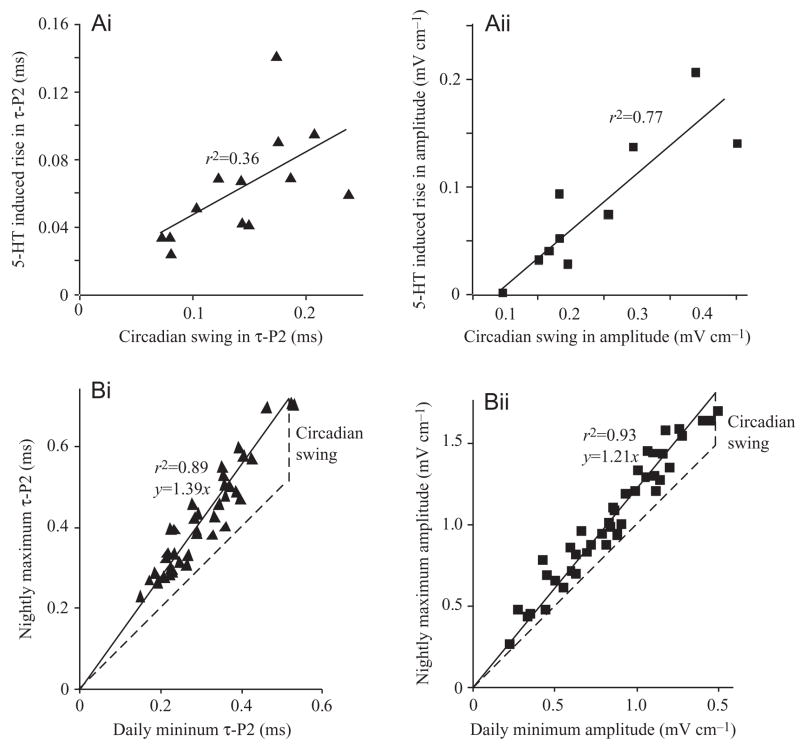
For saturating doses of serotonin (2.5 nmol g−1 and above), the rises in τ-P2 and amplitude associated strongly with the magnitudes of their respective circadian swings (Fig. 10A), parameters that have previously been shown to be under social control (Franchina et al., 2001). Linear regression showed that the magnitude of the endogenous circadian rhythm in amplitude accounted for approx. 75% of the variance in the serotonin-induced amplitude rise (r2=0.77; F=27; d.f.=1,8; P=0.0009). The magnitude of the endogenous rhythm in τ-P2 accounted for approx. one third of the variance in the 5-HT-induced rise in τ-P2 (r2=0.36; F=6.1; d.f.=1,11; P=0.03). Daily minima for all fish in the study were tightly associated with nightly maxima (Fig. 9B), (τ-P2: F=323; d.f.=1,39; P=3.5E-20), (amplitude: F=572; d.f.=1,41; P=2.6E-25). Thus the diurnal baseline is related to the circadian swing, and accordingly to the responsiveness to serotonin.
Fig. 10.
(A) The response of repolarization time (τ-P2) (Ai) and amplitude (Aii) to 5-HT doses ≥2.5 nmol g−1 varies as a function of the magnitude of the circadian swing. Thus individuals with large circadian oscillations in τ-P2 or amplitude were predisposed to increase τ-P2 or amplitude more when injected with 5-HT than were fish with smaller cycles. (B) Daily minima and nightly maxima of τ-P2 (Bi) and amplitude (Bii) are tightly correlated. Regression (solid) lines are forced through zero, and the unity slope (broken) line is shown for comparison. The difference between the solid regression line and broken unity slope line represents the day–night swing. Thus, the larger the baselines of τ-P2 or amplitude, the larger the nightly swings and the larger the responses to 5-HT.
Discussion
Our most unique discovery is the association between the magnitude of the circadian rhythm in the male’s EOD and the magnitude of the EOD’s rapid response to exogenous serotonin. Prior and ongoing research in our laboratory shows that the male’s social environment exerts a strong influence on the magnitude of the circadian rhythms in his waveform (Franchina et al., 2001). Social stimulation, particularly by a slightly smaller male, induces a rapid increase in amplitude baseline and circadian modulation over 2 h, followed by a continued increase over 3–9 days. τ-P2 increases over a period of 1 h, when a male is challenged (Fig. 5). EOD amplitude does the same, though with a more varied response course. The new finding of a strong relationship between the magnitude of the circadian swing and response to serotonin suggests that the social environment is regulating the EOD both via a short-term mechanism, perhaps serotonin release, and a longer term mechanism that could be mediated through the regulation of the serotonin receptors, their second messengers, or some other factor in the downstream chain to EOD generation. Clarification of serotonin’s role in modulation and regulation of the EOD waveform awaits further study. Moreover, we hope to determine whether circadian variation in EOD waveform is a cause or consequence of social position. Preliminary data suggest that the relationship is reciprocal.
The tight relationship between daily minima and nightly maxima (Fig. 10B) suggests that a common mechanism regulates both. For instance, EOD amplitude could be a function of the number of active voltage-gated sodium channels (or the number of contributing electrocytes), and a circadian modulator could alter the unitary conductance of those channels. Alternatively, EOD amplitude rhythms could function by taking electrocytes on and offline in a circadian rhythm, with a socially mediated regulator of the sodium current setting a fixed amplitude for every electrocyte.
Our data suggest τ-P2 is determined more by channel properties than by collective activity of electrocytes. If increases in τ-P2 were derived from desynchronization of the electrocytes, one would expect a drop in amplitude when τ-P2 increased and a rise in τ-P2 when amplitude decreased. Such effects were suggested by an early study that compared individuals rather than day–night changes within individuals (Hopkins et al., 1990). No such relationship is apparent in any of our data within individuals. Indeed τ-P2 frequently varies significantly while amplitude remains dead flat, but in over a thousand natural cycles examined to date, we have never seen an amplitude decline coupled to a τ-P2 increase. Thus we believe that modulation of τ-P2 derives in large part from changes in ion-channel kinetics at the level of individual electrocyte membranes.
Our general finding that serotonin enhances sexual dimorphism in the male’s EOD waveform might be surprising, in that serotonin is widely considered to be the neurotransmitter of social subordinates. Some researchers, however, now characterize serotonin in the broader context of social stress (Edwards and Kravitz, 1997; Overli et al., 1999; Summers, 2001). Numerous studies in other teleosts have shown that serotonin turnover is increased in the brainstem of socially subordinate individuals (Hoglund et al., 2000; Overli et al., 1999; Winberg and Lepage, 1998; Winberg et al., 1992, 1996, 1997, 2001). Yet we see here that serotonin quickly, but transiently, enhances a social signal typical of dominants, not subordinates (Hagedorn and Zelick, 1989). Our pharmacological characterization of the serotonin receptors in B. pinnicaudatus (P. K. Stoddard, M. R. Markham, V. L. Salazar and S. Allee, manuscript in preparation) suggests a solution to the paradox: we have found two pharmacologically distinct populations of postsynaptic serotonin receptors, a 5HT2A-like receptor that drives the EOD characters up, and 5HT1A-like receptor that drives them down. If the excitatory receptors have a shorter action latency or stronger binding affinity than the inhibitory receptors, the EOD could be initially enhanced by a surge in serotonin, then perhaps diminished if serotonin release continued for a long time. This differential time course would be consistent with results obtained in rainbow trout Oncorhynchus mykiss, where immediate serotonin activation in the telencephalon occurs in both members of a paired territorial encounter, but turnover remains high only in the loser (Overli et al., 1999), or in anole lizards Anolis carolinensis, where telencephalic serotonin turnover of social dominants increases briefly, but rises later in social subordinates and remains high longer (Summers et al., 1998). Alternately, if the 1A receptor is central and the 2A receptor peripheral, then our peripheral injections might be acting preferentially on the excitatory receptor. The action of serotonin via peripheral injection might be taken to indicate a peripheral action, because serotonin is thought not to cross the blood–brain barrier of adult mammals (Bouchaud, 1972; Deutch and Roth, 1999). In teleosts, however, the blood brain barrier is less exclusive and peripheral serotonin readily enters the brain (Genot et al., 1981). More recent studies show that the blood–brain barrier of rodents is made more permeable by serotonin, and by this mechanism serotonin can facilitate its own entry into the brain (Sharma et al., 1995; Winkler et al., 1995). Pending further investigation, we consider the CNS to be a plausible target for serotonin’s action, perhaps initiating the release of another substance that acts as the local modulator of EOD amplitude and duration. Different protein kinases have been shown to work in the periphery as part of second messenger systems that rapidly modulate the amplitude of sodium currents in other gymnotiform EODs (Emerick and Agnew, 1989; Gotter et al., 1997; McAnelly and Zakon, 1996), but the first messengers have yet to be identified.
Acknowledgments
We thank Maikel Couto for upkeep of the fish colony, David Berman for help in building the test enclosure, Vance Hodge for advice and logistic support with the pharmacology, Susan Allee, Jennifer Herrick, Carolina Hernandez for help with data collection and organization. Support and equipment were provided by NIH MBRS SCORE grant GM08205 to P.K.S. Experiments were approved in advance by the FIU IACUC and complied with the ‘Principles of Animal Care’ publication No. 86-23, revised 1985, of the National Institutes of Health.
References
- Bouchaud C. Démonstration par radiographie de l’existence d’une barrièr hématoencéphalique pour la 5-hydroxytryptamine [trans: Autoradiographic demonstration of an hematoencephalic barrier against 5-hydroxytryptamine] C R Acad Sci D. 1972;275:975–978. [PubMed] [Google Scholar]
- Curtis CC, Stoddard PK. Active mate choice in an electric fishBrachyhypopomus pinnicaudatus. Anim Behav in press. [Google Scholar]
- Deutch AY, Roth RH. Neurotransmitters. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San Diego: Academic Press; 1999. pp. 193–234. [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr Opin Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Emerick MC, Agnew WS. Identification of phosphorylation sites for adenosine 3′,5′-cyclic phosphate dependent protein kinase on the voltage-sensitive sodium channel from Electrophorus electricus. Biochemistry (Moscow) 1989;28:8367–8380. doi: 10.1021/bi00447a016. [DOI] [PubMed] [Google Scholar]
- Ferrari MB, McAnelly ML, Zakon HH. Individual variation in and androgen-modulation of the sodium current in electric organ. J Neurosci. 1995;15:4023–4032. doi: 10.1523/JNEUROSCI.15-05-04023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MB, Zakon HH. Conductances contributing to the action potential of Sternopygus electrocytes. J Comp Physiol A. 1993;173:281–292. doi: 10.1007/BF00212692. [DOI] [PubMed] [Google Scholar]
- Franchina CR. PhD thesis. Cornell University; USA: 1997. Ontogenetic, day-night, and socially mediated changes in the electric organ discharge waveform of a weakly electric fish (Gymnotiformes, Hypopomidae) [Google Scholar]
- Franchina CR, Salazar VL, Volmar CH, Stoddard PK. Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus. II. Social effects. J Comp Physiol A. 2001;187:45–52. doi: 10.1007/s003590000176. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Stoddard PK. Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus: I. Quantification of day-night changes. J Comp Physiol A. 1998;183:759–768. doi: 10.1007/s003590050299. [DOI] [PubMed] [Google Scholar]
- Genot G, Morfin R, Peyraud C. Blood-brain barrier for serotonin in the eel (Anguilla anguilla L.) Comp Biochem Physiol. 1981;68C:247–250. doi: 10.1016/0306-4492(81)90025-3. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Kaetzel MA, Dedman JR. A major second messenger mediator of Electrophorus electricus electric tissue is CaM kinase II. Comp Biochem Physiol. 1997;118A:81–91. doi: 10.1016/s0300-9629(96)00411-2. [DOI] [PubMed] [Google Scholar]
- Hagedorn M. The electric fish Hypopomus occidentalis can rapidly modulate the amplitude and duration of its electric organ discharges. Anim Behav. 1995;49:1409–1413. [Google Scholar]
- Hagedorn M, Zelick R. Relative dominance among males is expressed in the electric organ discharge characteristics of a weakly electric fish. Anim Behav. 1989;38:520–525. [Google Scholar]
- Hoglund E, Balm PH, Winberg S. Skin darkening, a potential social signal in subordinate arctic charr (Salvelinus alpinus): the regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. J Exp Biol. 2000;203:1711–1721. doi: 10.1242/jeb.203.11.1711. [DOI] [PubMed] [Google Scholar]
- Hopkins CD. Hypopomus pinnicaudatus (Hypopomidae) a new species of gymnotiform fish from French Guiana. Copeia. 1991;1991:151–161. [Google Scholar]
- Hopkins CD, Comfort NC, Bastian J, Bass AH. Functional analysis of sexual dimorphism in an electric fish, Hypopomus pinnicaudatus, order Gymnotiformes. Brain Behav Evol. 1990;35:350–367. doi: 10.1159/000115880. [DOI] [PubMed] [Google Scholar]
- Khan IA, Thomas P. Stimulatory effects of serotonin on maturational gonadotropin release in the Atlantic croaker, Micropogonias undulatus. Gen Comp Endocrinol. 1992;88:388–396. doi: 10.1016/0016-6480(92)90233-a. [DOI] [PubMed] [Google Scholar]
- Maler L, Ellis WG. Inter-male aggressive signals in weakly electric fish are modulated by monoamines. Behav Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- McAnelly L, Zakon HH. Protein kinase A activation increases sodium current magnitude in the electric organ of Sternopygus. J Neurosci. 1996;16:4383–4388. doi: 10.1523/JNEUROSCI.16-14-04383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnelly ML, Zakon HH. Coregulation of voltage-dependent kinetics of Na(+) and K(+) currents in electric organ. J Neurosci. 2000;20:3408–3414. doi: 10.1523/JNEUROSCI.20-09-03408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse M, Kawasaki M. Possible involvement of the ampullary electroreceptor system in detection of frequency-modulated electrocommunication signals in Eigenmannia. J Comp Physiol A. 1998;183:543–552. doi: 10.1007/s003590050280. [DOI] [PubMed] [Google Scholar]
- Overli O, Harris CA, Winberg S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol. 1999;54:263–275. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Westman J, Navarro JC, Dey PK, Nyberg F. Probable involvement of serotonin in the increased permeability of the blood-brain barrier by forced swimming. An experimental study using Evans Blue and 131I-sodium tracers in the rat. Behav Brain Res. 1995;72:189–196. doi: 10.1016/0166-4328(96)00170-2. [DOI] [PubMed] [Google Scholar]
- Simon NG. Hormonal processes in the development and expression of aggressive behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. Amsterdam: Academic Press; 2002. pp. 339–392. [Google Scholar]
- Stoddard PK. Electric signals: predation, sex, and environmental constraints. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ, editors. Advances in the Study of Behaviour. Vol. 31. London, New York: Academic Press; 2002. pp. 201–242. [Google Scholar]
- Stoddard PK, Rasnow B, Assad C. Electric organ discharges of the gymnotiform fishes: III. Brachyhypopomus. J Comp Physiol A. 1999;184:609–630. doi: 10.1007/s003590050359. [DOI] [PubMed] [Google Scholar]
- Summers CH. Mechanisms for quick and variable responses. Brain Behav Evol. 2001;57:283–292. doi: 10.1159/000047246. [DOI] [PubMed] [Google Scholar]
- Summers CH, Larson ET, Summers TR, Renner KJ, Greenberg N. Regional and temporal separation of serotonergic activity mediating social stress. Neuroscience. 1998;87:489–496. doi: 10.1016/s0306-4522(98)00144-4. [DOI] [PubMed] [Google Scholar]
- Teshiba T, Shamsian A, Yashar B, Yeh SR, Edwards DH, Krasne FB. Dual and opposing modulatory effects of serotonin on crayfish lateral giant escape command neurons. J Neurosci. 2001;21:4523–4529. doi: 10.1523/JNEUROSCI.21-12-04523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg S, Lepage O. Elevation of brain 5-HT activity, POMC expression, and plasma cortisol in socially subordinate rainbow trout. Am J Physiol. 1998;274:R645–654. doi: 10.1152/ajpregu.1998.274.3.R645. [DOI] [PubMed] [Google Scholar]
- Winberg S, Myrberg AA, Jr, Nilsson GE. Agonistic interactions affect brain serotonergic activity in an acanthopterygiian fish: the bicolor damselfish (Pomacentrus partitus) Brain Behav Evol. 1996;48:213–220. doi: 10.1159/000113199. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson GE, Olsen KH. Changes in brain serotonergic activity during hierarchic behavior in Arctic charr, Salvelinus alpinus (L.). are socially induced. J Comp Physiol A. 1992;170:93–99. doi: 10.1007/BF00190404. [DOI] [PubMed] [Google Scholar]
- Winberg S, Overli O, Lepage O. Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary L-tryptophan. J Exp Biol. 2001;204:3867–3876. doi: 10.1242/jeb.204.22.3867. [DOI] [PubMed] [Google Scholar]
- Winberg S, Winberg Y, Fernald RD. Effect of social rank on brain monoaminergic activity in a cichlid fish. Brain Behav Evol. 1997;49:230–236. doi: 10.1159/000112994. [DOI] [PubMed] [Google Scholar]
- Winkler T, Sharma HS, Stalberg E, Olsson Y, Dey PK. Impairment of blood-brain barrier function by serotonin induces desynchronization of spontaneous cerebral cortical activity: experimental observations in the anaesthetized rat. Neurosci. 1995;68:1097–1104. doi: 10.1016/0306-4522(95)00194-n. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci. 1997;17:697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]