Abstract
The metal concentrations in a copper mine tailings and Desert broom (Baccharis sarothroides Gray) plants were investigated. The metal concentrations in plants, soil cover, and tailings were determined using ICP-OES. The concentration of copper, lead, molybdenum, chromium, zinc, arsenic, nickel, and cobalt in tailings was 526.4, 207.4, 89.1, 84.5, 51.7, 49.6, 39.7, and 35.6 mg kg−1, respectively. The concentration of all elements in soil cover was 10~15% higher than that of the tailings, except for molybdenum. The concentration of copper, lead, molybdenum, chromium, zinc, arsenic, nickel, and cobalt in roots was 818.3, 151.9, 73.9, 57.1, 40.1, 44.6, 96.8, and 26.7 mg kg−1 and 1214.1, 107.3, 105.8, 105.5, 55.2, 36.9, 30.9, and 10.9 mg kg−1 for shoots, respectively. Considering the translocation factor, enrichment coefficient, and the accumulation factor, desert broom could be a potential hyperaccumulator of Cu, Pb, Cr, Zn, As, and Ni.
Keywords: Phytoremediation, Hyperaccumulator, Heavy metals, Mine tailings, Desert broom
1. Introduction
Utilization of the earth’s natural resources is fundamental to the survival and prosperity of society. However, removal of natural resources from one environment to another for utilization impacts both environments to some extent. Tailing from mining activities is one of the best examples of such a scenario. The excess of heavy metals and metalloids released from mine tailings may cause severe damage to ecosystems including plants, animals, micro-organisms and human health (Kim et al., 2003). Uncontrolled mining activities can generate a large amount of particulate emissions and waste containing heavy metals and metalloids that can contaminate the surroundings—soil, water and air. Such effects may be particularly serious and may pose a severe ecological and human health risk when mining activities are located in the vicinity of urban environments. Therefore, it is necessary to minimize or mitigate the impacts of resource utilization to the extent reasonably feasible.
A range of technologies has been used for the removal of metals for soil remediation. Many of these methods have high maintenance costs and may cause secondary pollution. A promising approach is the phytoremediation technology, where living plants are used to remove trace metals from impacted sites. Significant research has been conducted on phytoremediation for metal-sorption capacity (US EPA, 2000; Meagher, 2000; Mitch, 2002; Glick, 2003; Pulford and Watson, 2003). A series of fascinating scientific discoveries combined with an interdisciplinary research approach has allowed the development of this idea into a promising, low-cost and environmentally friendly technology (Chaney et al., 1997, 2000; Baker et al., 1991; Eapen and Dsouza, 2005; Krämer 2005). Phytoremediation can be applied to both organic and inorganic pollutants, present in soil substrates (e.g. soil), in liquid substrates (e.g. water), and in air (Salt et al., 1998; Adler et al., 1994). Phytoremediation is currently divided into several types: phytoextraction, phytodegradation, rhizofiltration, phytostabilization and phytovolatilization (Salt et al., 1998). This research is included in phytoextraction.
Hyperaccumulating plants that are often found growing in impacted areas can naturally accumulate higher quantities of heavy metals/metalloids in their shoots than in their roots. There are numerous references concerning hyperaccumulating plants (Berti and Cunningham, 1993; Brown et al., 1995; Shen and Liu, 1998; Ozturk et al., 2003). A hyperaccumulator has been defined as a plant that can accumulate, copper >1000 mg kg−1, lead >1000 mg kg−1, or zinc >10 000 mg kg−1 in their shoot dry matter. In hyperaccumulating plants, the metal concentrations in shoots are invariably greater than that in roots, demonstrating a special ability of the plant to absorb and transport metals and store them in their above-ground components (Baker and Brooks, 1989; Baker et al., 1994; Brown et al., 1994; Wei et al., 2002). Also, a hyperaccumulator is regarded as a plant in which the concentration of heavy metals in its above ground components is 10–500 times more than that in normal plants (Shen and Liu, 1998). The first hyperaccumulators to be characterized were members of the Brassicaceae and Fabaceae families (Salt et al., 1998). Therefore, it will be useful to identify plants having the ability to hyperaccumulate heavy metals.
There are numerous tailings impoundments containing copper and other metals in the vicinity of Claypool, Arizona, USA. Many plants [including desert broom (Baccharis sarothroides), mesquite (Prosopis spp.), desert Willow (Chilopsis linearis), and whitethorn acacia (Acacia constricta)] are established at the copper mine tailings reclamation project (CMTRP) near Claypool. Since these plant species are surviving at the CMTRP, it is important to identify their phytoremediation potential. To achieve this goal, the concentration of heavy metals and metalloids in the tailings and soil cover from the mining areas were analyzed. Then the concentration of those metals and metalloids was determined in the roots and shoots of desert broom which was collected from five different locations. The main objectives were as follows: (1) to determine the ability of desert broom to accumulate and tolerate heavy metals/metalloids such as Cu, Pb, Cr, Zn, As, and Ni; and (2) to determine the elemental ratios in desert broom that could identify this plant as a heavy metals/metalloids hyperaccumulator having the potential to remediate impacted tailings.
2. Materials and methods
2.1 Site description
The CMTRP is situated north of Claypool, Gila County, Arizona approximately 80 miles East of Phoenix, Arizona, USA in the Globe-Miami mining district (Fig. 1). The climate of the Globe-Miami district is generally mild and characterized as semi-arid. Maximum temperatures, occurring in June and July, generally range from 104°F (40°C) to 108°F (42°C)) and rarely exceed 110°F (43°C). December and January are normally the coldest months when minimum temperatures may fall within the range of 11°F (−11.7°C) to 22°F (−5.6°C) during the early morning hours. The annual average total precipitation is 18.05 in (45.8 cm) (Jones, 1991). The reclamation project, comprised of 6 tailing impoundments covering an area of approximately 1100 acres (445.2 ha), was initiated in 1989. Copper is the primary product mined in the Globe-Miami mining district.
Fig. 1.
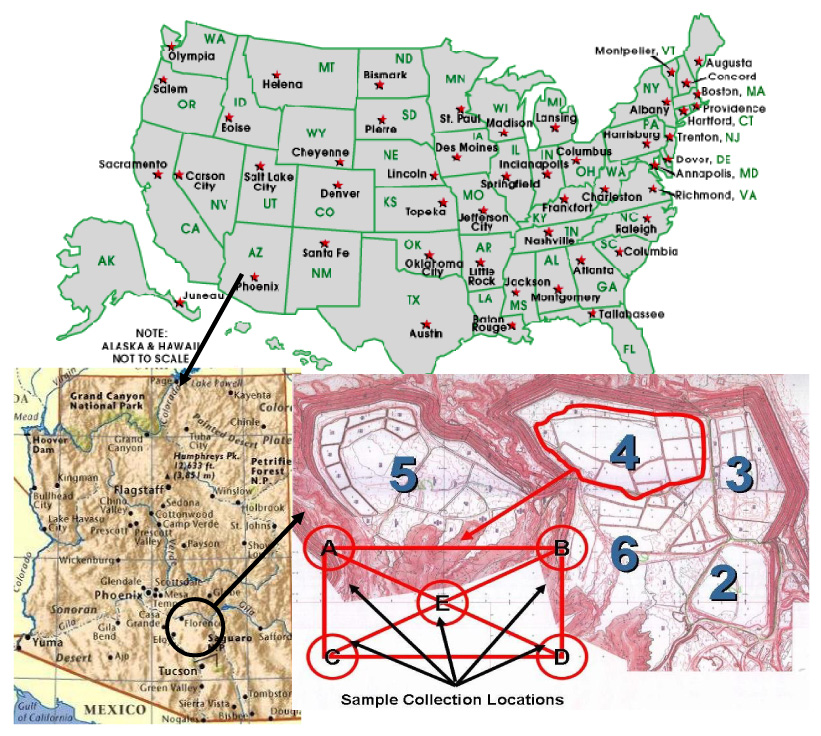
Location of the copper mine tailings reclamation project sites (2 to 6) and sampling locations (A to E) at Claypool, AZ, USA. Five of the six impoundments (No’s 2, 3, 4, 5 and 6) are contiguous. No. 1, the northernmost impoundment, is separate
2.2 Sampling
Sampling was carried out during March 2005 and among many plants, desert broom was collected from the mine tailings because it is an invasive species. Desert Broom is a multi-branched, erect or spreading evergreen shrub with very narrow linear leaves. According to Dimmit (2000), desert broom is one of the most dominant plants of the sandy flood plains throughout Arizona. It is a shrub of rapid growth that partial burial by sand does not interfere with its growth. It has a deep root system and high shoot biomass production. Collected plant samples were approximately eight to ten years old (average). At the CMTRP, there are 5 reclaimed tailing impoundments (Sites 2 to 6). Each site was divided into a geometrical shape as shown in Fig. 1. Then samples were collected at each intersection of Fig. 1. Three soil and plant samples were collected from each intersection location (A to E in Fig. 1) for enhanced statistical analysis. Samples were preserved and transported properly for analysis in the laboratory.
2.3 Soil analysis
On the surface of the sites, the top 8" (~20.4 cm) layer was considered soil cover overlaying the tailings. Collected soil cover and tailing samples were air-dried at 70 °C for 3 days, ground (only soil cover was ground because tailings were fine enough to sieve) and then sieved through a 2 mm mesh to yield a homogeneous mixture. The pH value was determined in a 1:2.5 (w:v) soil: deionized water slurry. Samples were then analyzed using an X-ray fluorescence (XRF, Fischer, Winsor, CT, USA) instrument to determine the elemental concentration of the available elements in the soil. Based on the concentration level, samples were classified as high and low level elements. To obtain a total extraction of high and low level elements, 0.5 g soil samples were digested with HNO3 (trace pure, SCP Science, New York, USA) using the EPA Method 3051 in a microwave oven (Multiwave 3000, Anton Paar, Ashland, VA, USA). The total concentrations of elements were then determined by inductively coupled plasma/optical emission spectroscopy (ICP/OES) (Perkin Elmer Optima 4300 DV, Shelton, CT, USA). A background equivalent concentration experiment was performed to test the instrument sensitivity and the following parameters were introduced: nebulizer flow, 0.7 l min−1; radio frequency power, 1450 W; sample introduction, 1.5 ml min−1; flush time, 20 s; delay time, 10 s; read time, 10 s; wash time, 30 s; replicates, 3. Standards were prepared by dilution of 1000 mg l−1 stock solutions and the calibration curve was obtained using 5 to 10 points including the blank. The ICP/OES uses Winlab32 as default package to calculate metal concentrations.
2.4 Plant analysis
Prior to analysis, plant samples were carefully washed with tap water and thoroughly rinsed with deionized water to remove any soil particles attached to the plant surfaces. After washing, the samples were oven-dried at 60° C for 24 h. The dried tissues were weighed and ground into fine powder for the determination of elemental concentration. A similar digestion procedure was followed, as above described for soil digestion. Finally, total concentrations of all elements of interest were determined by utilizing ICP/OES, as above described.
2.5 Translocation factor and enrichment Coefficient
The translocation factor (TF) of heavy metals/metalloids from roots to shoots and the enrichment coefficient (EC) of heavy metals/metalloids in a plant, as well as the accumulation factor (AF) of heavy metals/metalloids in shoots to that in shoots of plants from non-impacted environments (Zu et al., 2005) were calculated as follows:
| (1) |
| (2) |
| (3) |
All these factors were used to evaluate the heavy metals/metalloids accumulation capacity of plants.
2.6 Statistical analysis, quality assurance and quality control
The sampling and chemical analyses were run in triplicate in order to evaluate the experimental reproducibility. The confidence of data generated in the present investigations has been analyzed by standard statistical methods (SPSS 11.0, Chicago, IL, USA) to determine the mean values and standard deviation. Each data set was calculated at the 95 % confidence level (P < 0.05) to determine the error margin (Gardea-Torresdey et al., 1996). A correlation coefficient for the calibration curve of 0.994 or greater was obtained and computed as required to confirm the linear range. Certified standard reference materials of metals and metalloids (Metuchen, NJ, USA) were used for the calibration and quality assurance for each analytical batch. An external certified standard of each element was used after every 10 samples to monitor the matrix effect on the analytes for quality control. Reagent blanks and analytical triplicates were also used where appropriate to ensure the accuracy and precision in the analysis. The recovery rates were around 92 ± 8% for all of the metals and metalloids in the reference materials.
3. Results
3.1 Concentration of heavy metals and metalloids in the tailings and soil cover
A range of elements was identified in the tailings and soil cover collected from the CMTRP. The elements were classified based on the concentration found as high level elements (HLE): K, Al, Fe, S, Ca, Mg, Na, Cu, P and low level elements (LLE): Mn, Mo, Pb, Cr, Vn, Zn, Co, As, Ni. The concentrations of HLE and LLE are shown in Table 1 and 2, respectively. As shown in these tables, concentrations of Cu, Mo, and As were 526.4, 89.09, and 49.6 mg kg−1, respectively, which were higher than those in normal soils (Table 3). The concentrations of HLE and LLE in soil cover were higher (Appx. 10~15%) than that of the tailings except for Mo (112.51 mg kg−1). The pH value of soil cover samples ranges from 6.1 to 7.3 and the pH conditions were favorable for the plants to grow.
Table 1.
Concentration of high level elements (HLE) in the tailings (top row in each column) and soil cover (bottom row in each column) in mg kg−1.
| HLE | Location A | Location B | Location C | Location D | Location E | Average |
|---|---|---|---|---|---|---|
| K | 118 605.3 | 118 779.6 | 119 216.2 | 118 906.1 | 119 613.2 | 119 024.1 ± 201.3 |
| 105 714.2 | 105 886.4 | 105835.2 | 105 618.9 | 106 659.8 | 105 942.8 ± 209.6 | |
| Al | 23 256.6 | 23 199.6 | 23 265.9 | 23 256.9 | 23 254.9 | 23 246.8 ± 13.5 |
| 19 753.9 | 20 005.1 | 19 993.5 | 20 133.82 | 19 901.2 | 19 957.5 ± 71.2 | |
| Fe | 17 816.5 | 17 845.6 | 17 856.9 | 17 836.1 | 17 799.5 | 17 830.9 ± 11.6 |
| 15 697.3 | 15 429.6 | 15 599.3 | 15 589.3 | 15 690.9 | 15 601.3 ± 54.8 | |
| S | 3346.1 | 3377.9 | 3369.3 | 3349.6 | 3351.1 | 3358.8 ± 7.1 |
| 2948.1 | 2945.6 | 3011.9 | 2989.6 | 2979.3 | 2974.9 ± 14.3 | |
| Mg | 1211.3 | 1202.3 | 1214.9 | 1198.2 | 1208.6 | 1207.1 ± 3.4 |
| 1089.6 | 1098.6 | 1099.3 | 1072.8 | 1065.3 | 1085.1 ± 7.7 | |
| Ca | 1212.3 | 1214.3 | 1200.9 | 1198.7 | 1184.9 | 1202.2 ± 6.0 |
| 1045.3 | 1052.9 | 1078.9 | 1069.2 | 1087.5 | 1066.8 ± 8.9 | |
| Na | 1151.2 | 1134.8 | 1156.9 | 1125.9 | 1145.9 | 1142.9 ± 5.4 |
| 985.9 | 978.9 | 965.8 | 978.8 | 994.8 | 980.8 ± 1.9 | |
| Cu | 529.6 | 526.9 | 527.9 | 519.8 | 527.6 | 526.4 ± 1.9 |
| 456.3 | 455.8 | 452.9 | 450.2 | 459.9 | 455.1 ± 1.6 | |
| Pb | 205.9 | 203.8 | 209.8 | 209.8 | 207.7 | 207.4 ± 1.3 |
| 199.6 | 198.7 | 200.1 | 207.3 | 199.6 | 201.1 ± 1.8 | |
Table 2.
Concentration of low level elements (LLE) in the tailings (top row in each column) and soil cover (bottom row in each column) in mg kg−1.
| LLE | Location A | Location B | Location C | Location D | Location E | Average |
|---|---|---|---|---|---|---|
| P | 184.15 | 182.39 | 180.66 | 179.48 | 179.39 | 181.21 ± 1.1 |
| 170.65 | 165.87 | 166.74 | 168.92 | 165.76 | 167.59 ± 1.2 | |
| Mn | 158.32 | 159.26 | 160.08 | 149.89 | 159.36 | 157.38 ± 2.2 |
| 151.23 | 154.68 | 151.78 | 152.47 | 151.11 | 152.25 ± 0.8 | |
| Mo | 91.56 | 90.09 | 87.29 | 87.12 | 89.41 | 89.09 ± 1.1 |
| 107.31 | 108.37 | 110.21 | 111.37 | 125.31 | 112.51 ± 3.7 | |
| Cr | 89.02 | 86.36 | 84.52 | 85.25 | 81.87 | 84.51 ± 1.1 |
| 80.26 | 80.31 | 81.12 | 77.9 | 74.59 | 78.84 ± 1.3 | |
| Vn | 58.23 | 57.89 | 58.21 | 56.94 | 57.01 | 57.66 ± 0.3 |
| 50.12 | 49.99 | 51.26 | 48.56 | 50.21 | 50.03 ± 0.5 | |
| Zn | 55.23 | 51.02 | 52.03 | 49.96 | 50.12 | 51.67 ± 1.1 |
| 40.12 | 40.23 | 41.26 | 39.94 | 39.12 | 40.13 ± 0.4 | |
| As | 50.21 | 51.26 | 49.63 | 48.21 | 48.69 | 49.60 ± 0.6 |
| 41.25 | 40.23 | 40.36 | 39.9 | 38.21 | 39.99 ± 0.5 | |
| Ni | 41.12 | 40.12 | 39.23 | 38.95 | 38.99 | 39.68 ± 0.5 |
| 29.79 | 30.12 | 31.02 | 30.88 | 31.12 | 30.59 ± 0.3 | |
| Co | 34.25 | 35.69 | 36.02 | 35.98 | 36.01 | 35.59 ± 0.4 |
| 29.12 | 28.78 | 28.97 | 27.03 | 30.12 | 28.80 ± 0.6 | |
Table 3.
Normal concentration range of elements (mg kg−1) in the soil and plants.
3.2 Accumulation of heavy metals and metalloids in plants
The concentration of HLE and LLE in desert broom is shown in Table 4. The uptake of Cu, Pb, Mo, Cr, Zn, As, Ni and Co in the roots and shoots of desert broom was noteworthy. The accumulation of Cu in the shoots (1214.1 mg kg−1) tops the list, followed by Pb (107.3 mg kg−1), Mo (105.8 mg kg−1), Cr (105.5 mg kg−1), Zn (55.2 mg kg−1), As (36.9 mg kg−1), Ni (30.9 mg kg−1), and Co (10.9 mg kg−1). There were significant differences (P<0.05) in the average concentrations of the above elements, except for Zn, Mo, and As.
Table 4.
Concentration of HLE and LLE in roots and shoots of desert broom from the copper mine tailings reclamation project and accumulation factor (AF) (accumulation of heavy metals/metalloids in shoots to that in shoots of plants from non-impacted environments)f.
| HLE | Concentration (mg kg−1) |
LLE | Concentration (mg kg−1) |
||||
|---|---|---|---|---|---|---|---|
| Root | Shoot | AF | Root | Shoot | AF | ||
| K | 89 736 | 284 004 | -- | Mn | 344.5 | 2112.6 | -- |
| Al | 5420.9 | 1469.8 | -- | Mo | 73.9 | 105.8 | 21.2 |
| Fe | 6069.3 | 3354.9 | -- | Vn | 39.5 | 84.1 | -- |
| Mg | 3939.8 | 3470.2 | -- | Cr | 57.1 | 105.5 | 21.1 |
| Ca | 87 548 | 377 981 | -- | Zn | 40.1 | 55.2 | 2.8 |
| Cu | 818.3 | 1214.1 | 48.6 | As | 44.6 | 36.9 | 7.4 |
| Pb | 151.9 | 107.3 | 21.5 | Ni | 96.8 | 30.9 | 3.1 |
| Co | 26.7 | 10.9 | 5.5 | ||||
The elemental concentration in plants from non-impacted environment is shown in Table 2.
3.3 Translocation factor and enrichment coefficient
Fig. 2 shows the translocation factor (TF) and enrichment coefficient (EC) of Cu, Pb, Mo, Cr, Zn, As, Ni, and Co. As shown in Fig. 2, the TF values for Cu, Mo, Cr, and Zn are greater than 1, which indicates that these metals move more easily in the plants than Pb, As, Ni, and Co. The EC values for Cu, Mo, Cr, and Zn were greater than 1 as well. The highest EC value of all elements was for Cu (Fig. 2b) and Cr had the highest TF value (Fig. 2a).
Fig. 2.
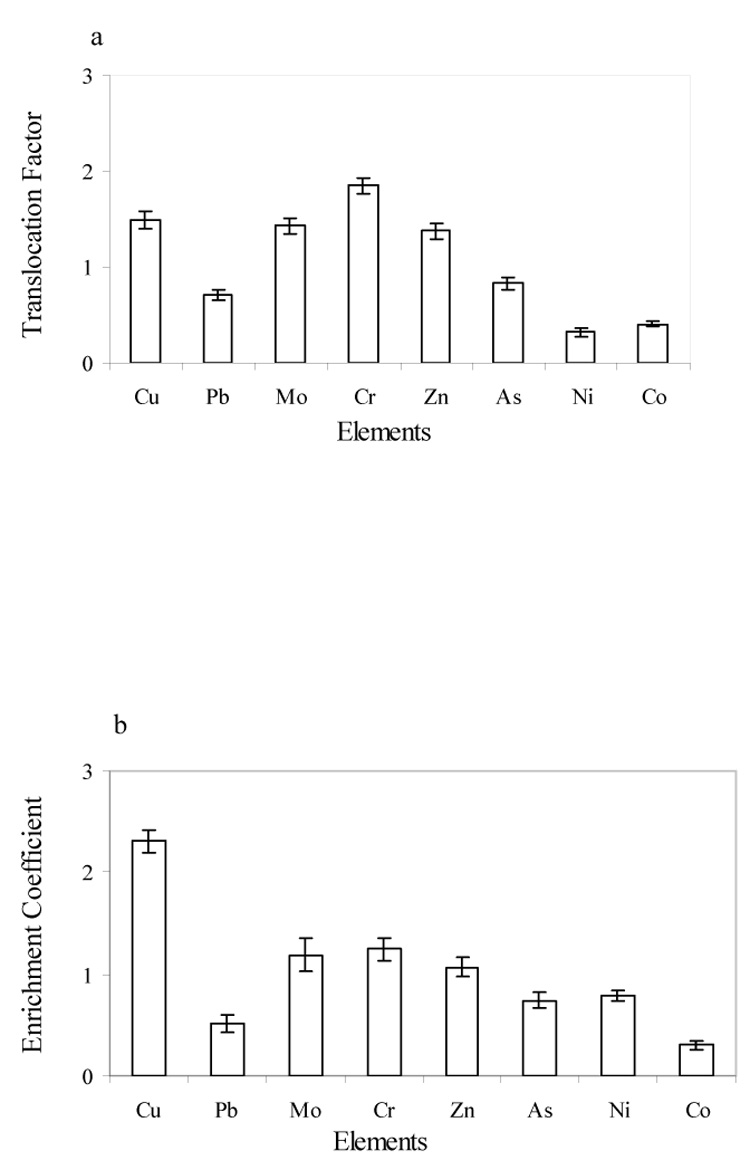
Translocation factor (a) and enrichment coefficient (b) of Desert broom.
4. Discussion
4.1 Uptake and accumulation
Table 1 and 2 shows the general trend that desert broom has within a broad range of metal and metalloid concentrations in the soil (Cu > Pb > Mn > Mo > Cr > Vn > Zn > As > Ni > Co). The concentration of Cu, Mo, and As in the soil of the CMTRP exceeded the ranges which were considered toxic to normal plants (Kabata-Pendias and Pendias, 1984), so desert broom growing in the impacted site exhibited strong metal adaptability. Wei et al. (2005) concluded in their research that the exclusion of metals from aboveground tissues has been regarded as a metal tolerant strategy. The excessive metal and metalloid concentration did not affect desert broom; nevertheless, it seemed that it possesses metal resistance capability according to Pichtel et al. (2000). Resistance of plants to heavy metals can be achieved by an avoidance mechanism, which includes the immobilization of a metal in roots and in cell walls (Garbisu and Alkorta, 2001). As shown in Fig. 2a, Pb, As, Ni, and Co accumulated by desert broom were retained in the roots and the TF values of less than 1 demonstrates the limited mobility of Pb, As, Ni, and Co in desert broom. Each plant species might have a unique mechanism against any metals; however, similar results were found in other research for Pb (Fitzgerald et al., 2003), As (Geng et al., 2006), Ni (Nkoane et al., 2005), and Co (Page et al., 2006). The elevated metal concentrations in roots and low translocation to the aboveground tissues in some investigated species might also suggest that they are capable of rather well-balanced uptake and translocation of metals under heavily metal-polluted conditions (Nkoane et al., 2005; Deng et al., 2004).
On the other hand, the TF values for Cu, Mo, Cr, and Zn were greater than 1, which clearly illustrates that the translocation of Cu, Mo, Cr, and Zn was higher from the roots to the shoots. TF values greater than 1 indicate a very efficient ability to transport metals from roots to shoots, most likely due to efficient metal transporter systems (Zhao et al, 2002) and probably sequestration of metals in leaf vacuoles and apoplast (Lasta et al., 2000). This high metal accumulation in desert broom indicates that an internal metal detoxification tolerance mechanism might exist in addition to its exclusion strategies (Baker, 1981).
Enrichment coefficients are a common important factor when considering the phytoremediation potential of a given species (Zhao et al., 2003). In this study, EC values of Cu, Mo, Cr, and Zn were greater than 1 (Fig. 2b) which indicated the phytoremediation potential of desert broom for these heavy metals from the CMTRP. Although EC (Eq. 2) mainly shows the metal concentration ratio between plant and soil, desert broom might adsorb/absorb some pollutants from the air because of the blowing dust around this area. On the other hand, EC values for Pb, As, Ni, and Co were found to be less than 1 in this study. The decrease in enrichment coefficients may be due to the saturation of metal uptake and/or root to shoot transport when internal metal concentrations were high. Baker (1981) concluded that any species may act as an accumulator, an indicator and excluder over different ranges of soil metal concentration and this seems to be the case for desert broom for Pb, As, Ni, and Co. Desert broom might behave differently with a higher concentration of Pb, As, Ni, and Co in the soil.
The normal concentrations of Cu, Mo, Pb, Cr, Zn, Co, As, and Ni are shown in Table 3. The normal concentrations of Cu, Mo, Pb, Cr, Zn, Co, As, and Ni are the concentration when the plants are grown in non-impacted environments. When plants are grown in non-impacted environment, then the concentration of above mentioned element is considered as a normal concentration. The concentrations of all these elements, except Zn, in desert broom (Table 4) were higher than that of normal plants (Table 3), which showed that desert broom had a strong ability to tolerate these elements. The detoxification tolerance to heavy metals and metalloids is based on the sequestration of heavy metal ions in vacuoles, on binding the metals by appropriate ligands like organic acids, proteins and peptides and on the presence of enzymes that can function at high levels of metallicions (Garbisu and Alkorta, 2001).
4.2 Hyperaccumulator and Potential Applications to Phytoremediation
Presently, there is no standard rule to determine whether or not any plant is a hyperaccumulator. However, four rules are being used successfully to determine hyperaccumulator criteria: 1) the concentrations of heavy metal in plant shoots reach hyperaccumulating level, Pb and Cu >1000 mg kg−1 (Baker et al., 1994), Zn >10 000 mg kg−1 (Brown et al., 1994), As >1000 mg kg−1 (Ma et al., 2001a), Ni and Co >1000 mg kg−1 (Brooks, 1998), Cr >1000 mg kg−1 (Reeves and Baker, 2000) and Mo >1500 mg kg−1 (Lombi et al., 2001), 2) the concentrations of some heavy metals in shoots are 10–500 times as much as those in a normal plant (Table 3) (Shen and Liu, 1998), 3) the metal concentration in shoots are invariably greater than that in roots (Baker et al., 1989, 1994) and 4) an enrichment coefficient >1 (Brown et al., 1994; Wei et al., 2002).
In this research, the accumulation of Cu in desert broom satisfied all the above mentioned criteria. Thus desert broom can be called a hyperaccumulating plant. For Mo, Cr, and Zn, desert broom can be considered as a hyperaccumulator considering the TF and EC value. Finally, according to the accumulated concentration in plant shoots and the concentration levels compared to plants from non-impacted environments, desert broom had hyperaccumulation capacity for Pb, although the TF and EC value were less than 1. The response of desert broom as a hyperaccumulator against Cu, Mo, Cr, Zn, and Pb might be by employing the strategy of accumulation and sequestration of metals because plants have high capacity to take up metals by roots and translocate and store them in the shoots (Baker et al., 2000; McGrath et al., 2001).
Based on the results of this study, two distinct strategies of phytoremediation can be applied at the CMTRP, phytoextraction and phytostabilization (Salt et al., 1998). Phytoextraction is the utilization of metal accumulating plants to transport and concentrate metals from impacted soils to shoots, followed by gathering the aboveground tissues by conventional methods. In phytostabilization, plants can stabilize pollutants in the soil around the root area by rendering them harmless (Eapen and Dsouza, 2005). According to this field investigation, desert broom exhibited a strong accumulative ability for Cu, Mo, Cr, Zn, and Pb and therefore it might be used to phytoremediate impacted soils at the CMTRP after further research in the accumulation mechanism. It is very important to point out that the desert broom plants naturally established themselves at this site.
Acknowledgement
The authors acknowledge the financial support of Phelps Dodge Miami, Inc., Claypool, Arizona, the National Institutes of Health (Grant S06GM8012-33), the University of Texas at El Paso’s Center for Environmental Resource Management, the HBCU/MI Environmental Technology Consortium that is funded by the Department of Energy (grant DE-FC0202EW15254), the NIH Center for the Border Health (grant No. 26-8600-29), and the National Institute of Environmental Health Sciences (Grant R01ES11367-01). J. Gardea-Torresdey also acknowledges the Dudley family for the Endowed Research Professorship in Chemistry.
Footnotes
Capsule: Desert broom, a potential hyperaccumulating plant to clean up Cu, Pb, Cr, Zn, As, Ni and Co from the mine tailings in AZ, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PR, Arora REI, Ghaouth A, Glenn DM, Solar JM. Bioremediation of phenolic compounds from water with plant root surface peroxidases. Journal of Environmental Quality. 1994;23:1113–1117. doi: 10.2134/jeq1994.00472425002300050038x. [DOI] [PubMed] [Google Scholar]
- Alloway BJ. Heavy Metals in Soils. second ed. London: Blackie Academic & Professional; 1995. [Google Scholar]
- Baker AJM. Accumulators and excluder-strategies in the response of plants to heavy metals. Journal of Plant Nutrition. 1981;3:643–654. [Google Scholar]
- Baker AJM, Brooks RR. Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- Baker AJM, Reeves RD, McGrath SP. In situ decontamination of heavy metal polluted soils using crops of metal-accumulating plants—a feasibility study. In: Hinchee RE, Olfenbuttel RF, editors. In situ bioreclamation. Stoneham, Massachusetts: Butterworth-Heinemann Publishers; 1991. pp. 539–544. [Google Scholar]
- Baker AJM, Reeves RD, Hajar ASM. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J & C Presl (Brassicaceae) New Phytologist. 1994;127:61–68. doi: 10.1111/j.1469-8137.1994.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Berti WR, Cunningham SD. Remediating soil Pb with green plants. International Conference on Environmental Geochemistry Health; July; New Orleans, LA. 1993. pp. 25–27. [Google Scholar]
- Nkoane BBM, Sawula GM, Wibetoe G, Lund W. Identification of Cu and Ni indicator plants from mineralized locations in Botswana. Journal of Geochemical Exploration. 2005;86:130–142. [Google Scholar]
- Bowen HJM. Environmental chemistry of the elements. London: Academic Press; 1979. [Google Scholar]
- Brooks RR. Plants that hyperaccumulate heavy metals. New York: Cambridge University Press; 1998. [Google Scholar]
- Brown SL, Chaney RL, Angle JS, Baker AJM. Phytoremediation potential of Thlaspi caerulescens and bladder campion for zinc- and cadmium contaminated soil. Journal of Environmental Quality. 1994;23:1151–1157. [Google Scholar]
- Brown SL, Chaney RL, Angle JS, Baker AJM. Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant Silene vulgaris grown on sludge-amended soils. Environmental Science & Technology. 1995;29:1581–1585. doi: 10.1021/es00006a022. [DOI] [PubMed] [Google Scholar]
- Chaney RL, Malik M, Li YM, Brown SL, Brewer EP, Angle JS, Baker AJM. Phytoremediation of soil metals. Current Opinion in Biotechnology. 1997;8:279–284. doi: 10.1016/s0958-1669(97)80004-3. [DOI] [PubMed] [Google Scholar]
- Chaney RL, Li YM, Angle JS, Baker AJM, Reeves RD, Brown SL, Homer FA, Malik M, Chin M. Improving metal hyperaccumulation wild plants to develop commercial phytoextraction systems: Approaches and progress. In: Terry N, Bañuelos GS, editors. Phytoremediation of contaminated soils and water. CRC Boca Raton, FL: CRC press; 2000. pp. 131–160. [Google Scholar]
- Deng H, Ye ZH, Wong MH. Accumulation of lead, zinc copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environmental Pollution. 2004;132:29–40. doi: 10.1016/j.envpol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Dimmit M. Sunflower family. In: Steven JP, Patricia WC, editors. A natural history of the Sonoran Desert. Tucson: Arizona-Sonora Desert Museum Press; 2000. pp. 174–175. [Google Scholar]
- Eapen S, Dsouza SF. Prospects of genetic engineering of plats for phytoremediation of toxic metals. Biotechnology Advances. 2005;23:97–114. doi: 10.1016/j.biotechadv.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EJ, Caffrey JM, Nesaratnam ST, McLoughlin P. Copper and lead concentrations in salt marsh plants on the Suir Estuary, Ireland. Environmental Pollution. 2003;123:67–74. doi: 10.1016/s0269-7491(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Garbisu C, Alkorta I. Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresource Technology. 2001;77:229–236. doi: 10.1016/s0960-8524(00)00108-5. [DOI] [PubMed] [Google Scholar]
- Gardea-Torresdey JL, Tiemann KJ, Gonzales JH, Hennig JA, Townsend MS. Ability of silica-immobilized medicago sativa (alfalfa) to remove copper ions from solution. Journal of Hazardous Materials. 1996;48:181–190. [Google Scholar]
- Geng CN, Zhu YG, Tong YP, Smith SE, Smith FA. Arsenate (As) uptake by and distribution in two cultivars of winter wheat (Triticum aestivum L.) Chemosphere. 2006;62:608–615. doi: 10.1016/j.chemosphere.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Glick BR. Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnology Advances. 2003;21:383–393. doi: 10.1016/s0734-9750(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Jones GL. Mill tailing reclamation at Cyprus Miami Mining Corporation. Proceeding of the National Meeting of the American Society for Surface Mining and Reclamation; May 13–17, 1991; Durango, CO. 1991. [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace Elements in Soils and Plants. Florida: CRC Press; 1984. [Google Scholar]
- Kim IS, Kang KH, Johnson-Green P, Lee EJ. Investigation of heavy metal accumulation in Polygonum thunbergii for Phytoextraction. Environmental Pollution. 2003;126:235–243. doi: 10.1016/s0269-7491(03)00190-8. [DOI] [PubMed] [Google Scholar]
- Krämer U. Phytoremediation: novel approaches to cleaning up polluted soils. Current Opinion in Biotechnology. 2005;16:133–141. doi: 10.1016/j.copbio.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lasta MM, Pence NS, Garvin DF, Ebbs SD, Kochina LV. Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2000;51:71–79. [PubMed] [Google Scholar]
- Lavado RS, Porcelli CA, Alvarez R. Nutrient and heavy metal concentration and distribution in corn, soybean and wheat as affected by different tillage systems in the Argentine Pampas. Soil & Tillage Research. 2001;62:55–60. [Google Scholar]
- Lombi E, Zhao FJ, Dunham SJ, McGrath SP. Phytoremediation of Heavy Metal, Contaminated Soils, Natural Hyperaccumulation versus Chemically Enhanced Phytoextraction. Journal of Environmental Quality. 2001;30:1919–1926. doi: 10.2134/jeq2001.1919. [DOI] [PubMed] [Google Scholar]
- Ma LQ, Komar KM, Kennelley ED. United States Patent 6280500. Methods for removing pollutants from contaminated soil materials with a fern plant Document Type and Number. 2001a http://www.freepatentsonline.com/6280500.html.
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED. A fern that hyperaccumulates arsenic. Nature. 2001b;409:579. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- Meagher RB. Phytoremediation of toxic elemental and organic pollutants. Current Opinion in Plant Biology. 2000;3:153–162. doi: 10.1016/s1369-5266(99)00054-0. [DOI] [PubMed] [Google Scholar]
- Mitch ML. Phytoextraction of toxic metals: a review of biological mechanism. Journal of Environmental Quality. 2002;31:109–120. [PubMed] [Google Scholar]
- Ozturk L, Karanlik S, Ozkutlu F, Cakmak I, Kochian LV. Shoot biomass and zinc/cadmium uptake for hyperaccumulator and non-accumulator Thlaspi species in response to growth on a zinc-deficient calcareous soil. Plant Science. 2003;164:1095–1101. [Google Scholar]
- Page V, Bayon RCL, Feller U. Partitioning of zinc, cadmium, manganese and cobalt in wheat (Triticum aestivum) and lupin (Lupinus albus) and further release into the soil. Environmental and Experimental Botany. 2006;58:269–278. [Google Scholar]
- Pichtel J, Kuroiwa K, Sawyerr HT. Distribution of Pb, Cd and Ba in soils and plants of two contaminated sites. Environmental Pollution. 2000;110:171–178. doi: 10.1016/s0269-7491(99)00272-9. [DOI] [PubMed] [Google Scholar]
- Pulford ID, Watson C. Phytoremediation of heavy metal-contaminated land by tree-a view. Environment International. 2003;29:529–540. doi: 10.1016/S0160-4120(02)00152-6. [DOI] [PubMed] [Google Scholar]
- Reeves RD, Baker AJM. Phytoremediation of Toxic Metals. In: Raskin I, Ensley BD, editors. Using Plants to Clean Up the Environment. New York: John Wiley and Sons Inc.; 2000. p. 193. [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation - Annual Review of Plant Physiology. Plant Molecular Biology. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Shen ZG, Liu YL. Progress in the study on the plants that hyperaccumulate heavy metal. Plant Physiology Communication. 1998;34:133–139. [Google Scholar]
- US EPA. Office of Research and Development. Introduction to Phytoremediation. 2000 EPA/600/R-99/107. [Google Scholar]
- Wei CY, Chen TB, Huang ZC. Cretan bake (Pteris cretica L): an Arsenicaccumulating Plant. Acta Ecologica Sinica. 2002;22:777–782. [Google Scholar]
- Wei SH, Zhou QX, Wang X. Identification of weed plants excluding the absorption of heavy metals. Environment International. 2005;31:829–834. doi: 10.1016/j.envint.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP. Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2002;53:535–543. doi: 10.1093/jexbot/53.368.535. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Lombi E, McGrath SP. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil. 2003;249:37–43. [Google Scholar]
- Zu YQ, Li Y, Chen JJ, Chen HY, Qin L, Schvartz C. Hyperaccumulation of Pb, Zn, and Cd in hervaceous grown on lead-zinc mining area in Yunnan, China. Environment International. 2005;31:755–762. doi: 10.1016/j.envint.2005.02.004. [DOI] [PubMed] [Google Scholar]


