Abstract
Recent work has added strong support to the long-standing hypothesis that stabilization of both long-term potentiation and memory require rapid reorganization of the spine actin cytoskeleton. This development has led to new insights into the origins of cognitive disorders, and raised the possibility that a diverse array of memory problems, including those associated with diabetes, reflect disturbances to various components of the same mechanism. In accord with this argument, impairments to long-term potentiation in mouse models of Huntington's disease and in middle-aged rats have both been linked to problems with modulatory factors that control actin polymerization in spine heads. Complementary to the common mechanism hypothesis is the idea of a single treatment for addressing seemingly unrelated memory diseases. First tests of the point were positive: Brain-Derived Neurotrophic Factor (BDNF), a potent activator of actin signaling cascades in adult spines, rescued potentiation in Huntington's disease mutant mice, middle-aged rats, and a mouse model of Fragile-X syndrome. A similar reversal impairments to long-term potentiation was obtained in the middle-aged animals by up-regulating BDNF production with brief exposures to ampakines, a class of drugs that positively modulate AMPA-type glutamate receptors. Work now in progress will test if chronic elevation of BDNF enhances memory in normal animals.
Keywords: long-term potentiation, ampakine, BDNF, stabilization, actin, synaptic plasticity, hippocampus
1. Introduction
Well over a hundred years ago Ribot proposed that memory is encoded by changes in connections between the brain's ‘nervous elements’ and becomes stabilized (resistant to disruption) during the first several minutes following its acquisition (Ribot, 1882). Confirmation of the first point, and descriptions of how the second might be accomplished, did not come quickly. It was not until 1973, and the advent of long-term potentiation (LTP)(Bliss and Lomo, 1973), that synapses were shown to possess the expected capability for rapid and persistent changes in efficacy. The subsequent discovery that potentiation is vulnerable to disruption for several minutes after induction (Arai et al., 1990; Barrionuevo et al., 1980) endowed LTP with the third member of an unlikely combination of properties required of a memory substrate: synapse specificity, extraordinary stability, and a rapid onset consolidation process. The links to memory were further strengthened by evidence that LTP occurs during learning (Roman et al., 1987) and that agents which block the effect cause amnesia (Morris et al., 1986). As evidence of these types gradually accumulated, a sizeable group of investigators began to use LTP as a surrogate in the search for the cellular processes that encode and consolidate memory.
Efforts to isolate the synaptic events responsible for various aspects of LTP have accelerated in recent years, in part because of new technologies and in part because of past successes in sharpening the focus of the search. Increasing attention is now being given to the possibility of using the growing body of information about LTP to investigate the causes of, and potential treatments for, various memory and cognitive disorders. Related to this are LTP-based projects concerned with the design of memory enhancing drugs (Lynch, 2002). In the following sections we will consider these developments beginning with new evidence on the synaptic processes that express and stabilize LTP.
2. Substrates of LTP
Experiments showing that induction of LTP requires increases in dendritic calcium concentrations (Lynch et al., 1983) led to the early assumption that potentiation is expressed by a post-synaptic change, most probably to the number of glutamate receptors (Lynch and Baudry, 1984). That LTP is accompanied by ultrastructural changes to the post-synaptic density (Chang and Greenough, 1984; Desmond and Levy, 1986; Geinisman et al., 1993; Harris et al., 2003; Lee et al., 1980; Yuste and Bonhoeffer, 2001) also pointed to increased numbers of receptors as a likely expression mechanism. These observations and hypotheses prompted efforts to identify cellular processes that could rapidly reorganize a dendritic spine and its specialized post-synaptic membrane.
2.1 Reorganization of the spine cytoskeleton stabilizes LTP
Changes in the structure of small processes on various kinds of cells are accomplished through reorganization of the actin cytoskeleton and it was accordingly of interest to ask if such an effect occurs in individual, adult spines following LTP-inducing afferent stimulation (Fischer et al., 1998; Fukazawa et al., 2003; Lisman, 2003; Matus, 2000; Okamoto et al., 2004). Because LTP appears in less than 1 min (Gustafsson and Wigstrom, 1990), and undergoes considerable stabilization in the subsequent 10-15 min (Abraham and Williams, 2003; Lynch et al., 2007b), the hypothesized LTP-related actin changes would need to be reasonably rapid. Tests of these points became possible when it was found that phalloidin, a toxin that binds selectively to filamentous (F)-actin, discretely labeled spines when applied to adult hippocampal slices in situ during the course of routine physiological experiments (Lin et al., 2005). Using this technique, we found that theta burst stimulation produces a several-fold increase in the number of spines with dense concentrations of polymerized actin in the target dendritic lamina (Fig. 1).
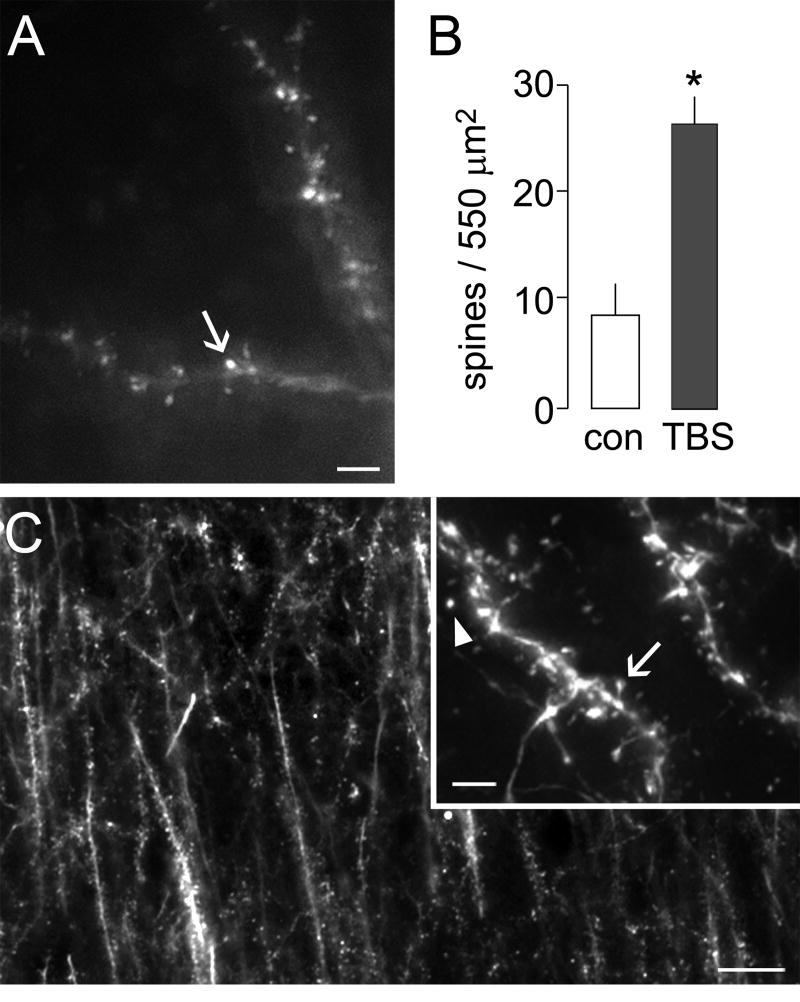
Figure 1. Theta burst stimulation induces actin polymerization in hippocampal dendritic spines.
Long-term potentiation was induced in adult hippocampal slices by theta burst stimulation (TBS) of Schaffer-collateral afferents to field CA1. F-actin was labeled in situ by application of rhodamine-phalloidin to identify stimulation effects on levels of polymerized actin in dendritic spines within the zone of potentiated synapses. (A) Photomicrograph shows labeling of F-actin by rhodamine-phalloidin applied through a patch-clamp electrode in a slice receiving theta stimulation. Numerous densely labeled spines are observed along the lengths of labeled dendrites (arrow). Calibration bar: 2 μm. (B) Plot shows the numbers of spines densely labeled with intracellular application of phalloidin to cells in control slices (con) and those receiving theta stimulation. Slices receiving theta stimulation showed nearly 3-fold greater numbers of densely labeled spines (*p<0.0001). (C) Low-power photomicrograph shows typical labeling of F-actin rich spines after extracellular application of fluorescent-tagged phalloidin at 20 min after theta stimulation. Calibration bar: 10 μm. Inset shows examples of isolated spines (arrowhead) and those with a clear head and neck attached to its parent dendrite (arrow). Calibration bar: 5 μm.
Subsequent experiments confirmed that spine actin polymerization is essential for LTP stabilization. Latrunculin A at concentrations sufficient to block polymerization within spines (Krucker et al., 2000) completely eliminated LTP stabilization (Rex et al., 2007), while post-theta stimulation treatments that block consolidation (e.g., adenosine) also disrupted polymerization (Kramár et al., 2006). Moreover, the actin effect had a threshold (number of theta bursts) comparable to that for inducing LTP and developed quickly enough (∼2 min) to participate in LTP consolidation. Notably, the different manipulations used to disrupt newly formed actin filaments become progressively less effective during the first 10-15 min following LTP induction. Low frequency stimulation, for example, completely eliminates actin polymerization and LTP when applied 1 min, but not 15 min, after theta burst stimulation (Kramár et al., 2006). These results suggest that the filaments formed in response to theta stimulation are initially dynamic, with a high level of actin turnover, but are then stabilized by end-capping, crosslinking, and bundling. We propose that the transition from the dynamic to the stable configuration is the event that consolidates LTP.
2.2. Increases in synapse size express LTP
The above results suggested a means for testing, across very large numbers of synapses, whether LTP is associated with an increase in synapse size. The F-actin effect, closely linked to LTP as it is, could be used as a marker for potentiated spines, while immunostaining for post-synaptic density proteins would provide a means for estimating synapse size. Combining the two methods (double labeling) would permit comparisons of synapses situated on labeled (potentiated) vs. non-labeled spines. We attempted experiments of this type using rhodamine-phalloidin to label spines containing dense F-actin but found that the phalloidin labeling method does not work well in conjunction with conventional immunocytochemistry (Rex et al., 2007). This led us to investigate signaling cascades leading from the membrane to the actin cytoskeleton with the goal of finding a marker for recently potentiated synapses appropriate for co-immunostaining.
The p21-activated kinase (PAK) ≫ LIM-kinase ≫ Cofilin signaling pathway regulates actin assembly under a variety of conditions (Carlisle and Kennedy, 2005; Meng et al., 2003) and its activity can be assessed by measuring the phosphorylation levels of its component proteins. While immunostaining for total (phosphorylated and unphosphorylated) PAK and Cofilin shows that these proteins are present in a large percentage of the spine population in field CA1 of the adult rat hippocampus (Chen et al., 2007; Racz and Weinberg, 2006; Rex et al., 2007), spines containing dense concentrations of the phosphorylated (p-) proteins were uncommon (Fig. 2A-D). Since Cofilin, which promotes the disassembly of actin filaments, is inactivated by phosphorylation (Bamburg et al., 1999), this is consonant with the relative absence of spines with high concentrations of F-actin. Theta burst stimulation of the Schaffer collateral afferents of field CA1 stratum radiatum produced a marked increase in the number of pPAK and pCofilin positive spines; the effect was present at 2 min, but not 30 seconds, after stimulation, reached its peak at about 7 min, and then declined (Fig. 2E)(Chen et al., 2007). As might be expected, the phosphorylation events show none of the persistence found for actin polymerization, which was unchanged at 2 hours after theta stimulation (Rex et al., 2007).
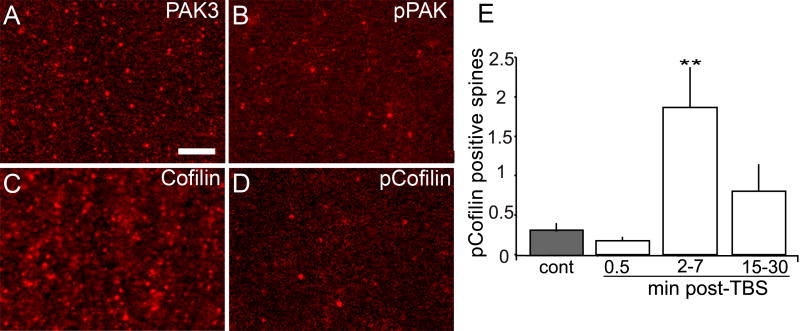
Figure 2. Theta burst stimulation increases numbers of spines containing dense pPAK and pCofilin.
(A-D) Laser confocal micrographs show spine-like profiles containing immunoreactivity for PAK3 (A), pPAK (B), Cofilin (C) and pCofilin (D) in CA1 str. radiatum of control hippocampal slices receiving only baseline stimulation. Note that elements containing dense PAK3 and Cofilin immunoreactivity are more numerous than those containing pPAK and pCofilin. Calibration bar: 5 μm. (E) Plot shows numbers of pCofilin-positive spines in proximal str. radiatum of hippocampal slices receiving control stimulation (cont, gray bar) or theta burst stimulation (TBS, white bars) with harvest at 0.5, 2-7, or 15-30 min after stimulation. The number of pCofilin-positive spines was significantly increased at 2-7 min post-theta stimulation (**p=0.008 vs cont, ANOVA followed by Tukey's HSD, n=8/group).
No difficulties were encountered in using pPAK or pCofilin antibodies in conjunction with those against PSD-95, an integral synaptic scaffold protein, to identify spine synapses (Hunt et al., 1996), that were or were not associated with phosphoproteins (Fig. 3A). Therefore, double labeling for these antigens was used to evaluate the effects of theta burst stimulation on the size of PSD-95+ synapses associated with recently activated (e.g., pCofilin containing) spines in adult rat hippocampal slices. In parallel analyses, images of immunolabeled profiles were collected using either (i) confocal microscopy and flattened Z-stacks or (ii) wide field microscopy coupled with restorative deconvolution to generate 3-D images of spines and PSD-95+ synapses; counts and measures of PSD size were done with locally developed software (Chen et al., 2007; Rex et al., 2007). The results were unambiguous: after theta stimulation, synapses situated on densely pPAK or pCofilin immunolabeled spines were about 60% larger than surrounding synapses that were not associated with these phosphoproteins (Fig. 3B).
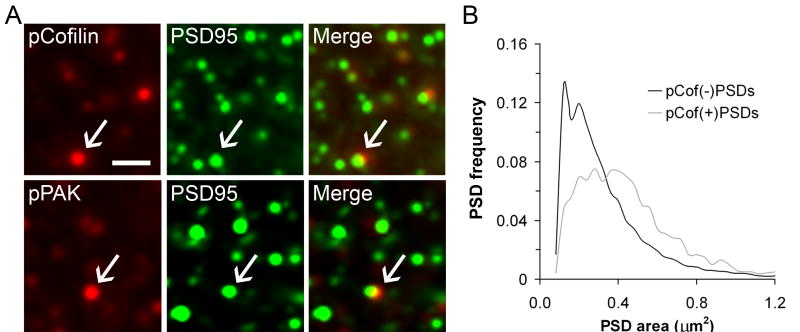
Figure 3. Synapses on spines containing phosphoproteins (pPAK, pCofilin) are larger then their neighbors.
Hippocampal slices received theta burst or control stimulation, were harvested at 7 min, and then tissue was processed for double immunofluorescence for PSD-95 and pCofilin or pPAK. (A) Micrographs show that PSD-95 is colocalized with pCofilin and pPAK in spine-like puncta within CA1 str. radiatum. Micrographs show the individual localization of the phosphoprotein (red, left) and PSD-95 (green, middle) and the overlay of these images (“Merge”, right). In the merged images double labeling appears yellow; arrows indicate individual double-labeled elements. Calibration Bar: 1μm. (B) Plot shows the size frequency distribution of PSD-95 immunoreactive synapses that were associated with pCofilin (“pCof(+)PSDs”, gray line) and those not associated with pCofilin (“pCof(-)PSDs”, black line) in str. radiatum of theta stimulated slices. Note that the size distribution for PSDs associated with pCofilin is shifted toward the right, denoting larger sizes, and has a more normal distribution. A similar rightward shift in the size frequency distribution was found for PSDs associated with pPAK.
Electron microscopic work from different laboratories has shown that the number of AMPA-type glutamate receptors found in a synapse scales linearly with the size of the synapse (Ganeshina et al., 2004; Nusser et al., 1998b; Racca et al., 2000; Takumi et al., 1999). The effects just described thus provide a simple explanation for the expression of LTP: theta stimulation causes a rapid increase in the area of the synapse, most likely as a result of altering the synapse shape (Chen et al., 2007), and thereby presents a larger pool of receptors opposite the transmitter release zone.
3. LTP-related changes to spines and synapses occur during learning
The above results describe mechanisms that could potentially encode and stabilize memory: they are associated with a change in synaptic strength, occur quickly in a synapse-specific fashion, and contribute to a structural modification. We tested for their occurrence during learning using an unsupervised paradigm in which young adult rats gain familiarity with a complex environment (Fedulov et al., 2007). The animals were handled extensively over a five day period and then placed into one of three groups: a) transported from the home cage but not placed in the novel environment; b) pretreated with the NMDA receptor antagonist (±)-3-(2-carboxypiperzain-4-yl)propyl-1-phosphonic acid (CPP) to block LTP and then allowed to explore for 30 min; or c) pretreated with vehicle and allowed to explore. All animals were sacrificed promptly at the end of the 30 min test period and tissue sections through hippocampus were immunostained for pCofilin and the synaptic marker PSD-95. A defined zone in the apical dendritic field of hippocampal field CA1 (stratum radiatum) was analyzed in detail using the same computerized microscopic and image analysis systems and software employed in LTP experiments described above. This analysis demonstrated that numbers of spines densely labeled for pCofilin were increased by approximately 30% in the vehicle/exploration group relative to either the home-cage controls or the NMDA receptor antagonist/exploration group.
Spines with high concentrations of pCofilin were uncommon in the in vivo study, just as was the case for the slice experiments. It follows from this that the increases observed in the vehicle/exploration rats, while substantial in a relative sense, in fact involved a very small percentage of the total spine population. This accords with the view that a learning mechanism can only engage small fractions of the encoding elements if it is to satisfy the capacity requirements imposed by memory (Furber et al., 2004; Granger et al., 1994).
Having found that learning produces an NMDA-receptor dependent, LTP-like increase in pCofilin-positive spines, we next asked if it also changes the size of synapses. Restorative deconvolution was used to generate 3-D images of double-labeled profiles in the CA1 sample field from the three treatment groups. As with the LTP studies (Chen et al., 2007), PSD-95 immunolabeled synapses located on pCofilin positive spines were significantly larger than the much more numerous PSD-95+ synapses that were not situated on such spines (Fedulov et al., 2007). Thus, it appears from these results that a 30-min period of intense learning produces the same effects on synapse size as LTP, at least in apical field CA1 of hippocampus. Provisionally, then, we suggest that the observed effects are the physical substrates of memory.
4. A common target for diseases of memory?
The above results, combined with other findings not discussed here, can be assembled into a reasonably specific hypothesis about the formation of memory (Fig. 4). The argument begins with the presence of three quite different classes of receptors in the synapse: a) transmitter, b) adhesion, and c) modulatory. A very substantial body of work indicates that LTP consolidation (but not induction and expression) requires signaling from adhesion receptors belonging to the integrin family (Gall and Lynch, 2004; 2005). It is assumed in the model that the integrins are engaged by appropriate activation of the transmitter receptors. Integrins exert a profound influence over the actin cytoskeleton in all types of cells and neutralizing antibodies against hippocampal integrins completely block theta stimulation-induced actin polymerization and LTP consolidation (Kramár et al., 2006). Building on this, and on observations common to much of cell biology, the model assumes that integrins engage multiple pathways, including the PAK ≫ Cofilin cascade, leading to actin polymerization. As discussed earlier, there is ample evidence indicating that the theta stimulation-induced actin networks are initially dynamic but then are stabilized; it is here assumed that conventional events (capping, crosslinking, etc.) produce this effect. Finally, we include receptors belonging to a class that is modulatory in the sense that its members either accelerate or depress the links leading to cytoskeletal reorganization. The two most prominent examples are the adenosine A1 receptor, which blocks polymerization and LTP consolidation (Gall and Lynch, 2005), and Brain-Derived Neurotrophic Factor's TrkB receptor, which potently facilitates both effects (Rex et al., 2007).
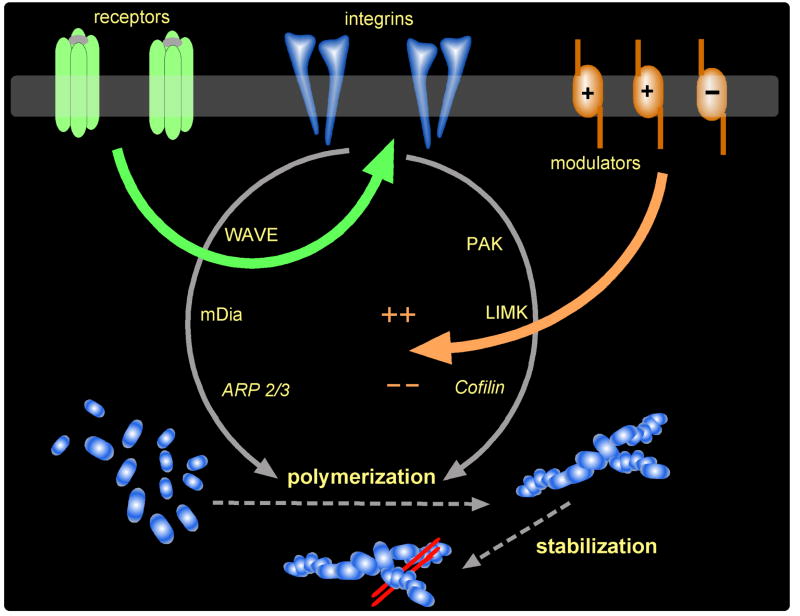
Figure 4. Proposed signaling pathways through which synaptic activity controls spine actin polymerization in association with LTP and memory.
As shown, polymerization is controlled by three receptor classes including the neurotransmitter receptors (labeled ‘receptors’ here), adhesion receptors represented by the integrins, and modulatory receptors including those that facilitate and inhibit polymerization and LTP (prime examples here being the TrkB receptor for BDNF and the A1 adenosine receptor, respectively). In this model, neurotransmitter/glutamate receptor activity leads to integrin activation. The integrins then signal through multiple pathways, including the PAK > LIM Kinase > Cofilin path discussed in the text, to control actin polymerization. Signaling from the modulatory receptors converges on integrin signaling to facilitate or inhibit polymerization.
There are multiple reasons for suspecting that various memory disorders involve defects to one or more aspects of the vastly complicated machinery that links patterned afferent activity to the spine cytoskeleton. Both syndromic and non-syndromic forms of mental retardation are reportedly accompanied by unusual spine morphologies (Newey et al., 2005; Purpura, 1974; Purpura, 1975; Segal et al., 2003), something that is highly suggestive of cytoskeletal defects. Related to this, there are disturbances to Rho GTPase signaling (Newey et al., 2005; Ramakers, 2002); and PAK protein levels (Boda et al., 2006) in patients suffering from certain cognitive disorders; these enzymes play a critical role in linking integrins and modulatory receptors to filament assembly and stabilization. The above described methods for assessing activity-induced actin signaling and polymerization within individual, adult spines has opened the way to testing for defects in cytoskeletal reorganization in animal models of psychiatric diseases involving memory and cognition. The following sections describe the first results from efforts of this kind.
4.1. Plasticity deficits in mouse models of Huntington's Disease
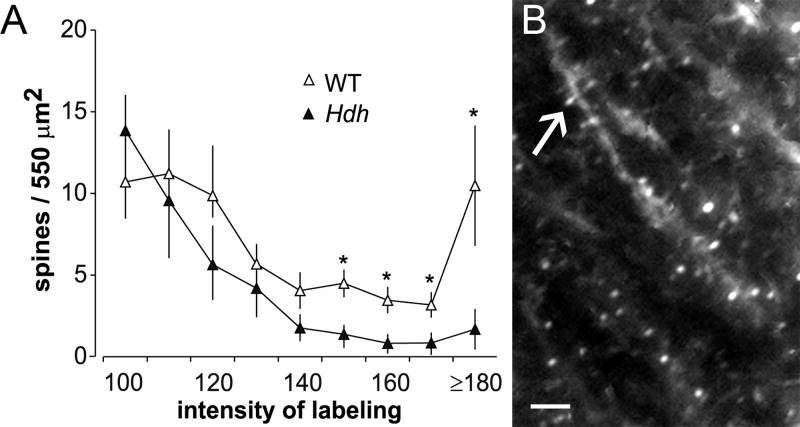
Asymptomatic carriers of the Huntington's disease mutation commonly exhibit problems with memory and cognition (Ho et al., 2003). Mouse models of the disease also exhibit learning deficits well in advance of the onset of locomotor problems (Van Raamsdonk et al., 2005). Both patients and the mice are reported to have reduced BDNF levels (Duan et al., 2003; Ferrer et al., 2000; Zuccato et al., 2001), something that could lead to deficits in spine actin polymerization and thus LTP consolidation (see Fig 4). We tested these ideas using hippocampal slices prepared from different full-length knock-in models of Huntington's disease (i.e., HdhQ92, HdhQ111 and CAG 140); the physiological analyses were conducted in young adult animals, months in advance of the onset of movement disorders. LTP was severely impaired in the mutants (Lynch et al., 2007a), in accord with earlier reports (Murphy et al., 2000a; Usdin et al., 1999). Theta burst stimulation produced a strong initial potentiation that rapidly decayed back to baseline, indicating that the consolidation processes were either defective or not properly set in motion in the minutes following induction. As expected from the recordings, theta-induced polymerization of spine actin was also markedly reduced in the Huntington's disease mutant mice relative to paired wild-type controls (Fig. 5A).
Figure 5. Huntington Disease mutant mice exhibit deficits in LTP-related actin polymerization.
Hippocampal slices prepared from HdhQ111 mice were labeled in situ by extracellular application of fluorescent-tagged phalloidin following theta burst stimulation and electrophysiological recording. (A) Plot shows labeling intensity distributions for phalloidin-labeled spines in the region of theta burst afferent stimulation for wild type (WT, open symbols) and mutant (Hdh, black symbols) mice. As shown, in slices from Hdh mice there were significantly fewer intensely labeled (150 - ≥180 pixel intensity units) spines as compared to measures from WT controls (*p<0.05); the profile shown for HdhQ111 mice receiving theta stimulation was not significantly different from HdhQ111 slices receiving control stimulation (not shown). (B) Photomicrograph shows abundant phalloidin-labeled spines (arrow) in a slice from HdhQ11 mouse receiving theta stimulation following 1 hr treatment with 2 nM BDNF. The neurotrophin restored theta-induced actin polymerization in the HdhQ111 to WT levels.
If the subnormal levels of BDNF found in the mutants were largely responsible for the failure of theta stimulation to trigger actin polymerization, then infusion of the neurotrophin should rescue both the activity-induced skeletal changes and LTP consolidation. These predictions were confirmed. Pretreating the Huntington's disease mutant hippocampal slices with 2 nM BDNF completely restored both the increase in spines densely labeled for F-actin normally found after a train of theta bursts (Fig. 5B) and the consolidation of LTP (Lynch et al., 2007a). These effects represented a true rescue of structural and physiological plasticity because BDNF at these low concentrations did not detectably affect LTP in wild-type mice. It thus appears that the Huntington's disease-associated synaptic impairments can be traced to reduced levels of a factor that positively modulates cytoskeletal changes needed to stabilize potentiation.
4.2. Age-related deficits in LTP
A substantial body of evidence indicates that memory losses occur as part of normal aging and thus can be detected by middle age in rat (Deupree et al., 1993) and in man (Davis et al., 2003). This prompted us to ask if LTP deficits appear in rat hippocampus during the transition from young adulthood to early middle age. A conventional train of theta bursts produced about the same degree of potentiation, with no evident differences in stability, in the apical dendrites of field CA1 in slices prepared from 7-9 month old rats as was found in slices from 2-3 month old animals (Rex et al., 2005). Memory impairments in middle-aged animals thus appear not to be due to global losses of LTP. We then shifted the search to the basal dendrites of the CA1 pyramidal neurons, a zone not previously investigated in aging studies but one in which plasticity differs in a number of regards from that found in the apical fields (Kramár and Lynch, 2003; Leung and Shen, 1999; Roth and Leung, 1995). LTP in the basal dendrites was clearly impaired in slices from middle-age rats relative to those from young adults; while the degree of potentiation in the older slices was equivalent in the two age groups immediately after theta stimulation, it was reduced by 2/3rds in the older vs. younger slices one hour later (Rex et al., 2005).
The above pattern of results suggests that normal aging affects plasticity in a highly differentiated fashion: in this example, it affects some but not all populations of synapses on a neuron, and disrupts the consolidation but not the induction and initial expression of LTP. Given these conclusions, we asked if the observed impairments could be attributed to a defect in some aspect of the model described in figure 4. Work described in the literature suggests that processing of the negative modulator adenosine changes with age (Murillo-Rodriguez et al., 2004; Sperlagh et al., 1997). This suggested the possibility that enhanced adenosine signaling may have prevented the stabilization of potentiation in the older group. Tests with the adenosine A1 receptor antagonist DPCPX reinforced this view: basal dendritic LTP was equal to young adult levels in middle aged slices treated with the antagonist (Rex et al., 2005). Moreover, rescue was obtained when the antagonist was applied shortly after theta stimulation and therefore was not due to drug effects on induction and initial expression of potentiation. Further study showed that the effects of adenosine on transmission in the basal dendrites of middle-aged slices are more pronounced than is the case for their younger counterparts. As there was no evident difference in A1 receptor density between the groups, it is likely that adenosine clearance, either through uptake or breakdown, slows with aging in the basal dendrites. In all, age-related impairments to LTP consolidation likely arise from excessive activity of a negative modulator of actin assembly.
4.3. LTP deficits in Fragile-X mental retardation syndrome
Spine abnormalities are a characteristic feature of Fragile-X syndrome and are found, albeit in a seemingly less pronounced form, in mouse models of the disease (Comery et al., 1997; Segal et al., 2003). The condition thus seems to be one in which disturbances in the regulation of the spine cytoskeleton and LTP consolidation would likely be present. Surprisingly, however, hippocampal LTP is not reported to be impaired in the mouse model of Fragile-X (Godfraind et al., 1996; Larson et al., 2005). We re-examined the issue and found that potentiation elicited by a conventional train of ten theta bursts is indeed not detectably different from that obtained in wild-type mice (Lauterborn et al., 2007). However, a pronounced deficit became evident when the theta train was reduced to five bursts, a stimulation pattern that more closely approximates the firing patterns of pyramidal neurons during learning (Otto et al., 1991). There were no evident differences between wild-types and mutants in the composite responses to the theta bursts, NMDA receptor mediated responses, or in the amount of potentiation recorded in the first minutes after theta stimulation. The Fragile-X deficit is thus largely in the stability of potentiation (Lauterborn et al., 2007), as was the case for Huntington's disease mutant mice and middle-aged rats.
Efforts to pin down the nature of the defect in LTP consolidation have not been successful, although the number of possibilities has been somewhat narrowed. BDNF levels are not reduced in the mutants, and there is no evidence of excessive adenosine activity. Moreover, theta burst stimulation-induced increases in polymerization of spine actin appear to occur normally (Lauterborn et al., 2007). A statistical difference in percent potentiation was present at about 5 min after stimulation, a time period that seems short for the mobilization of protein synthesis. In any event, it appears that the impairment occurs at a consolidation step that follows filament assembly—if so, defective capping or crosslinking would be the most likely explanations for why LTP decays in the mutant if indeed actin mechanisms are involved.
5. Treating learning-related defects in cytoskeletal plasticity
The above sections described evidence that changes to the spine cytoskeleton consolidate LTP and that similar events occur during the formation of stable memory. Results were also summarized suggesting that defects in these LTP/learning processes are found in mouse models of three different human conditions involving disturbances to memory and cognition. The question now arises as to whether it will be possible to use this information to design novel therapeutics. One possibility in this direction would be to enhance positive, or reduce negative, modulation. As described, infusions of BDNF produced a complete restoration of actin polymerization and LTP consolidation in the mouse model of Huntington's disease. However, this could represent a special case because the Huntington mutation reduces BDNF production: the results obtained with the Huntington's disease mutant mice may not generalize to situations in which neurotrophin levels are normal. We addressed the issue by measuring the effects of BDNF infusion on LTP impairments in middle-aged rats and Fragile-X mice.
5.1. BDNF rescues LTP in the presence of normal neurotrophin levels
As noted, the stability of LTP in the basal dendrites of hippocampal field CA1 is impaired at relatively early stages of normal aging. This deficit was eliminated by infusion of low (2 nM) concentrations of BDNF (Rex et al., 2007). BDNF at this dosage has no measurable effect on the size or shape of synaptic responses, or on levels of spine F-actin, but did activate signaling through the PAK/Cofilin pathway (Rex et al., 2007). It remains now to determine if this is the route over which the neurotrophin restores the stability of LTP to middle-aged hippocampus. But the results so far collected are sufficient to demonstrate that BDNF has a potent, positive effect on plasticity under conditions in which its levels are not reduced.
Comparable results were obtained in experiments using Fragile-X mice. The amount and stability of potentiation induced by a single train of five theta bursts were the same in mutant and wild-type slices exposed to 2 nM BDNF. The neurotrophin had no effect on input/output curves, the post-synaptic responses to an individual theta burst, or the degree to which burst responses changed during a train (Lauterborn et al., 2007).
5.2. Up-regulating BDNF as a strategy for treating memory disorders
While the above results are highly suggestive, it seems unlikely that BDNF protein can be used as a therapeutic for psychiatric diseases: there are difficulties with passage across the blood brain barrier and earlier trials elicited negative side effects. Moreover, bulk infusion would undoubtedly elicit undesirable effects due to aphysiological presentation of the compound. An alternative approach would be to find a clinically plausible means for increasing the production of endogenous BDNF and then counting on activity-dependent release (Balkowiec and Katz, 2000) to produce the same types of effects found with infusions. It has been known for more than a decade that neuronal activity regulates BDNF expression (Gall, 1992; Gall and Lauterborn, 2000), which raises the possibility that increased excitatory transmission could have the effect of increasing BDNF concentrations. Tests of this idea became possible with the development of positive allosteric modulators of AMPA receptors that enhance fast, excitatory transmission at central synapses (Staubli et al., 1994b; Staubli et al., 1994a). These ‘ampakine’ drugs produce a variety of acute effects, including lowered thresholds for LTP and accelerated learning, with surprisingly few side-effects (Lynch, 2006; Lynch and Gall, 2006). Subsequent work found that the drugs can be used to increase BDNF production both in vitro and in vivo (Lauterborn et al., 2000). Additional studies led to the discovery that brief, even very brief, ampakine treatments can produce elevated BDNF protein levels lasting for surprisingly long periods (i.e., for 2 to 3 days following a pulse ampakine exposure) (Lauterborn et al., 2003). With these latter findings, the way was open to asking if up-regulating BDNF expression can be used to treat deficits in LTP and learning.
We chose the LTP deficits that emerge in early middle age as a test case. Middle aged (8-10 month old) rats were injected I.P. with an ampakine variant that has a half-life of about 15 min and then placed in a social, complex environment for 30 min. This procedure was repeated twice daily for four days; animals were sacrificed and hippocampal slices were prepared 18 hours after the last drug (or vehicle) injection. There were no detectable differences between the behaviors of the ampakine- vs. vehicle-treated rats during their sessions in the complex environment. Nonetheless, BDNF protein levels in parietal neocortex and hippocampus were elevated 50-100% above vehicle control levels at the time of slice preparation (Rex et al., 2006). It bears repeating that the physiological tests began almost a day after the last administration of a drug with a 15-min half-life.
Elevating endogenous hippocampal BDNF levels produced the same effects as infusion of the neurotrophin: LTP in the middle-aged slices was restored to the same levels found in young adult slices. The rescue was selective in that the daily ampakine treatments did not affect input/output curves, theta burst responses, or within-train changes in theta burst responses (Rex et al., 2006). Taken together, these results constitute a first demonstration that chronic increases in BDNF can produce a selective, by both behavioral and physiological measures, restoration of synaptic plasticity. Whether it accomplishes this effect via its established action on the actin assembly machinery, as proposed by the general model being advanced here, is a critical next question.
6. Discussion
Consolidation
The idea that the extreme persistence of LTP reflects changes to spine anatomy, and thus to the spine cytoskeleton, was advanced during the early years of LTP research (Lee et al., 1980; Lynch and Baudry, 1984; Matus, 2000). The recent work described here provides strong evidence in support of the hypothesis. Threshold levels of theta burst stimulation, an experimental treatment that produces the type of synapse–specific potentiation assumed to be responsible for memory in big-brained mammals, triggered actin polymerization in spines located in the dendritic zone containing potentiated synapses (Kramár et al., 2006). The effect had the same activation threshold (number of theta bursts) as LTP and emerged in the time frame expected for an LTP stabilization process. Blocking polymerization within the spine heads thoroughly and selectively blocked LTP consolidation (Rex et al., 2007), while treatments known to erase recently induced potentiation produced the same effect on polymerization (Kramár et al., 2006). Finally, and perhaps most critically, the newly formed actin filaments undergo an, as yet, poorly understood transformation such that they become resistant to disruption. This effect occurs over the same post-stimulation period that LTP is stabilized. Combined, these points make a strong case for reorganization of the spine cytoskeleton as being the event that consolidates LTP.
These arguments take on added significance with the recent demonstration that the same spine cytoskeletal changes found after induction of LTP occur during learning (Fedulov et al., 2007). Critically, the same increase in synapse size found in the LTP experiments was also obtained in the learning studies. Experiments using immunogold electron-microscopy have confirmed the intuitive idea that synapse area dictates receptor number (Nusser et al., 1998a; Racca et al., 2000). The expanded synapses sitting on top of potentiated spines, in either LTP or behavioral experiments, are therefore likely to have more receptors and therefore greater potency. Greater synaptic potency, as shown in modeling studies, is sufficient to provide for the encoding of memory (Granger et al., 1994).
Defects
The above points lead inevitably to the question of whether memory disorders arise from defects in the incredibly complicated pathways controlling the spine cytoskeleton and synapse configuration. Results from initial tests of the question have been intriguing. Pre-symptomatic Huntington's disease mutant mice have substantial LTP impairments and are reported to have below normal scores on various learning tasks (Murphy et al., 2000b; Van Raamsdonk et al., 2005). These effects are not unexpected given the memory and cognition problems described for human subjects during the very early stages of Huntington's disease. The work described here indicates that theta-driven polymerization of spine actin is defective in the mouse models (Lynch et al., 2007a); this observation explains the failure of LTP consolidation in the mutants and suggests that memory problems in early Huntington's disease do in fact originate with disturbances to the sub-synaptic cytoskeleton. A likely explanation for the synaptic defect in Huntington's disease arose when it was discovered that BDNF released from axon terminals promotes theta stimulation-induced actin polymerization in mature spines (Rex et al., 2007). The reduced neurotrophin levels could thus make it impossible for conventional theta burst trains to trigger the assembly of spine actin filaments and thus to stabilize recently induced potentiation. In agreement with this, infusion of low concentrations of BDNF rescued both actin polymerization and stable LTP in the Huntington's disease mutant mice.
Other attempts to test if problems in reorganizing the spine cytoskeleton are a common cause of memory disorders have not advanced as far as has the work with Huntington's disease. The age-related loss of LTP consolidation in certain lamina of hippocampus appears to involve aberrant processing of adenosine, a negative modulator of theta-induced actin polymerization (Rex et al., 2005). Moreover, infusions of the positive modulator BDNF restored LTP in middle-aged slices (Rex et al., 2005). However, there appear to have been no direct tests of whether aging affects spine cytoskeletal changes, and whether this can be corrected by appropriate manipulations to positive or negative modulators. Results from studies on mouse models of Fragile-X syndrome are even more ambiguous with regard to the spine cytoskeleton hypothesis of memory disorders. Consolidation of hippocampal LTP was impaired in the mutants but this was not accompanied by evidence for cytoskeletal abnormalities up to the stage of actin polymerization (Lauterborn et al., 2007). Tests are needed of whether the mutation affects processes that transfer the assembled actin filaments from a dynamic to a stable state; unfortunately, very little is known about this event at mature dendritic spines, a point which illustrates how further information on the spine actin networks will gradually fill in the many blanks in the cytoskeletal hypothesis for memory disorders.
Treatment
Whether it will be possible to overcome diverse defects in cytoskeletal reorganization with a single treatment is a key question for future research. That BDNF fully reversed deficits in LTP consolidation arising from three completely unrelated causes (middle-aging, Fragile-X, Huntington's Disease) is highly encouraging in this regard because it suggests that the events controlling filament assembly and stabilization are, to a significant degree, redundant. This would mean that failures or excessive activity in one signaling pathway can be compensated for by manipulating activity in other cascades. Explicit tests of this idea should become possible as more is learned about the actin regulatory processes set in motion by patterned afferent activity.
The broad corrective effects of low BDNF concentrations emphasize the potential therapeutic utility of treatments that up-regulate production of the neurotrophin without producing unacceptable side effects. Efforts in this direction have already been prompted by the possibility of using elevated BDNF levels to treat degenerative conditions (O'Neill et al., 2005) or depression (Duman, 2004; Li et al., 2003). The discovery that brief exposures to ampakines produce significant and long-lasting increases in BDNF levels is encouraging in this regard. Notably, the effects were obtained in animals engaged in exploration and social interactions (Rex et al., 2006), and thus may be applicable to circumstances in which animals, including humans, normally find themselves. It is also the case that ampakines have been extensively tested in clinical trials.
The idea of using up-regulation of BDNF expression to treat impairments in synaptic plasticity has so far only been tested in middle-aged rats. In that case, daily treatments with a short (15 min) half-life ampakine elevated BDNF concentrations and rescued LTP in tests carried out 18 hours after the last drug treatment. These findings indicate that the general strategy of chronically increasing BDNF expression with minimal disruption is feasible and can produce a desired outcome. It remains to be seen if restoring plasticity corrects learning impairments in middle-aged rats and if ampakines are equally effective in counteracting other conditions that disrupt LTP and memory consolidation. Success along these lines would have significant impact on emerging strategies for treating diseases of memory and cognition.
Enhancement
New results showing that learning triggers LTP-related changes in spines and associated synapses have important implications for attempts at accelerating learning or increasing the strength of memory. For one, they provide a biological marker to use in conjunction with behavior in evaluating the effects of potential enhancers. The search for such compounds logically begins with the modulators described in figure 4 because of evidence that these can affect structural and functional plasticity in the normal hippocampus. BDNF, for example, reduces the number of theta bursts needed to trigger actin polymerization in spines and to induce stable LTP in normal rat hippocampal slices (Rex et al., 2007). It also nearly doubles the maximum potentiation produced by full-length trains (Kramár et al., 2004). Based on these results it seems reasonable to ask if chronic increases in BDNF expression reduce the amount of training needed to produce evidence of modified spines and synapses in the cortical telencephalon.
Similarly, the LTP-related spine and synapse changes are obvious markers for investigations into the mode of action of a diverse array of agents reported to acutely enhance memory encoding (Diaz Brinton, 1998; Harvey et al., 2005; Riedel and Jolles, 1996). It will be particularly interesting to determine if various hormones and peptides (e.g., estrogen, insulin, vasopressin) reported to influence learning should be added to the list of positive and negative modulators of spine cytoskeletal plasticity.
The ability to visualize LTP-like changes during learning may also make it possible to ask how potential cognitive enhancers affect the distribution of memory-related changes across the cortical telencephalon. The time needed to map labeled spines and measure synapses on brain sections can be expected to decrease drastically with time; if so, then it may become feasible to describe ‘engrams’ for particular learning problems and to ask whether they are modified or expanded by putative cognitive enhancers. This would allow for distinctions between agents that accelerate learning (reduce the number of training trials) vs. those that affect the strength and/or nature of encoding. Candidates for each hypothetical class have already been identified. Ampakines act acutely to enhance AMPA receptor mediated synaptic currents, an effect that serves to increase the depolarization needed to unblock co-localized NMDA receptors and, as expected, promote the induction of LTP. Some of the drugs simply increase the amplitude of the EPSC while others both increase and prolong post-synaptic responses (Arai and Kessler, 2007; Lynch, 2006; Lynch and Gall, 2006). Members of the first of these categories acutely reduce the number of theta bursts needed to induce LTP but have no effect on the magnitude of potentiation elicited by full-length theta trains; in other words, they lower LTP's threshold but do not affect its ceiling. The other ampakines markedly raise the ceiling as well as lowering the threshold (Arai et al., 2004), an effect that may involve inducing LTP in synapses that normally cannot be potentiated by the stimulated group of afferents.
Increasing the number of potentiated synapses would happen if many dendritic branches targeted by the stimulated afferents do not receive a sufficient number of synapses to produce the depth of depolarization required to engage NMDA receptors at individual synapses. By prolonging the duration of AMPA receptor mediated depolarization, the second group of ampakines would generate greater temporal summation in the target dendrites and hence compensate for the insufficient number of activated synapses. This analysis predicts that amapkines which prolong excitatory currents will a) substantially increase the number of spines in which actin polymerization occurs after a conventional theta burst train and b) cause modified spines and synapses to appear in regions where they are not normally found after a given type of learning. A result of the latter type would indicate that some drugs will not only affect the ease of storage but also the quality and relationships of the resultant memory. In some instances this would likely be a negative effect, but in others it would allow for cognitive architectures that are normally precluded by the design of cortical networks and the strictures imposed by LTP-based synaptic learning rules. Expanded encoding could, in other words, bring us closer to true cognitive enhancement.
Acknowledgments
This work was supported in part by NINDS grants NS045260, NS051823 and NS37799. C.S. Rex was supported by National Institutes of Aging grant AG00258.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Williams JM. Properties and mechanisms of LTP maintenance. The Neuroscientist. 2003;9:463–474. doi: 10.1177/1073858403259119. [DOI] [PubMed] [Google Scholar]
- Arai A, Larson J, Lynch G. Anoxia reveals a vulnerable period in the development of long-term potentiation. Brain Research. 1990;511:353–357. doi: 10.1016/0006-8993(90)90184-d. [DOI] [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Suzuki E. Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus. Neurosci. 2004;123:1011–1024. doi: 10.1016/j.neuroscience.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999;9:364–370. doi: 10.1016/s0962-8924(99)01619-0. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Schottler S, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:334–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda B, Nikonenko I, Alberi S, Muller D. Central Nervous System Functions of PAK Protein Family: From Spine Morphogenesis to Mental Retardation. Mol Neurobiol. 2006;34:67–80. doi: 10.1385/mn:34:1:67. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Chang FLF, Greenough WT. Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Res. 1984;309:35–46. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery T, Harris J, Willems P, Oostra B, Irwin S, Weiler I, Greenough W. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Small SA, Stern Y, Mayeux R, Feldstein SN, Keller FR. Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003;39:1063–1091. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Changes in the postsynaptic density with long-term potentiation in the dentate gyrus. J Comp Neurol. 1986;253:476–482. doi: 10.1002/cne.902530405. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R. Vasopressin in the mammalian brain: the neurobiology of a mnemonic peptide. Prog Brain Res. 1998;119:177–199. doi: 10.1016/s0079-6123(08)61570-8. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sc USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J Neurosci. 2007;27:8031–8039. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Furber SB, Bainbridge WJ, Cumpstey JM, Temple S. Sparse distributed memory using N-of-M codes. Neural Netw. 2004;17:1437–1451. doi: 10.1016/j.neunet.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Gall C. Regulation of brain neurotrophin expression by physiological activity. Trends Pharmacol Sci. 1992;13:401–403. doi: 10.1016/0165-6147(92)90123-n. [DOI] [PubMed] [Google Scholar]
- Gall CM, Lauterborn JC. Regulation of BDNF Expression: Multifaceted, region-specific control of a neuronal survival factor in the adult CNS. In: Mocchetti I, editor. Neurobiology of the Neurotrophins. FP Graham Publishing Co.; Johnson City, TN: 2000. pp. 541–579. [Google Scholar]
- Gall CM, Lynch G. Integrins, synaptic plasticity, and epileptogenesis. In: Scharfman H, Binder D, editors. Recent Advances in Epilepsy Research: Molecular Mechanisms of Epileptogenesis. Vol. 548. Kluver Academic; 2004. pp. 12–33. [DOI] [PubMed] [Google Scholar]
- Gall CM, Lynch G. Consolidation: a view from the synapse. In: Stanton PK, Bramham C, Scharfman HE, editors. Synaptic Plasticity and Transsynaptic Signaling. Springer Science + Business Media; New York: 2005. pp. 469–494. [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 2004;125:615–623. doi: 10.1016/j.neuroscience.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Heller RE, Rossi M, Parshall RF. Structural synaptic correlate of long-term potentiation: formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus. 1993;3:435–445. doi: 10.1002/hipo.450030405. [DOI] [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice. Am J Med Genet. 1996;64:246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Granger R, Whitson J, Larson J, Lynch G. Non-Hebbian properties of long-term potentiation enable high-capacity encoding of temporal sequences. Proc Natl Acad Sci U S A. 1994;91:10104–10108. doi: 10.1073/pnas.91.21.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Wigstrom H. Long-term potentiation in the hippocampal CA1 region: its induction and early temporal development. Prog Brain Res. 1990;83:223–232. doi: 10.1016/s0079-6123(08)61252-2. [DOI] [PubMed] [Google Scholar]
- Harris KM, Fiala JC, Ostroff L. Structural changes at dendritic spine synapses during long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:745–748. doi: 10.1098/rstb.2002.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Shanley LJ, O'Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33:1029–1032. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- Ho AK, Sahakian BJ, Brown RG, Barker RA, Hodges JR, Ane MN, Snowden J, Thompson J, Esmonde T, Gentry R, Moore JW, Bodner T. Profile of cognitive progression in early Huntington's disease. Neurology. 2003;61:1702–1706. doi: 10.1212/01.wnl.0000098878.47789.bd. [DOI] [PubMed] [Google Scholar]
- Hunt CA, Schenker LJ, Kennedy MB. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci. 1996;16:1380–1388. doi: 10.1523/JNEUROSCI.16-04-01380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci USA. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Lynch G. Developmental and regional differences in the consolidation of long-term potentiation. Neuroscience. 2003;118:387–398. doi: 10.1016/s0306-4522(02)00916-8. [DOI] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 2005;25:9460–9469. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar EA, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain derived neurotrophic factor rescues synaptic plasticity in a mouse model of Fragile X syndrome. J Neurosci. 2007 doi: 10.1523/JNEUROSCI.2624-07.2007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lee KS, Schottler F, Oliver M, Lynch G. Brief bursts of high-frequency stimulation produce two types of structural changes in rat hippocampus. J Neurophysiol. 1980;44:247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- Leung LS, Shen B. N-methyl-D-aspartate receptor antagonists are less effective in blocking long-term potentiation at apical than basal dendrites in hippocampal CA1 of awake rats. Hippocampus. 1999;9:617–630. doi: 10.1002/(SICI)1098-1063(1999)9:6<617::AID-HIPO2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Li X, Witkin JM, Need AB, Skolnick P. Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell Mol Neurobiol. 2003;23:419–430. doi: 10.1023/A:1023648923447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Actin's actions in LTP-induced synapse growth. Neuron. 2003;38:361–362. doi: 10.1016/s0896-6273(03)00257-5. [DOI] [PubMed] [Google Scholar]
- Lynch G. Memory enhancement: the search for mechanism-based drugs. Nature Neurosci. 2002;5:1035–1038. doi: 10.1038/nn935. [DOI] [PubMed] [Google Scholar]
- Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol. 2006;6:82–88. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lynch G, Baudry M. The biochemistry of memory: A new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Lynch G, Gall CM. Ampakines and the three-fold path to cognitive enhancement. Trends Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease. J Neurosci. 2007a;27:4424–4434. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Gall CM. LTP consolidation: substrates, explanatory power, and functional significance. Neuropharm. 2007b;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–758. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev Neurosci. 2003;14:233–240. doi: 10.1515/revneuro.2003.14.3.233. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–370. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Murphy KP, Carter RJ, Lione LA, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation. J Neurosci. 2000a;20:5115–5123. doi: 10.1523/JNEUROSCI.20-13-05115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KP, Carter RJ, Lione LA, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation. J Neurosci. 2000b;20:5115–5123. doi: 10.1523/JNEUROSCI.20-13-05115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ, Murray TK, Clay MP, Lindstrom T, Yang CR, Nissenbaum ES. LY503430: pharmacology, pharmacokinetics, and effects in rodent models of Parkinson's disease. CNS Drug Review. 2005;11:77–96. doi: 10.1111/j.1527-3458.2005.tb00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto KI, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H, Wiener SI, Wible CG. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Purpura DP. Dendritic spine “dysgenesis” and mental retardation. Science. 1974;186:1126–1128. doi: 10.1126/science.186.4169.1126. [DOI] [PubMed] [Google Scholar]
- Purpura DP. Normal and aberrant neuronal development in the cerebral cortex of human fetus and young infant. UCLA Forum Med Sci. 1975:141–169. doi: 10.1016/b978-0-12-139050-1.50014-8. [DOI] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Spatial organization of cofilin in dendritic spines. Neuroscience. 2006;138:447–456. doi: 10.1016/j.neuroscience.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Ramakers GJA. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Rex CS, Kramar EA, Colgin LL, Lin B, Gall CM, Lynch G. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci. 2005;25:5956–5966. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes LTP-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot T. Diseases of Memory. Apppleton-Century-Crofts; New York: 1882. [Google Scholar]
- Riedel WJ, Jolles J. Cognition enhancers in age-related cognitive decline. Drugs Aging. 1996;8:245–274. doi: 10.2165/00002512-199608040-00003. [DOI] [PubMed] [Google Scholar]
- Roman F, Staubli U, Lynch G. Evidence for synaptic potentiation in a cortical network during learning. Brain Res. 1987;418:221–226. doi: 10.1016/0006-8993(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Roth LR, Leung LS. Difference in LTP at basal and apical dendrites of CA1 pyramidal neurons in urethane-anesthetized rats. Brain Res. 1995;694:40–48. doi: 10.1016/0006-8993(95)00767-k. [DOI] [PubMed] [Google Scholar]
- Segal M, Kreher U, Greenberger V, Braun K. Is fragile X mental retardation protein involved in activity-induced plasticity of dendritic spines? Brain Res. 2003;972:9–15. doi: 10.1016/s0006-8993(03)02410-7. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Zsilla G, Baranyi M, Kekes-Szabo A, Vizi ES. Age-dependent changes of presynaptic neuromodulation via A1-adenosine receptors in rat hippocampal slices. Int J Dev Neurosci. 1997;15:739–747. doi: 10.1016/s0736-5748(97)00028-2. [DOI] [PubMed] [Google Scholar]
- Staubli U, Perez Y, Xu F, Rogers G, Ingvar M, Stone-Elander S, Lynch G. Centrally active modulators of glutamate (AMPA) receptors facilitate the induction of LTP in vivo. Proc Natl Acad Sci USA. 1994b;91:11158–11162. doi: 10.1073/pnas.91.23.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA. 1994a;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nature Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Usdin MT, Shelbourne PF, Myers RM, Madison DV. Impaired synaptic plasticity in mice carrying the Huntington's disease mutation. Hum Mol Genet. 1999;8:839–846. doi: 10.1093/hmg/8.5.839. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J Neurosci. 2005;25:4169–4180. doi: 10.1523/JNEUROSCI.0590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Ann Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]