ABSTRACT
OBJECTIVE
To describe the clinical presentation and delays in diagnosis of patients with cystic fibrosis (CF) with the goal of raising physicians’ awareness of CF and establishing baseline data for comparison with outcomes of patients who undergo newborn screening for CF.
DESIGN
Retrospective review of hospital medical records and CF clinic charts of newly diagnosed CF patients younger than 18 years who had attended the CF clinic at the BC Children’s Hospital in Vancouver between January 1, 1993, and January 1, 2005. Age at diagnosis of CF was ascertained for 24 adult patients diagnosed during the same period from the CF clinic at St Paul’s Hospital in Vancouver, BC.
SETTING
Cystic fibrosis clinic at the BC Children’s Hospital.
PARTICIPANTS
All newly diagnosed CF patients from mainland BC and northern Vancouver Island (N = 122).
MAIN OUTCOME MEASURES
Mean age at diagnosis; mean delay in diagnosis; weight and height or length at diagnosis; vitamin E status; mean head circumference; types of symptoms before diagnosis; Pseudomonas aeruginosa status; and number of days spent in tertiary care hospitals before diagnosis.
RESULTS
Excluding the adult patients and patients with meconium ileus, mean age at diagnosis of CF was 3.6 years, and mean delay in diagnosis after first symptoms was 2.1 years. Weight at diagnosis was ≤ 5th percentile in 37% of cases, and height or length was ≤ 5th percentile in 26% of cases. Excluding those with meconium ileus and those taking vitamin E supplementation, 70% of the children were vitamin E deficient at diagnosis. These children had a mean head circumference substantially smaller than that of children who had adequate levels of vitamin E. About 95% of children had gastrointestinal (GI) or malnutrition symptoms before diagnosis; 15% had GI symptoms only. About 81% of patients had respiratory symptoms, but only 4% had respiratory symptoms as the only evidence of CF before diagnosis. Around 9% were colonized with P aeruginosa at diagnosis. Before being diagnosed, 79% of patients had required tertiary care hospitalization for a group total of 320 hospital days.
CONCLUSION
Considerable delays in diagnosis of children with CF occur when the disease is identified solely on clinical presentation. Morbidity is often severe enough to require hospital admission before CF is diagnosed. Symptoms that occurred before diagnosis were often GI or malnutritional in nature rather than respiratory, but all such symptoms were associated with diagnostic delays.
RÉSUMÉ
OBJECTIF
Décrire le mode de présentation clinique et les retards de diagnostic de la mucoviscidose (MV) pour sensibiliser le médecin de famille à cette maladie et recueillir des données de base pour comparer les issues des sujets qui ont eu un dépistage de MV à la naissance.
TYPE D’ÉTUDE
Examen rétrospectif de dossiers d’hôpitaux et de cliniques de MV des patients de moins de 18 ans avec un diagnostic récent de MV qui avaient été vus à la clinique de MV du BC Children’s Hospital de Vancouver entre le 1er janvier 1993 et le 1er janvier 2005. On a également établi l’âge au diagnostic pour 24 patients adultes vus durant la même période à la clinique de MV du St-Paul’s Hospital de Vancouver, BC.
CONTEXTE
La clinique de MV du BC Children’s Hospital.
PARTICIPANTS
Tous les cas de MV nouvellement diagnostiqués en Colombie-Britannique continentale et dans la partie nord de l’île de Vancouver (N=122).
PRINCIPAUX PARAMÈTRES ÉTUDIÉS
Âge moyen au diagnostic; retard moyen pour le diagnostic; poids et taille ou longueur au diagnostic; état relatif à la vitamine E; circonférence moyenne de la tête; type de symptômes avant le diagnostic; état relatif au Pseudomonas aerginosa; et nombre de jours passés dans un hôpital de soins tertiaires avant le diagnostic.
RÉSULTATS
Si on exclut les patients adultes et les cas d’iléus méconial, l’âge moyen au diagnostic de MV était de 3,6 ans et le délai moyen entre les premiers symptômes et le diagnostic, de 2,1 ans. Le poids au diagnostic était égal ou inférieur au 5e centile dans 37% des cas, et la taille ou la longueur était égale ou inférieure au 5e centile dans 26% des cas. En excluant ceux qui avaient un iléus méconial ou qui prenaient des suppléments de vitamine E, 70% des enfants présentaient une déficience en vitamine E au diagnostic. La circonférence de la tête de ces enfants était considérablement inférieure à celle des enfants présentant des niveaux adéquats de vitamine E. Environ 95% des enfants avaient des symptômes digestifs ou des signes de malnutrition avant le diagnostic; 15% avaient seulement des symptôme digestifs. Environ 81% des patients présentaient des symptômes respiratoires, mais dans seulement 4% des cas, ces symptômes étaient les seuls indices de MV avant le diagnostic. Environ 9% étaient colonisés par Paeruginosa au diagnostic. Avant le diagnostic, 79% des patients avaient dû séjourner un total de 320 jours pour le groupe dans un hôpital de soins tertiaires.
CONCLUSION
Chez les enfants atteints de MV, le diagnostic subit un retard considérable quand il se fonde uniquement sur la présentation clinique. La morbidité est souvent suffisamment sévère pour nécessiter une hospitalisation avant l’établissement du diagnostic. Les symptômes apparus avant le diagnostic avaient souvent rapport au système digestif ou à la malnutrition plutôt qu’au système respiratoire, mais tous étaient liés à un retard de diagnostic.
Cystic fibrosis (CF) is a common lethal autosomal recessive disorder seen in 1/3000 to 1/4200 live births in Canada and the United States.1–3 It is difficult to diagnose clinically because early symptoms are often minimal or nonspecific. An Australian study from the 1980s found a mean delay in diagnosis of CF from onset of symptoms of 2.6 years.4 A more recent US study found considerable morbidity and delays in diagnosis of CF patients identified clinically despite modern advances in health care.2 No similar studies have been carried out in Canada.
The objective of our study was to characterize the clinical presentation of pediatric patients with CF by describing their symptoms, age at diagnosis, duration of symptoms before diagnosis, and morbidity including days in tertiary care hospitals before diagnosis. Our goal was to increase physicians’ awareness and knowledge of the clinical presentation of CF in Canada in the hope of improving clinical identification and to establish baseline data for comparison in any discussion of newborn screening for CF. Our study is the first to document such data in a provincial setting typical of the Canadian health care system.
Newborn screening for CF is the standard of care in Australia and New Zealand, much of Europe, and some US states.5–9 The US Centers for Disease Control and Prevention and the American College of Medical Genetics have recently endorsed newborn screening for CF, and the Canadian Cystic Fibrosis Foundation (CCFF) has recently issued a statement of support for screening.10–12 Newborn screening is currently available in only 2 provinces in Canada (Alberta and Ontario). The CCFF statement highlighted the need for more data on the resources used to diagnose CF when its identification is based on the current “clinical suspicion” method.12
METHODS
This retrospective study involved review of all CF cases (N = 122) in mainland BC and northern Vancouver Island diagnosed between January 1, 1993, and January 1, 2005. Because access to funded services in BC, such as supply of pancreatic enzymes, requires that diagnosis be confirmed by a CF clinic, we believed that all CF patients from these regions were known to the CF clinics. Data from southern Vancouver Island were not included because patients from that area attend separate pediatric and adult clinics in Victoria. Diagnoses of CF were confirmed by positive sweat test results at the Vancouver reference centre according to the method of Gibson and Cooke.13 Data collection was anonymous and the data collected consisted of standard clinical and laboratory features of CF as described in the CF literature. For 98 pediatric patients (ie, younger than 18 years), CF clinic records were reviewed for family history of CF, symptoms (Table 1), age at onset of symptoms, number of hospitalizations and days in hospital before diagnosis, as well as height, weight, head circumference, and vitamin E levels at diagnosis.14 Vitamin E (α-tocopherol) was measured in deproteinized serum by hexane extraction followed by reversed-phase high-performance liquid chromatography with ultraviolet detection. Data were collected by a research assistant and one of the authors (M.S.) and reviewed for accuracy and completeness by all the authors.
Table 1.
Clinical records were reviewed for the following signs and symptoms of cystic fibrosis in pediatric patients at the time of diagnosis
| SIGNS AND SYMPTOMS |
|---|
Previous respiratory diagnoses
|
Respiratory signs and symptoms
|
| Gastrointestinal or malnutrition signs and symptoms |
Weight ≤ 5th percentile.
Fecal fat excretion > 20 mmol/d on 72-h collection.
Hemoglobin < 90 g/L at 0–5 mo, < 100 g/L at 6–11 mo, < 106 g/L at 1–5 y, < 117 g/L at 6–18 y.
Vitamin E level < 7 μmol/L at 0–12 mo, < 10 μmol/L at 1.1–6 y, < 13 μmol/L at 6.1–18 y.
Age at diagnosis of 24 adult CF patients diagnosed within the same time period was ascertained from records at the adult CF clinic at St Paul’s Hospital in Vancouver.
Data were compiled using a Microsoft Access database and analyzed with the Statistical Package for the Social Sciences, version 13.0. Unless otherwise specified, results are reported excluding those diagnosed owing to meconium ileus, because neonatal intestinal obstruction is a well-recognized symptom of CF, and virtually all children who present with meconium ileus have diagnostic testing for CF during the first weeks of life. All results were calculated including children diagnosed because of family history, as that information might have become available only through diagnosis of a sibling.
RESULTS
Mean number of live births per year in mainland BC and northern Vancouver Island during the study period was 37 340,15 giving a mean rate of CF of 1:3673 live births during the 12 years evaluated. During these 12 years, a mean of 8 new pediatric cases of CF were diagnosed per year (range 5 to 17 cases). Mean age at diagnosis of CF including both adult and pediatric patients was 8.5 years; median age at diagnosis of CF was 2.5 years (range 2 days to 63 years). Table 2 shows ages and duration of symptoms before diagnosis of CF for the 98 pediatric patients. Seventeen patients presented with meconium ileus. Fifty-one children with CF (52%) remained undiagnosed at 1 year old.
Table 2.
Age at diagnosis of cystic fibrosis and time between onset of symptoms and diagnosis
| TIMING OF DIAGNOSIS | ALL PEDIATRIC PATIENTS (N = 98) | PEDIATRIC PATIENTS WITHOUT MECONIUM ILEUS (N = 81) |
|---|---|---|
| Age at diagnosis | ||
| • Mean | 3.0 y | 3.6 y |
| • Median | 1.2 y | 2.2 y |
| • Range | 0 d–16.3 y | 2 d–16.3 y |
| Delay in diagnosis | ||
| • Mean | 1.8 y | 2.1 y |
| • Median | 0.5 y | 0.8 y |
| • Range | 0 d–14 y | 0 d–14 y |
About 79% (64/81) of children without meconium ileus were hospitalized in a tertiary care facility before diagnosis (Table 3) for a group total of 320 days in hospital. This does not include community hospital admissions or hospitalizations after diagnosis for treatment of complications of CF related to diagnostic delays.
Table 3.
Tertiary care hospitalization of children (excluding those with meconium ileus) before diagnosis of cystic fibrosis: 64 out of 81 (79%) children were hospitalized.
| DAYS IN TERTIARY CARE | NO. OF DAYS |
|---|---|
| Range | 1–30 |
| Median | 3 |
| Mean | 5 |
| Total | 320 |
Table 4 shows categories of symptoms experienced before diagnosis. Common gastrointestinal (GI) presentations included steatorrhea in 58/98 (59%) patients, meconium ileus in 17/98 (17%) patients, and rectal prolapse in 8/98 (8%) patients. Malnutrition was profound in 3 infants who presented with the triad of edema due to hypoalbuminemia, hemolytic anemia due to vitamin E deficiency, and an extensive rash due to zinc and fatty acid deficiency. About 81% of patients had respiratory symptoms (Table 1), but respiratory symptoms were less common than GI or malnutrition symptoms. Only 4% of patients presented with respiratory symptoms only (Table 4). Sputum cultures were positive for Pseudomonas aeruginosa in 7/81 (9%) children without meconium ileus at diagnosis, but this should be considered a minimal estimate, as only pharyngeal or sputum cultures were obtained. Twelve of the 98 children (12%) had positive family histories of CF. Only 1 case was diagnosed on the basis of family history; the patient had no symptoms at diagnosis.
Table 4.
Clinical presentation at diagnosis
| SYMPTOM CATEGORY | SIGNS AND SYMPTOMS | % OF PATIENTS |
|---|---|---|
| Growth | • Weight ≤ 5th percentile | 37 |
| • Height or length ≤ 5th percentile | 26 | |
| Gastrointestinal | • Meconium ileus | 17 |
| • Isolated gastrointestinal symptoms (excluding meconium ileus) | 12 | |
| • Gastrointestinal symptoms with other symptoms | 95 | |
| Respiratory | • Isolated respiratory symptoms | 4 |
| • Respiratory symptoms with other symptoms | 81 | |
| Family history | • Family history only | 1 |
| • Diagnosis owing to symptoms | 11 |
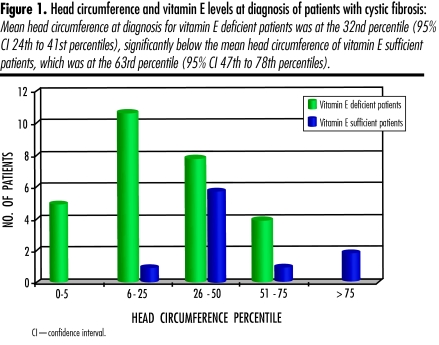
Failure to thrive was common; growth variables at diagnosis are shown in Table 4. Excluding patients with meconium ileus and those receiving total parenteral nutrition or other sources of vitamin E supplementation, 47/67 (70%) CF patients were vitamin E deficient at time of diagnosis. Mean head circumference of these patients was in the 32nd percentile (95% confidence interval 24th to 41st percentiles). This is significantly smaller than the mean head circumference of children with adequate levels of vitamin E whose mean head circumference was at the 63rd percentile (95% confidence interval 47th to 78th percentiles) (Figure 1).
Figure 1. Head circumference and vitamin E levels at diagnosis of patients with cystic fibrosis.
Mean head circumference at diagnosis for vitamin E deficient patients was at the 32nd percentile (95% CI 24th to 41st percentiles), significantly below the mean head circumference of vitamin E sufficient patients, which was at the 63rd percentile (95% CI 47th to 78th percentiles).
DISCUSSION
This study documents morbidity at diagnosis of children with CF in a mixed urban and rural area in Canada and highlights the challenges of clinical identification and diagnosis. To our knowledge, no similar analysis has been published in Canada, although 1 previous study focused on the Asian CF population in the metropolitan Toronto area.16 It is also noteworthy that 24 of the 122 newly diagnosed CF patients were adults. An analysis of their morbidity and experiences is planned for a separate report.
The incidence of CF per live birth in our study was similar to that reported in a recent article based on Canada-wide CF registry data.3 The mean age of 3.0 years at diagnosis of CF for children in BC (including those with meconium ileus) is consistent with the Canadian mean of 3.5 years as reported for 2002 in the CCFF Patient Data Registry.17 Excluding CF patients who presented with meconium ileus, the mean delay in diagnosis of 2.1 years after onset of symptoms is also consistent with the literature2,4,6,8,9,18,19 and reflects the difficulty of diagnosing CF in a timely manner. In view of the morbidity documented in our patients at diagnosis, it is not surprising that we found that most had spent time in tertiary care hospitals before diagnosis of CF. These periods would have been extremely distressing for families; we did not systematically survey parents’ attitudes or recollections of this period, but such issues have been documented elsewhere.20 In addition to the personal and psychosocial costs of hospitalizations, the actual monetary costs of prediagnosis hospitalizations should be added to those mentioned in a recent analysis from Wisconsin, which concluded that it is more economic to identify and diagnose CF patients by newborn screening than by traditional clinical means.21
The time during which a child is sick but has no definitive diagnosis can not only involve hospitalizations and investigations, but also lead to increased illness because of delays in instituting appropriate management. The clinical condition of 3 patients with severe malnutrition at diagnosis was sufficiently severe to be life threatening. It seems reasonable to suspect that others had died in infancy of similar complications without the diagnosis having been made or their data having been included in this analysis. This possibility is supported by results of a randomized controlled trial in Wisconsin that compared the outcomes of patients who had been screened for CF as newborns with those of patients diagnosed by clinical means. One in 15 patients died among those diagnosed by clinical means; there were no early deaths in the screened group. Similarly, in a UK study that randomized infants to be screened or not screened during a 5-year period, Doull et al22 found there were 4 early deaths among 59 patients with CF who were not screened and no deaths among the 74 patients who were screened.
Although CF is often perceived to be a primarily respiratory condition, our findings indicate that, at the time of diagnosis, more CF patients had GI or malnutrition symptoms (95%) than respiratory symptoms (81%). Excluding those diagnosed owing to neonatal intestinal obstruction, 15% of patients had GI or malnutrition symptoms as their only indication of CF. Meconium ileus in itself should trigger testing for CF, but our findings indicate that, in older infants and children, other persistent and unexplained GI symptoms, such as failure to thrive, anemia, diarrhea or steatorrhea, and rectal prolapse, should also raise clinical suspicion, regardless of whether a child has respiratory symptoms.
Growth retardation was an early and prominent feature of CF in our study. More than one-third of our CF patients were at ≤ 5th percentile for weight, and more than one-quarter were at ≤ 5th percentile for length or height. A large, randomized, prospective study in Wisconsin showed that children diagnosed with CF on the basis of symptoms alone continued to have growth retardation relative to children who had been screened and treated for CF as newborns until at least 10 years of age.2 This is of concern because being below ideal body weight has been shown to be an independent predictor of mortality in CF.23
Vitamin E deficiency was also common among our patients. Vitamin E deficiency at diagnosis of CF has been associated with decreased head circumference and long-term impaired cognitive functioning.24,25 Our patients with vitamin E deficiency had smaller head circumferences than patients who had adequate levels of vitamin E (Figure 1), which lends further support to the reported association between vitamin E deficiency and decreased head circumference and later cognitive problems.
Pulmonary disease is currently the leading cause of morbidity and mortality among CF patients,26 and chronic pulmonary infection with P aeruginosa is associated with more rapidly declining pulmonary function.27 It has now been established that early aggressive treatment at first isolation of P aeruginosa can prevent or postpone chronic colonization and have a beneficial effect on maintenance of pulmonary function.28,29 In our study, at least 7/81 (9%) patients already demonstrated colonization with P aeruginosa at diagnosis, suggesting that the opportunity for early treatment with its potential long-term benefits might have been lost for these patients.
Mounting evidence of the health benefits of newborn screening for CF has been documented in recent years. Results from the Wisconsin trial have been published in a series of papers that demonstrate that newborn screening prevents severe malnutrition and improves long-term growth2 and cognitive function.24 In a UK trial, Doull et al22 observed a more than 50% reduction in mortality from causes other than meconium ileus early in life in screened patients compared with patients in whom CF had been identified clinically. Another UK study19 comparing clinically identified and screened CF patients, all of whom were homozygous for the F508 mutation, demonstrated a 50% lower prevalence of chronic P aeruginosa infection (P < .005) and significantly better pulmonary outcomes, as indicated by chest radiograph scores (P < .05), in patients who underwent newborn screening. These results were achieved despite reduced treatment intensity in the screened cohort.30 Based on the positive results of these and earlier studies, newborn screening for CF is now the standard of care in Australia and New Zealand, much of Europe, and many US states. It seems likely that there will be increasing pressure to implement similar newborn screening programs in Canada.
Limitations
There might have been some CF patients who were not known to our clinic because, without newborn screening, as many as 1 in 15 CF patients die without being diagnosed2,22; any such patients would not, therefore, have been included in our analysis. Some living CF patients might not be known to us also; however, access to funded services in BC, such as supply of pancreatic enzymes, requires that diagnosis be confirmed by a CF clinic, making this possibility less likely. The proportion of patients found to be colonized with P aeruginosa is likely to be an underestimation since the predictive value for a “negative” P aeruginosa culture from a throat swab is thought to be only 70%.31,32
Conclusion
Our study has documented considerable morbidity and delay in diagnosis of CF when identification of the disease was based on clinical suspicion. By time of diagnosis, some patients were already seriously ill. Despite the common perception that CF is primarily a respiratory disease, we found that more patients had GI or malnutrition symptoms before diagnosis. Testing triggered by these symptoms might help decrease the diagnostic delays we have described. With the recent implementation of newborn screening for CF in Alberta and Ontario and the expected implementation of it in BC, as recently recommended by the BC Newborn Screening Advisory Committee, our documentation of the morbidity of CF diagnosed clinically will be useful for assessing the health benefits of early detection of CF in Canada.
Acknowledgment
We wish to acknowledge the help and support of the members of the CF Clinic, particularly Joyce Schmidt RN for her help with data gathering, Drs Lillquist, Peacock, and Kielska, nurses Anna Gravelle and Shelagh Jenkins, and secretary Pamela Seldon.
EDITOR’S KEY POINTS
Although cystic fibrosis (CF) is a common lethal autosomal recessive disorder that can be difficult to diagnose in the early stages, only 2 provinces (Alberta and Ontario) routinely screen newborns for CF.
In this study, investigators reviewed all diagnoses of CF in British Columbia made within a 12-year period and found that the mean delay in diagnosis was more than 2 years. Patients often experienced morbidity severe enough to require tertiary care hospitalization before the disease was diagnosed.
Although CF is often thought of as a respiratory disease, physicians should also suspect CF in patients with growth impairment, gastrointestinal symptoms, or nutritional deficiencies.
POINTS DE REPÈRE DU RÉDACTEUR
Bien que la mucoviscidose (MV) soit une affection à caractère autosomique récessif létal relativement fréquente et qu’elle puisse être difficile à diagnostiquer précocement, seulement 2 provinces, l’Alberta et l’Ontario, en font le dépistage systématique chez le nouveau-né.
Dans cette étude, les auteurs ont relevé tous les diagnostics de MV effectués en Colombie-Britannique sur une période de 12 ans; le délai moyen pour le diagnostic excédait 2 ans. Les patients avaient souvent présenté une morbidité suffisamment grave pour nécessiter des soins hospitaliers tertiaires avant l’établissement du diagnostic.
Même si la MV est souvent vue comme une maladie respiratoire, le médecin devrait y penser en présence d’un retard de croissance, de symptômes digestifs ou de malnutrition.
Footnotes
Cet article a fait l’objet d’une révision par des pairs.
Contributors
Drs Davidson, Steinraths, and Vallance contributed to concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
This article has been peer reviewed.
References
- 1.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol. 1996;143(10):1007–17. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 2.Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai HC, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics. 2001;107(1):1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Dupuis A, Hamilton D, Cole DE, Corey M. Cystic fibrosis birth rates in Canada: a decreasing trend since the onset of genetic testing. J Pediatr. 2005;147(3):312–5. doi: 10.1016/j.jpeds.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Wilcken B, Towns SJ, Mellis CM. Diagnostic delay in cystic fibrosis: lessons from newborn screening. Arch Dis Child. 1983;58(11):863–6. doi: 10.1136/adc.58.11.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilfond BS, Gollust SE. Policy issues for expanding newborn screening programs: the cystic fibrosis newborn screening experience in the United States. J Pediatr. 2005;146(5):668–74. doi: 10.1016/j.jpeds.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Siret D, Bretaudeau G, Branger B, Dabadie A, Dagorne M, David V, et al. Comparing the clinical evolution of cystic fibrosis screened neonatally to that of cystic fibrosis diagnosed from clinical symptoms: a 10-year retrospective study in a French region (Brittany) Pediatr Pulmonol. 2003;35(5):342–9. doi: 10.1002/ppul.10259. [DOI] [PubMed] [Google Scholar]
- 7.Waters DL, Wilcken B, Irwing L, Van Asperen P, Mellis C, Simpson JM, et al. Clinical outcomes of newborn screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed. 1999;80(1):F1–7. doi: 10.1136/fn.80.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merelle ME, Schouten JP, Gerritsen J, Dankert-Roelse JE. Influence of neonatal screening and centralized treatment on long-term clinical outcome and survival of CF patients. Eur Respir J. 2001;18(2):306–15. doi: 10.1183/09031936.01.00080101. [DOI] [PubMed] [Google Scholar]
- 9.Chatfield S, Owen G, Ryley HC, Williams J, Alfaham M, Goodchild MC, et al. Neonatal screening for cystic fibrosis in Wales and the West Midlands: clinical assessment after five years of screening. Arch Dis Child. 1991;66(1 Spec):29–33. doi: 10.1136/adc.66.1_spec_no.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosse SD, Boyle CA, Botkin JR, Comeau AM, Kharrazi M, Rosenfeld M, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep. 2004;53(RR13):1–36. [PubMed] [Google Scholar]
- 11.Maternal and Child Health Bureau, US Department of Health and Human Services. Newborn screening: towards a uniform screening panel and system. [Accessed 2008 Mar 1];Fed Regist. 2005 70(44):1–329. Available from: http://mchb.hrsa.gov/screening/
- 12.Montgomery M, Berthiaume Y, Buchanan J, Cantin A, Corey M, Lands L, et al. Newborn screening programs for cystic fibrosis. Report of the working group. Toronto, ON: Canadian Cystic Fibrosis Foundation; 2005. [Google Scholar]
- 13.Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23(3):545–9. [PubMed] [Google Scholar]
- 14.Lockitch G, Halstead AC, Wadsworth L, Quigley G, Reston L, Jacobson B. Age- and sex-specific pediatric reference intervals and correlations for zinc, copper, selenium, iron, vitamins A and E, and related proteins. Clin Chem. 1988;34(8):1625–8. [PubMed] [Google Scholar]
- 15.British Columbia Ministry of Health, Vital Statistics Agency. 2004 annual report. Victoria, BC: British Columbia Ministry of Health, Vital Statistics Agency; 2005. [Accessed 2006 May 9]. Available from: www.vs.gov.bc.ca/stats/annual/index.html. [Google Scholar]
- 16.Mei-Zahav M, Durie P, Zielenski J, Solomon M, Tullis E, Tsui LC, et al. The prevalence and clinical characteristics of cystic fibrosis in South Asian Canadian immigrants. Arch Dis Child. 2005;90(7):675–9. doi: 10.1136/adc.2003.042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Cystic Fibrosis Foundation. Canadian patient data registry: national report, 2002. Toronto, ON: Canadian Cystic Fibrosis Foundation; 2002. [Google Scholar]
- 18.Rosenstein BJ, Langbaum TS, Metz SJ. Cystic fibrosis: diagnostic considerations. Johns Hopkins Med J. 1982;150(3):113–20. [PubMed] [Google Scholar]
- 19.Sims EJ, McCormick J, Mehta G, Mehta A Steering Committee of the UK Cystic Fibrosis Database. Neonatal screening for cystic fibrosis is beneficial even in the context of modern treatment. J Pediatr. 2005;147(3 Suppl):S42–6. doi: 10.1016/j.jpeds.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Merelle ME, Huisman J, Alderden-van der Vecht A, Taat F, Bezemer D, Griffioen RW, et al. Early versus late diagnosis: psychological impact on parents of children with cystic fibrosis. Pediatrics. 2003;111(2):346–50. doi: 10.1542/peds.111.2.346. [DOI] [PubMed] [Google Scholar]
- 21.Lee DS, Rosenberg MA, Peterson A, Makholm L, Hoffman G, Laessig RH, et al. Analysis of the costs of diagnosing cystic fibrosis with a newborn screening program. J Pediatr. 2003;142(6):617–23. doi: 10.1067/mpd.2003.209. [DOI] [PubMed] [Google Scholar]
- 22.Doull IJ, Ryley HC, Weller P, Goodchild MC. Cystic fibrosis-related deaths in infancy and the effect of newborn screening. Pediatr Pulmonol. 2001;31(5):363–6. doi: 10.1002/ppul.1059. [DOI] [PubMed] [Google Scholar]
- 23.Sharma R, Florea VG, Bolger AP, Doehner W, Florea ND, Coats AJ, et al. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax. 2001;56(10):746–50. doi: 10.1136/thorax.56.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koscik RL, Farrell PM, Kosorok MR, Zaremba KM, Laxova A, Lai HC, et al. Cognitive function of children with cystic fibrosis: deleterious effect of early malnutrition. Pediatrics. 2004;113(6):1549–58. doi: 10.1542/peds.113.6.1549. [DOI] [PubMed] [Google Scholar]
- 25.Koscik RL, Lai HJ, Laxova A, Zaremba KM, Kosorok MR, Douglas JA, et al. Preventing early, prolonged vitamin E deficiency: an opportunity for better cognitive outcomes via early diagnosis through neonatal screening. J Pediatr. 2005;147(3 Suppl):S51–6. doi: 10.1016/j.jpeds.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 27.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32(4):277–87. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 28.Lillquist YP, Davidson AG, Gravelle A, Jenkins S, Peacock D, McIlwaine M. Pulmonary function outcome after aggressive intervention protocol for first growth Pseudomonas aeruginosa: 9-year experience 1995–2004. Pediatr Pulmonol. 2004;38(Suppl 27):290. [Google Scholar]
- 29.Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997;23(5):330–5. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Sims EJ, McCormick J, Mehta G, Mehta A UK CF Database Steering Committee. Newborn screening for cystic fibrosis is associated with reduced treatment intensity. J Pediatr. 2005;147(3):306–11. doi: 10.1016/j.jpeds.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey BW, Wentz KR, Smith AL, Richardson M, Williams-Warren J, Hedges DL, et al. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. Am Rev Resp Dis. 1991;144(2):331–7. doi: 10.1164/ajrccm/144.2.331. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28(5):321–8. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]