Abstract
Weakly electric fish have long been known to express day–night oscillations in their discharge rates, and in the amplitude and duration of individual electric organ discharges (EODs). Because these oscillations are altered by social environment and neuroendocrine interactions, electric fish are excellent organisms for exploring the social and neuroendocrine regulation of circadian rhythm expression. Previous studies asserting that these oscillations are circadian rhythms have been criticized for failing to control temperature and randomize feeding regimes, or for running the fish under constant conditions for just 2–3 days. Here we show that the day–night oscillations in the EODs of the neotropical gymnotiform fish Brachyhypopomus pinnicaudatus free-run for over a week under constant photic and thermal conditions, and randomized food provisioning. Sex differences were apparent in strength and magnitude of the circadian oscillations; male oscillations were stronger and larger. All three parameters retain a common oscillation period while differing in the persistence of oscillation strength and magnitude, a difference consistent with proposals by others that declines of behavioral circadian rhythms may result from breakdowns downstream of the central oscillator.
Keywords: Circadian rhythms, Electric fish, Sex differences, Animal models
1. Introduction
Gymnotiform electric fish in the family Hypopomidae show pronounced day–night changes in their electric waveforms, changes that enhance the communication value of the signal at night when the fish are active and that save energy during the day when the fish are quiescent [1–4]. At the behavioral level, the day–night oscillations in the electric organ discharge (EOD) of male hypopomid electric fish are strongly influenced by social conditions [5]. Social environment appears to mediate changes in signal structure through the serotonergic system. The shape of the waveform is modulated over minutes at two anatomical levels, by serotonin acting in the brain, and by melanocortin peptides such as ACTH and alpha-MSH acting in the periphery [6,7]. The magnitudes of day–night oscillations differ between the sexes, and these sex differences can be enhanced by the application of non-aromatizable androgens [8].
The electric waveform is an elegant window into the physiology of the animal because it can be monitored instantaneously and non-invasively as these fish rest, swim, and interact socially and because its shape and oscillation are regulated by classic vertebrate neuroendocrine systems. In particular, gymnotiform electric fish can serve as a useful model system for understanding how the hypothalamic–pituitary–gonadal axis (HPG) interacts with the hypothalamic–pituitary–adrenal/interrenal axis (HPA/HPI) to regulate sex differences in circadian rhythms in melanocortins and glucocorticoids, circulating hormones that influence innumerable behavioral and physiological functions.
The full utility of this system as a broader vertebrate model hinges on whether the day–night oscillations in EOD waveform are true circadian rhythms, an assertion which has been called into question. The literature does contain examples of similar day–night oscillations entrained by Zeitgebers that have not been controlled in previous studies, e.g., behavioral rhythms that synchronize to a daily temperature oscillation of 0.9 °C [9], or endocrine rhythms that do not free-run in the absence of external entrainment [10,11]. Extensive evidence shows that circadian rhythms synchronize to feeding rhythms [12,13] and that feeding can entrain the molecular clocks in the master oscillators [14,15]. The touchstone of a true circadian rhythm is that it free-runs, i.e., continues to oscillate under constant conditions and in the absence of periodic entrainers in the environment such as cycles in light, temperature, disturbance, or food availability.
A few studies of gymnotiforms held under constant photic conditions have shown day–night oscillation in EOD waveform shape, discharge rate, and activity [6,16,17]. Franchina and Stoddard [2] ruled out locomotor activity as a driver of waveform rhythms, but none of these studies had excluded other possible Zeitgebers such as temperature or food availability. In this study we set out to determine whether the day–night changes in the EOD waveforms of the hypopomid electric fish, Brachyhypopomus pinnicaudatus, are in fact true circadian rhythms, persisting in the absence of periodic entrainment by light, temperature, food, or disturbance.
Previous reports have differed on whether EOD waveforms of female B. pinnicaudatus showed pronounced day–night oscillations or not. Franchina and Stoddard [2] reported rhythms to be missing or negligible in females, but Silva et al. [3] reported significant rhythms in female waveforms, albeit of lower magnitude than those seen in males. To resolve this conflict, we included equal numbers of males and females in this study.
2. Methods
2.1. Subjects
Our subjects were 8 male and 8 female sexually mature F10 and F11 captive-bred B. pinnicaudatus. Fish were reared and maintained in outdoor pools, then brought indoors 2 weeks prior to each experiment. Once indoors, fish were housed in 284 l aquaria in small social groups comprised of one mature male, one mature female, and one small female. Fish were fed small oligochaete “blackworms” ad libitum on a pre-determined, randomized schedule. Enough food was provided so that it was available at all times. During this pre-experimental period, we placed fresh water hyacinths in the indoor tanks every 2 days. Water conductivity was maintained at 100 μS/cm. The light cycle was approximately synchronous with the outdoor photoperiod (12:12 during winter and 14:10 during the end of summer). The water was kept at 27–28 °C by air exchange in the measurement room.
2.2. Experimental conditions
Fish were tested in two batches. In experiment 1, F10s (4 males, 4 females) were tested in the winter after the breeding season 6–26 Jan 2004. In experiment 2, F11s (4 males, 4 females) were tested near the end of the breeding season toward the end of Miami’s summer, 24 Sep–25 Oct 2004. We set the winter LD cycle 12:12 to match that of spring when the fish start to breed, and the late summer LD cycle to 14:10 to mimic peak summer photic conditions rather than equinox conditions of summer’s end when breeding normally ends. The light regimes were:
Expt. 1 LD 7 days, DD 7 days, LD 7 days, LL 7 days.
Expt. 2 LD 8 days, LL 9 days, LD 6 days, DD 8 days. (LL = constant light, DD = constant dark)
With the lights on, light intensity at the top of the aquarium was 265 l× in Experiment 1. Experiment 2 was conducted in a new test room with two tiers of aquaria; the light intensity was either 1325 or 53 l×, depending on whether fish were in the upper or lower aquarium racks. Males and females were evenly distributed through upper and lower racks. In both experiments, light levels experienced by the fish were often lower because they hid in unglazed ceramic shelter tubes during their subjective day when they were inactive. When the lights were off, the room was absolutely dark. Light baffles on the double-doorways and the air exchange vents prevented entry of stray light at any time. Aschoff’s rule [18] predicts stronger rhythms and period shifts under more intense light. The fact that our fish hide in dark tubes during their subjective days, combined with their extreme sensitivity to light [17], make them poor subjects in which to explore effects of light intensity on rhythmicity. For these reasons we did not examine effect of light intensity on rhythmicity. During LD periods, daily temperature oscillations of 1 °C were caused by the electric lights, but under experimental conditions of constant light or constant darkness, ongoing temperature recordings showed no measurable oscillation (resolution 0.1 °C). Other than at randomized times for feeding and plant replacement, nobody entered the experimental chamber during the experiment.
2.3. Social environment
Social stimulation by conspecifics can entrain rhythms [19], maintain rhythms [5,20,21], tighten their synchrony [20,22], or in some cases may have little effect at all [23]. Different sized social groups can exert opposite effects on period: Atlantic killifish (Fundulus heteroclitus) show more variable period lengths in circadian rhythms of color change when kept in groups of 5 than when kept singly or in groups of 25 in the same space [24]. We wished to measure unperturbed circadian rhythms, so we wanted individual fish to be socially isolated from one another. We have shown already that males’ day–night oscillations diminish sharply over a week’s time in social isolation [5] so we knew we were not likely to obtain reliable month-long recordings of isolated fish. We compromised by placing a small juvenile conspecific in the center compartment where it could interact electrically but could not contact the focal fish. We did so for males and female subjects alike to avoid any systematic bias. Presumably juveniles show circadian rhythms in their activity patterns, however the juveniles we chose lacked the size or social status to socially challenge an adult fish of either sex, an assumption we based on our observations that juveniles avoid adults and that larger males do not change the timing of their signal changes in the presence of smaller males (unpublished data). Our intention was that a juvenile would provide enough stimulation to the focal fish to keep oscillations sufficiently large to measure after a month’s time without engaging the focal animal enough to entrain its rhythm. Whether the activity of juveniles actually alters entrainment of adults cannot be ascertained at this time.
2.4. Measurement system
Our automated system for recording calibrated EODs from freely swimming fish is described in detail elsewhere [6]. For EOD recordings, fish were placed in the automated measurement tank, 120 × 44 × 44 cm, located in the light- and temperature-controlled room described above. EODs were amplified and digitized from carbon electrodes at opposite ends of the tank only when the fish was centered in the tank to accurately measure amplitude. We recorded EODs around the clock at intervals of ~1 min. Determination of fish position was done electronically. The system collected the first 9 EODs per sample that met threshold criteria within a 1 s window. Where fish amplitudes were particularly low (e.g., some females) the fish position detector thresholds were set close to the noise floor, and were more prone to mis-detection, resulting in noisy amplitude data. Likewise overlap between EODs of focal fish and juvenile companion fish produced some false triggering, particularly at night. False triggers were a sufficiently small proportion of the data that they could be removed with smoothing spline functions applied to the data clusters (described below).
2.5. Data analysis
We analyzed three signal parameters (Fig. 1) already known to oscillate on a day–night rhythm: EOD discharge rate and the EOD waveform’s amplitude and duration [2,17]. For each sample of 9 EOD pulses (inside a 1 s window), we took the median value as representative. EOD rate was computed as the reciprocal of the intervals in those samples in which EOD pulses were successive with no detection gaps (Fig. 1). EOD waveform amplitude was measured peak-to-peak. Waveform duration was parameterized as tauP2, the time constant of an inverse exponential function fit to the repolarization phase of the time-varying 2nd phase of the EOD (equation in Fig. 1) [6]. tauP2 should not be confused with the period of the circadian rhythm.
Fig. 1.
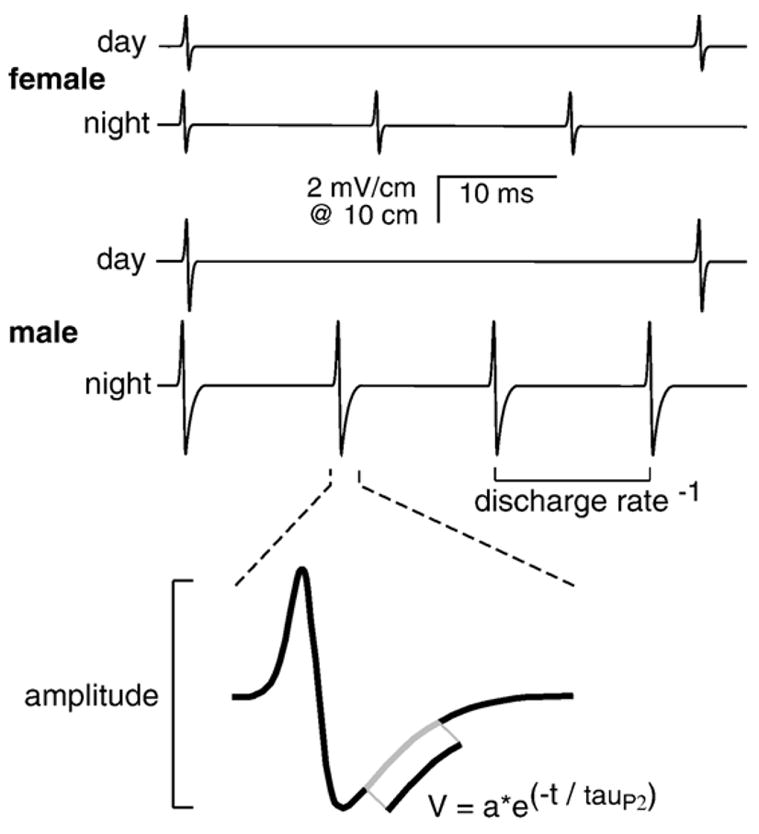
Three parameters measured for each EOD sample are discharge rate, peak-to-peak amplitude, and tauP2, the time constant of repolarization of the 2nd phase (see equation in figure). Male and female Brachyhypopomus pinnicaudatus have sexually distinct waveforms. Both sexes discharge faster at night than during the day.
The computer collected and measured EODs on irregular intervals of approximately a minute, determined by when the fish passed through the calibrated cylinder in the center of each tank. Irregularly sampled data do not constitute the true time series needed for periodic analysis. To obtain a regular time series, we fit a smoothing cubic spline to the data (csaps.m, Spline Toolbox v3.2.2 for MATLAB, Mathworks, Natick, MA), then resampled at 1 min intervals (Fig. 2). In two individuals we were unable to fit splines to one of their 12 light-treatment× variable blocks because of poorly sampled data. These were deleted from subsequent analysis.
Fig. 2.
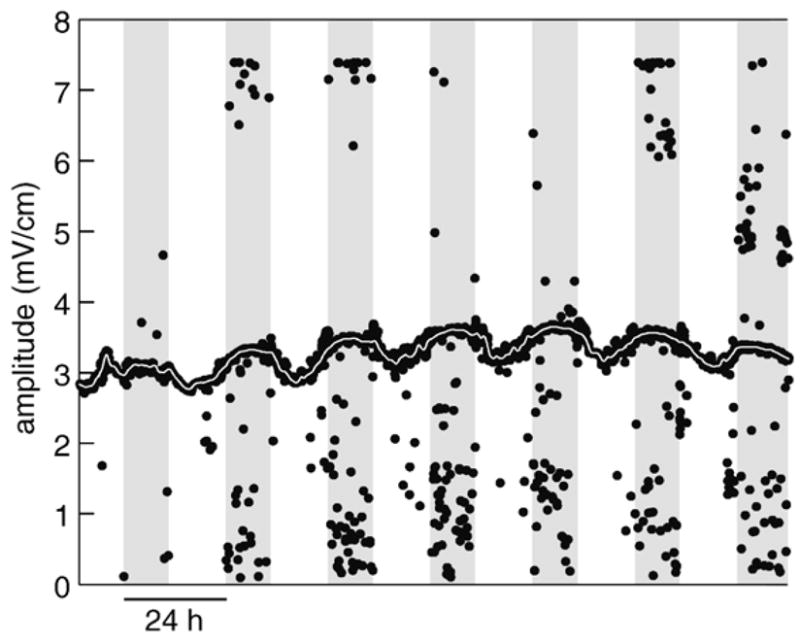
Raw data (black dots) and spline-fit data (thin white line) are shown for 1 week of amplitude recordings for one individual male. The raw data points that fall far from the clusters are most common when the EODs of the juvenile companion fish overlapped with those of the focal fish. Insensitivity of the smoothing spline algorithm to outliers is evidenced by how well the spline-fit line stays within the raw point cluster.
To explore the envelope of the circadian rhythms, we detrended the data by subtracting a version put through a 4-pole Butterworth lowpass filter with a corner set to 2.5 days. A forward–backward filter algorithm double-filtered the data without altering spectral phase (filtfilt.m, Signal Processing Toolbox v. 6.4 for MATLAB, Mathworks). We eliminated the first period of each light condition block to eliminate the transients that often occur following a light shift [25]. Detrended data are shown in Fig. 3 for all subjects in the experiment.
Fig. 3.
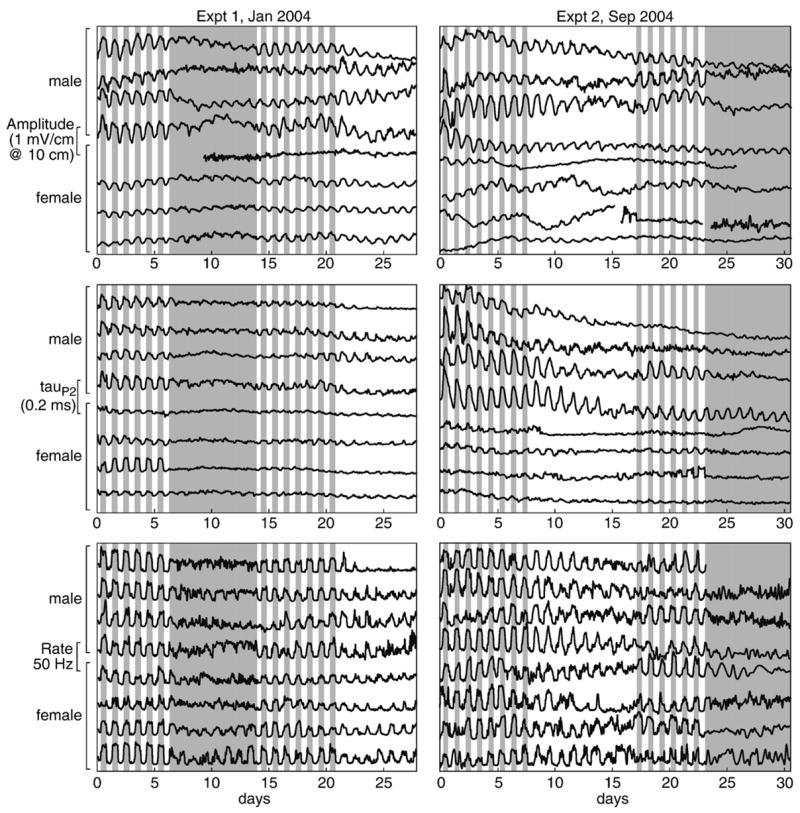
Detrended circadian oscillations in the 3 measured parameters are shown throughout the month long sample periods for each experiment. The gray-shaded areas indicate lights-out condition. In a few instances, a tank produced too many bad data points to resolve the pattern with a smoothing-spline fit: unresolved data are represented here by gaps in the lines. Rhythms free-ran under constant light and constant dark but generally remained stronger under constant light than under constant darkness. Likewise, rhythms were generally larger in magnitude for males than females, although females showed rhythms in discharge rate similar to those of males. Female rhythms free-ran much stronger for amplitude than for tauP2 where they seemed to disappear entirely in some individuals.
Strength of the circadian oscillation was determined from the autocorrelation index at the peak that lay 2 periods (~48 h) away from the center peak (xcorr.m, Signal Processing Toolbox v. 6.4 for MATLAB, Mathworks, called by autoco.m, Fly Toolbox for MATLAB, Jeff Hall lab, Brandeis Univ.). Magnitude of the circadian oscillation was calculated from the peak-to-peak variation of smoothed data within a period. We calculated the magnitude of the circadian oscillation by smoothing the spline-fit data (filtered as above except the corner was set to 3 h), then selecting the maximum and minimum for each ~24 h period. Oscillation magnitude, or “swing”, within a week-long experimental period is the mean of the corresponding 24 h maxima minus the mean of the 24 h minima.
Circadian period was determined by two methods for each fish for each analysis block (e.g., LD1, DD, LD2, LL): autocorrelation (xcorr.m, Signal Processing Toolbox v. 6.4 for MATLAB, Mathworks, called by autoco.m, Fly Toolbox for MATLAB, Jeff Hall lab, Brandeis Univ.), basing our estimate on the peak nearest 48 h [26], and maximum spectral entropy analysis or MESA (mesa.m Signal Processing Toolbox v. 6.4 for MATLAB, Mathworks, called by d_mesa.m, Fly Toolbox). All the time series points were used for autocorrelation, but trial-and-error experimentation revealed that downsampling to 30 min intervals gave the best resolution of circadian period by MESA. After correcting for period doubling and halving by MESA, comparison of the periods measured with the two methods still showed that autocorrelation values were more tightly clustered (autocorrelation S.D. = 1.32 vs. MESA S.D. = 2.37), suggesting that the MESA analysis was less reliable for these data (Fig. 4). For this reason, we relied on autocorrelation for further analysis of period. Statistical analysis was performed with routines in the Statistics Toolbox v. 5.1 for MATLAB (Mathworks). F statistics were produced by multiway ANOVA with light treatment, EOD parameter, sex, and experiment number as fixed effects. All listed error terms are standard deviations.
Fig. 4.
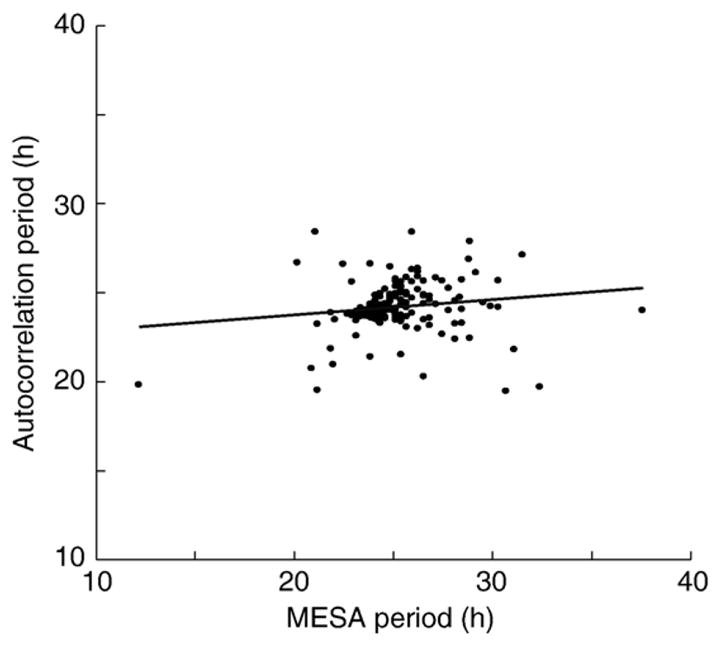
We compared two methods of period estimation. MESA produced more scatter than autocorrelation. The slope of the comparison (m = 0.078) was closer to zero than to the predicted 1.0. For this reason, we used autocorrelation to estimate period, rather than MESA or a combination of the two methods.
3. Results
Before evaluate in circadian oscillation of electric signal parameters we must be sure they show circadian periodicity. Degree of periodicity, the strength of a circadian rhythm, is quantified by the index of the autocorrelation function [26]. Plots of autocorrelation period by autocorrelation index (Fig. 5) show that as the index drops below about 0.33, the period becomes more variable, and the measurement of period becomes less reliable.
Fig. 5.
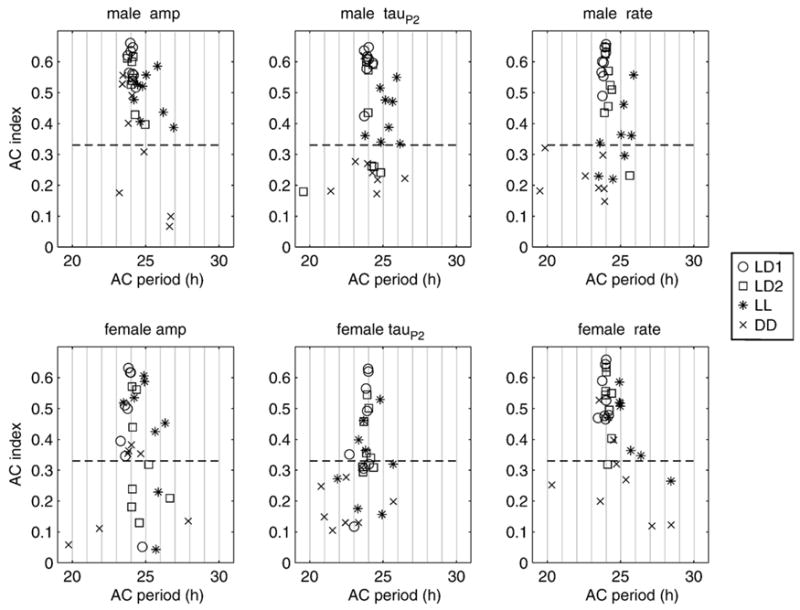
Plots of autocorrelation strength (AC index) as a function of autocorrelation period show that rhythms in waveform parameters and discharge rate were robust in both sexes under all light conditions except constant darkness where they tended to break down (low AC index). Periods under constant light (* symbols) ran longer than under constant dark or photoentrained conditions. Rhythm periods appear most consistent when the AC index is above 0.33 (dashed lines).
Rhythms were weaker (autocorrelation index was lower) in the 2nd experiment than in the first (X̄ = 0.44 ± 0.16 vs. X̄ = 0.39 ± 0.17, F = 7.88, P = 0.006), consistent with our subjective impression that waveform rhythms begin to weaken late in the season. Significant sex differences in autocorrelation indices indicate that males have stronger rhythmicity than females overall (sex: F = 11.08, 1 df, P = 0.001), an effect that became more apparent in the 2nd experiment (expt × sex interaction: F = 8.66, 1 df, p = 0.004).
Strong rhythms with autocorrelation indices above 0.33 were sustained under free-running conditions, both constant light and constant dark (Fig. 5). Rhythms for all three parameters were significantly stronger under photoentrained conditions than free-running and were stronger under constant light than under constant darkness (Table 1), effects that did not differ by sex (sex × light: F = 1.4, 3 df, P = 0.24) or by experiment (expt × light: F = 0.44, 3 df, P = 0.73).
Table 1.
Autocorrelation strengths (mean autocorrelation index ± S.D.) are compared across all three parameters with 2-sample t-tests
| (a)
| |||||
|---|---|---|---|---|---|
| LD1 and LL2 | LL and DD | t | df | P | |
| Amplitude | 0.48 ± 0.16 | 0.38 ± 0.17 | 2.38 | 61 | 0.02 |
| tauP2 | 0.45 ± 0.16 | 0.31 ± 0.14 | 3.80 | 62 | < 0.001* |
| Rate | 0.54 ± 0.10 | 0.33 ± 0.14 | 3.19 | 61 | < 0.001* |
| (b)
| |||||
| LL | DD | t | df | P | |
|
| |||||
| Amplitude | 0.46 ± 0.12 | 0.29 ± 0.17 | 3.19 | 29 | 0.003* |
| tauP2 | 0.38 ± 0.12 | 0.23 ± 0.12 | 3.54 | 30 | 0.001* |
| Rate | 0.40 ± 0.12 | 0.25 ± 0.11 | 3.63 | 29 | 0.001* |
Rhythms are stronger under photoentrainment than free-running, and are stronger free-running under constant light than under constant darkness. Two-tailed alpha of 0.05, when corrected for 6 comparisons by the conservative Bonferroni-adjustment, is 0.0083 (* significant in table). Neither sex nor experiment interactions terms were significant in multiway ANOVA, so these data are pooled across both terms.
Differences between parameters were so large in the multiway ANOVA that they swamped all other effects, so each parameter was analyzed separately. Experiments 1 and 2 were not significantly different, so results were pooled across the two experiments. In parallel with period strength, period magnitude differed strikingly by sex. Here we considered LD1 and LL as representative of photo-entrained and free-running conditions.
Under both photoentrained and constant light conditions absolute day–night swing was significantly larger in males than in females for waveform amplitude and tauP2, but not for discharge rate (Fig. 6). Sex differences were most striking in the initial LD1 period: mean swing in male EOD amplitude was twice that of females, and mean swing in male tauP2 was three times that of females (Fig. 6). Relative day–night swing, the swing expressed as a fraction of the baseline, showed the same pattern, but the effects were neither as large nor as statistically robust (Fig. 6).
Fig. 6.
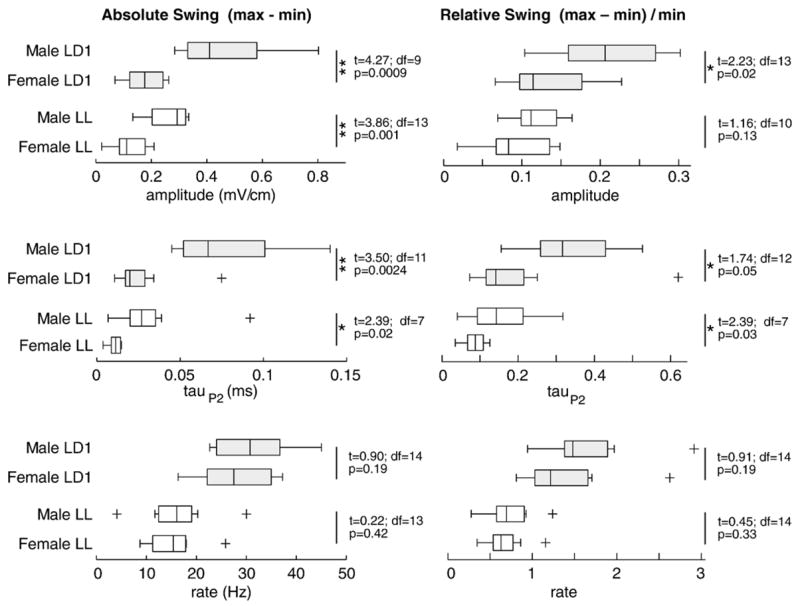
Absolute and relative magnitude of the circadian oscillation (daily max–min) is compared for the initial photoentrained period (LD1) and free-running under constant light (LL) with Tukey boxplots and two-sample t-tests assuming unequal variance. For waveform amplitude and tauP2 absolute swing is larger in males than females under photoentrained conditions. The differences become smaller under constant light. Relative swing is also larger in males under photoentrained conditions, but the sex differences diminish further under constant light. Sex differences in the day–night swing of discharge rate were not significant. The boxes have lines at the lower quartile, median, and upper quartile values; whiskers extend from each end of the boxes to show the extent of the rest of the data; outliers beyond 3 quartiles are indicated by ‘+’ symbols. One asterisk (*) indicates one-tailed significance with alpha = 0.05; two asterisks (**) indicate significance with alpha = 0.0083 (0.05 Bonferroni-adjusted for 6 comparisons). Although parameters are lower under LL than LD1, the differences could be due to increased duration in the apparatus or light treatment, and thus are not interpreted further.
To avoid confounding our measurements of period length with non-periodic data, we removed period length records where the autocorrelation index was less than 0.27, the highest threshold that retained enough data for full rank multiway ANOVA with the terms of interest. Because females had weaker rhythms, they lost more data, so we have tried to be careful not to base conclusions on interaction terms that may have lost too many data. For example, female DD data are especially weak for tauP2 (Fig. 5), so their necessary removal overweighs male tauP2 under DD on interaction terms involving EOD parameter, sex, and light treatment.
Period length did not differ across the experiments (X̄ = 24.17 h ± 0.54 vs. X̄ = 24.27 h ± 1.09, F = 0.13, P = 0.72), nor between the sexes (male X = 24.29 h ± 0.91 vs. female X̄ = 24.14 h ± 0.76, F = 0.07, P = 0.79), so the two experiments and the two sexes were pooled for further analysis of period length. Period lengths differed significantly by light treatment (F = 38.88, 3 df, P < 0.0001), so we evaluated period differences between light treatments with post-hoc tests (Table 2).
Table 2.
Post-hoc comparison using 2-sample t-tests show that autocorrelation period runs longer under constant light (LL) than when photoentrained (LD1) or under constant darkness (DD) for each EOD variable and for the mean of all three variables
| (a)
| |||||
|---|---|---|---|---|---|
| LD1 | LL | t | df | P | |
| Amplitude | 23.89 ± 0.26 | 25.11 ± 0.95 | 4.77 | 27 | b0.0001* |
| tauP2 | 23.84 ± 0.35 | 24.63 ± 1.20 | 2.46 | 27 | 0.021 |
| Rate | 23.89 ± 0.17 | 25.15 ± 0.73 | 6.73 | 27 | b0.0001* |
| Mean | 23.88 ± 0.17 | 25.00 ± 0.80 | 5.48 | 30 | b0.0001* |
| (b)
| |||||
| LL | DD | t | df | P | |
|
| |||||
| Amplitude | 25.11 ± 0.95 | 23.99 ± 1.20 | 3.23 | 21 | 0.004* |
| tauP2 | 24.63 ± 1.20 | 23.35 ± 0.58 | 2.27 | 17 | 0.037 |
| Rate | 25.15 ± 0.72 | 23.30 ± 1.98 | 2.99 | 16 | 0.009 |
| Mean | 25.00 ± 0.80 | 23.52 ± 1.31 | 3.71 | 26 | 0.001* |
Two-tailed alpha of 0.05, when corrected for 8 comparisons is 0.0063 (* significant in table). Neither sex nor experiment were significant in multiway ANOVA, so period data are pooled across both terms.
LL periods were significantly longer (by 1.52 h) than DD periods (Table 2). This effect is clearly evident from visual inspection of Fig. 5 in which the LL symbols (*) are consistently to the right of the others. Double raster plots (Fig. 7) of each variable for a representative male and female in each experiment show the pronounced phase lag during LL compared with LD or DD.
Fig. 7.
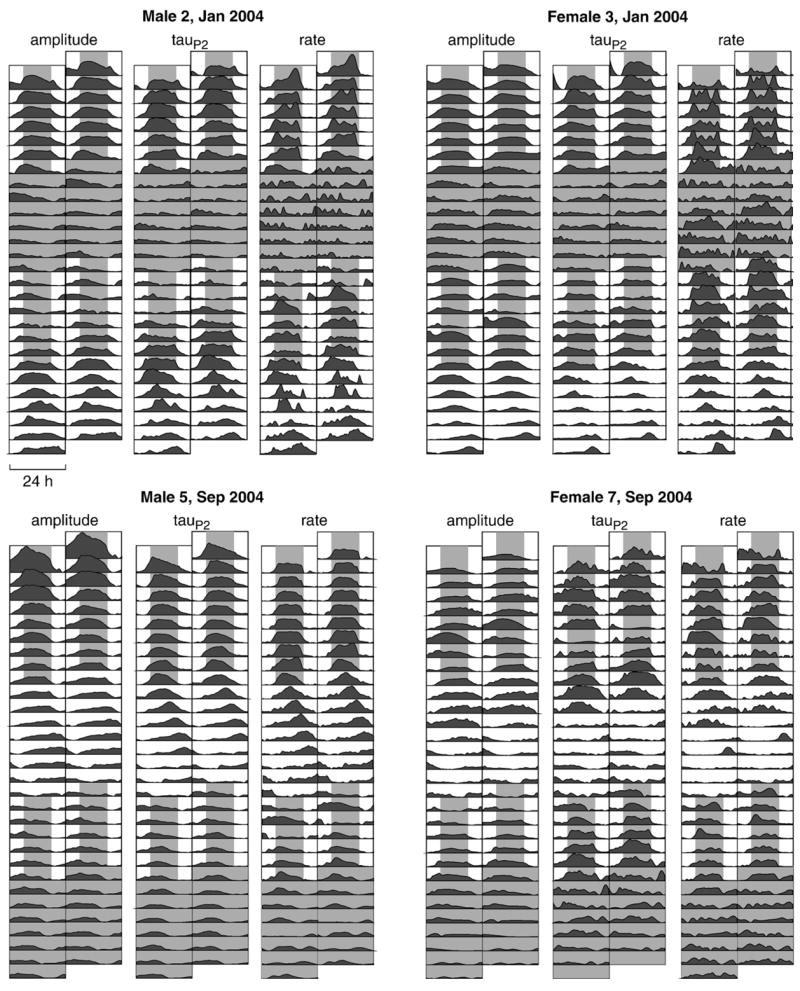
Double raster plots of all three variables are shown here for two representative males and females. Other than 24 h time-binning, data are the same detrended data as shown in Fig. 3. Phase lag is particularly noticeable during constant light, and a slight phase advance is seen under constant darkness. Female 3 maintained robust rhythms under constant photic conditions, but rhythms in female 7 disappear after 2–4 days under constant photic conditions.
To determine whether phase differed across the three parameters (amplitude, tauP2, rate), we calculated within-individual differences in phase between each pair of parameters in the 1st LD period when we expected fully entrained rhythms, and in the LL period when rhythms were free-running. Non-periodic data were removed by culling any detrended block with an autocorrelation index below 0.15, the highest threshold that retained enough data for a full rank multiway ANOVA with the light treatment and individual as fixed effect terms. Phase differences were calculated as the temporal offset of the peak of the cross-correlation function. Phase differences were further transformed by taking the absolute value, with a square-root transform to restore normality. The alternate hypothesis of separate oscillators would result in larger mean offsets under free-running conditions than entraining conditions. Fig. 8 shows the lack of a systematic difference between intra-individual phase comparisons. Likewise, multiway ANOVA with fixed effects of light and individual revealed no significant difference in either main effect (light: F = 1.18, 1 df, p = 0.28; individual: F = 1.37, 15 df, p = 0.19) nor in the key interaction term (light×individual: F = 1.08, 15 df, p = 0.40). Thus, the three signal parameters appear to follow a common oscillation, a finding consistent with visual inspection of representative data in the 2-period raster plot (Fig. 7).
Fig. 8.
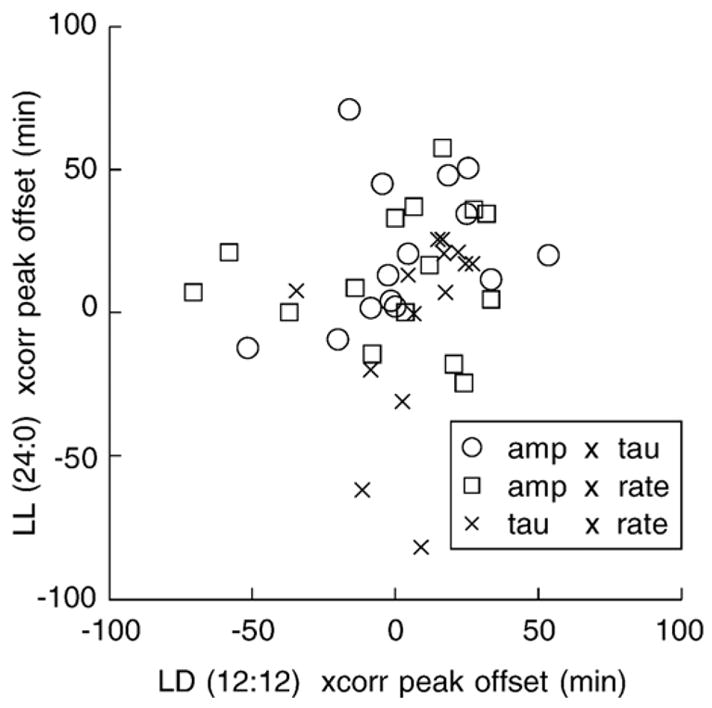
Phase differences between parameters are evaluated by comparing peak offsets of cross-correlations. Phase differences do not appear to differ systematically between photoentrained (abscissa) and free-running constant light conditions (ordinate). Lack of a systematic difference is consistent with the hypothesis that the three parameters, amplitude, tauP2, and rate, are driven by common or coupled oscillators.
4. Discussion
Circadian rhythms in electric signal parameters (amplitude, tauP2, and discharge rate) do not conform to the standard predictions of Aschoff’s rule for a typical nocturnal animal. Atypical of a nocturnal animal, their free-running periods ran longer than 24 h and longer under LL than DD. Typical of a nocturnal animal, their free-running rhythms were stronger under LL than DD [18]. Schwassmann [17] found the same pattern for activity records in two gymnotiforms Gymnorhamphichthys sp. and “Hypopomus sp.” (probably Brachyhypopomus sp.).
The three rhythms examined appear linked in some ways and not in others. Within individuals, all three signal parameters free-ran at the same period and phase suggesting they are driven by common or tightly linked oscillators. But in spite of a common oscillation period for EOD amplitude, tauP2, and rate, the magnitudes of the circadian oscillations vary independently. The periodicity of the oscillations strengthened across the two LD periods even though the absolute magnitudes of the oscillations weakened over these same periods, particularly for males. The fact that all three parameters retain a common oscillation period while differing in the persistence of oscillation strength (autocorrelation index) and peak-to-peak magnitude suggests these declines in rhythmic oscillation are not caused by a breakdown in the rhythm of a central oscillator, but occur in the output stages, most likely downstream of the central oscillator. This phenomenon may be a general one. Declines in circadian rhythmicity in aging humans are also believed to result from changes downstream from the pacemaker [27].
EOD amplitude, tauP2, and discharge rate are generated in different structures and modulated locally by different chemicals. Signal amplitude and tauP2 are generated at the cellular level in the peripheral electric organ. Melanocortin peptides (e.g., ACTH and alpha-MSH) act directly on electrocytes and act via the cAMP/PKA phosphorylation pathway to regulate the kinetics of voltage-gated sodium and potassium channels [7,28,29]. Centrally, however, these waveform parameters appear to be regulated by serotonin acting at 5HT1A and 5HT2A receptors [6,30]. Signal discharge rate, in contrast to the waveform parameters, is set in the pacemaker nucleus of the medulla, which in turn is regulated by glutamatergic and GABAergic input from diencephalic prepacemaker nuclei [31,32]. We have seen that EOD rate is also modulated by the same 5HT1A-selective drugs that modulate waveform amplitude and tauP2, and is in the same direction: the agonist 8-OH-DPAT decreases amplitude, tauP2, and discharge rate, whereas the antagonist WAY100635 increases all three (unpublished data). In the gymnotiform Apteronotus leptorhynchus, both the periventricular region of hypothalamus and the prepacemaker region receive serotonergic input, consistent with the possibility that a common circadian oscillation is conveyed to both control regions by serotonergic projections [33].
Free-running waveform rhythms were highly variable in females, undeniably robust in some individuals (e.g., female 3 in Fig. 7), while virtually absent in others. While rhythms in waveform were less periodic and lower in magnitude overall in females than in males, we can no longer discount them as insignificant. Indeed, sex differences in magnitudes of the amplitude and tauP2 rhythms were less pronounced when the magnitudes were expressed relative to the daily minimum (Fig. 6). That the sex differences in oscillation magnitude are reduced when expressed as a percentage of the minimum suggests that much of the sex difference in oscillation magnitude is due to males having larger waveform parameters (greater amplitude and longer 2nd phase) than females [2,34]. The sex differences in absolute oscillation magnitudes thus appear to reflect baseline sex differences in waveform. However, some sex difference does remain in the relative magnitude of the oscillation, suggesting that the rhythms in the humoral agents that modulate amplitude and tauP2 (likely the melanocortins alpha-MSH and ACTH [35]) also differ in magnitude between the sexes.
Our use of juveniles as social companions achieved its intended result: we were able to record rhythms throughout most of the month in most individuals. In Expt. 2, few individuals lost all evidence of rhythmicity by the end. In prior experiments on males without a juvenile conspecific as a tank companion, sharp declines in day–night oscillation magnitudes of amplitude and tauP2 occurred in just a week [2,5]. Silva et al. [36] also report a small but consistent decline in scotophase discharge rate when sexually mature B. pinnicaudatus are housed apart from sexually mature conspecifics. All available evidence suggests that the nature of the social environment determines the coupling of the central circadian oscillator to output structures in these fish, making them a valuable model species for investigating social modulations of pacemaker–effector coupling. The effect of social environment on the nature and strength of this coupling is the focus of our current work.
Acknowledgments
The research was supported by NIH grants MBRS GM08205 to PKS and MH064550 to MRM. Heather Gamper provided excellent care of the animals. Software development was accelerated by Jeff Hall’s lab and their FlyToolbox for circadian rhythms analysis. Dusty Dowse and Joel Levine graciously assisted PKS through the nuances of MESA analysis. Experiments were approved in advance by the FIU IACUC and complied with the “Principles of Animal Care” publication No. 86-23, revised 1985, of the National Institutes of Health. This paper is contribution 113 to the Program in Tropical Biology at Florida International University.
References
- 1.Hagedorn M. The electric fish Hypopomus occidentalis can rapidly modulate the amplitude and duration of its electric organ discharges. Anim Behav. 1995;49(5):1409–13. [Google Scholar]
- 2.Franchina CR, Stoddard PK. Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus: I. Quantification of day–night changes. J Comp Physiol A Sens Neural Behav Physiol. 1998;183(6):759–68. doi: 10.1007/s003590050299. [DOI] [PubMed] [Google Scholar]
- 3.Silva A, Quintana L, Galeano M, Errandonea P, Macadar O. Water temperature sensitivity of EOD waveform in Brachyhypopomus pinnicaudatus. J Comp Physiol A Sens Neural Behav Physiol. 1999;185(2):187–97. [Google Scholar]
- 4.Salazar VL. The energetic cost of bioelectrogenesis in the pulse-type gymnotiform fish Brachyhypopomus pinnicaudatus [Masters Thesis] Florida International University; 2003. [Google Scholar]
- 5.Franchina CR, Salazar VL, Volmar CH, Stoddard PK. Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus: II. Social effects. J Comp Physiol A Sens Neural Behav Physiol. 2001;187(1):45–52. doi: 10.1007/s003590000176. [DOI] [PubMed] [Google Scholar]
- 6.Stoddard PK, Markham MR, Salazar VL. Serotonin modulates the electric waveform of the gymnotiform electric fish Brachyhypopomus pinnicaudatus. J Exp Biol. 2003;206(Pt 8):1353–62. doi: 10.1242/jeb.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markham MR, Stoddard PK. Adrenocorticotropic hormone enhances the masculinity of an electric communication signal by modulating the waveform and timing of action potentials within individual cells. J Neurosci. 2005;25(38):8746–54. doi: 10.1523/JNEUROSCI.2809-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoddard PK, Zakon H, Markham MR, McAnelly L. Regulation and modulation of electric waveforms in gymnotiform electric fish. J Comp Physiol A Sens Neural Behav Physiol. 2006 doi: 10.1007/s00359-006-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman K. Zum einfluss der zeitgeberstarke auf die phasenlage der synchronisierten circadianen periodic. Z Vgl Physiol. 1969;62:93–110. [Google Scholar]
- 10.Ferraro JS, Steger RW. Diurnal variations in brain serotonin are driven by the photic cycle and are not circadian in nature. Brain Res. 1990;512(1):121–4. doi: 10.1016/0006-8993(90)91179-k. [DOI] [PubMed] [Google Scholar]
- 11.Kemppainen RJ, Sartin JL. Evidence for episodic but not circadian activity in plasma concentrations of adrenocorticotrophin, cortisol and thyroxine in dogs. J Endocrinol. 1984;103(2):219–26. doi: 10.1677/joe.0.1030219. [DOI] [PubMed] [Google Scholar]
- 12.Boulos Z, Terman M. Food availability and daily biological rhythms. Neurosci Biobehav Rev. 1980;4(2):119–31. doi: 10.1016/0149-7634(80)90010-x. [DOI] [PubMed] [Google Scholar]
- 13.Lamont EW, Diaz LR, Barry-Shaw J, Stewart J, Amir S. Daily restricted feeding rescues a rhythm of period2 expression in the arrhythmic suprachiasmatic nucleus. Neuroscience. 2005;132(2):245–8. doi: 10.1016/j.neuroscience.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Castillo MR, Hochstetler KJ, Tavernier RJ, Jr, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R551–5. doi: 10.1152/ajpregu.00247.2004. [DOI] [PubMed] [Google Scholar]
- 15.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17(4):284–92. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 16.Deng T-S, Tseng T-C. Evidence of circadian rhythm of electric discharge in Eigenmannia virescens system. Chronobiol Int. 2000;17(1):43–8. doi: 10.1081/cbi-100101030. [DOI] [PubMed] [Google Scholar]
- 17.Schwassmann HO. Circadian activity patterns in gymnotid electric fish. In: Menaker M, editor. Biochronometry. Friday Harbor, Washington: National Academy of Sciences; 1971. pp. 186–99. [Google Scholar]
- 18.Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Goel N, Lee TM. Sex differences and effects of social cues on daily rhythms following phase advances in Octodon degus. Physiol Behav. 1995;58(2):205–13. doi: 10.1016/0031-9384(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 20.Müller K. Locomotor activity in whitefish shoals (Coregonus lavaretus) In: Thorpe JE, editor. Rhythmic activity of fishes. New York: Academic Press; 1978. pp. 91–104. [Google Scholar]
- 21.Kavaliers M. Circadian activity of the white sucker, Catostomus commersoni: comparison of individual and shoaling fish. Can J Zool. 1980;58(8):1399–403. doi: 10.1139/z80-192. [DOI] [PubMed] [Google Scholar]
- 22.Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298(5600):2010–2. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- 23.Chen WM, Naruse M, Tabata M. The effect of social interactions on circadian self-feeding rhythms in rainbow trout Oncorhynchus mykiss Walbaum. Physiol Behav. 2002;76(2):281–7. doi: 10.1016/s0031-9384(02)00700-x. [DOI] [PubMed] [Google Scholar]
- 24.Kavaliers M. Social groupings and circadian activity of the killifish, Fundulus heteroclitus. Biol Bull. 1980;158:69–76. [Google Scholar]
- 25.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: I. The stability and lability of spontaneous frequency. J Comp Physiol A Sens Neural Behav Physiol. 1976;106:223–52. [Google Scholar]
- 26.Levine JD, Funes P, Dowse HB, Hall JC. Signal analysis of behavioral and molecular cycles. BioMed Central Neurosci. 2002;3(1):1. doi: 10.1186/1471-2202-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monk TH, Kupfer DJ. Circadian rhythms in healthy aging-effects downstream from the pacemaker. Chronobiol Int. 2000;17(3):355–68. doi: 10.1081/cbi-100101051. [DOI] [PubMed] [Google Scholar]
- 28.McAnelly L, Zakon HH. Protein kinase A activation increases sodium current magnitude in the electric organ of Sternopygus. J Neurosci. 1996;16 (14):4383–8. doi: 10.1523/JNEUROSCI.16-14-04383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAnelly ML, Zakon HH. Coregulation of voltage-dependent kinetics of Na(+) and K(+) currents in electric organ. J Neurosci. 2000;20(9):3408–14. doi: 10.1523/JNEUROSCI.20-09-03408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoddard PK. Plasticity of the electric organ discharge waveform: contexts, mechanisms, and implications for electrocommunication. In: Ladich F, Collin SP, Moller P, Kapoor BG, editors. Fish communication. Enfield, N.H.: Science Publisher, Inc.; 2006. pp. 623–46. [Google Scholar]
- 31.Kennedy G, Heiligenberg W. Ultrastructural evidence of GABA-ergic inhibition and glutamatergic excitation in the pacemaker nucleus of the gymnotiform electric fish, Hypopomus. J Comp Physiol A Sens Neural Behav Physiol. 1994;174(3):267–80. doi: 10.1007/BF00240210. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki M, Heiligenberg W. Different classes of glutamate receptors and GABA mediate distinct modulations of a neuronal oscillator, the medullary pacemaker of a gymnotiform electric fish. J Neurosci. 1990;10(12):3896–904. doi: 10.1523/JNEUROSCI.10-12-03896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston SA, Maler L, Tinner B. The distribution of serotonin in the brain of Apteronotus leptorhynchus: an immunohistochemical study. J Chem Neuroanat. 1990;3(6):429–65. [PubMed] [Google Scholar]
- 34.Hopkins CD, Comfort NC, Bastian J, Bass AH. Functional analysis of sexual dimorphism in an electric fish, Hypopomus pinnicaudatus, order Gymnotiformes. Brain Behav Evol. 1990;35(6):350–67. doi: 10.1159/000115880. [DOI] [PubMed] [Google Scholar]
- 35.Markham MR, Haskell-Luevano C, Stoddard PK. A melanocortin receptor modulates electrocyte action potentials via a cAMP/PKA pathway. Soc Neurosci Abstr. 2004:334.7. [Google Scholar]
- 36.Silva A, Perrone R, Macadar O. Modulation of EOD basal rate across seasons in the weakly electric fish Brachyhypopomus pinnicaudatus. submitted for publication. [Google Scholar]


