Abstract
Rationale: Hyponatremia is associated with decompensated heart failure and poor prognosis in patients with left ventricular systolic dysfunction.
Objectives: We sought to determine if hyponatremia is associated with right heart failure and worse prognosis in patients with pulmonary arterial hypertension (PAH).
Methods: We prospectively followed 40 patients with PAH and examined the relationship between serum sodium and right heart function as well as survival.
Measurements and Main Results: Subjects with hyponatremia (Na ⩽ 136 mEq/L) were more symptomatic (11/13 World Health Organization [WHO] class III/IV vs. 12/27 WHO class III/IV; P = 0.02), had more peripheral edema (69 vs. 26%; P = 0.009), and had higher hospitalization rates (85 vs. 41%; P = 0.009) than normonatremic subjects. Hyponatremic subjects had higher right atrial pressure (14 ± 6 vs. 9 ± 3 mm Hg; P < 0.001), lower stroke volume index (21 ± 7 vs. 32 ± 10 ml/m2; P < 0.01), larger right ventricular:left ventricular area ratio (1.8 ± 0.4 vs. 1.3 ± 0.4; P < 0.001), and lower tricuspid annular plane systolic excursion (1.4 ± 0.3 vs. 2.0 ± 0.6 cm; P = 0.001), despite similar mean pulmonary artery pressure (49 ± 10 vs. 47 ± 12 mm Hg; P = 0.60). The 1- and 2-year survival estimates were 93% (95% confidence interval [CI], 73–98%) and 85% (95% CI, 65–94%), and 38% (95% CI, 14–63%) and 15% (95% CI, 2–39%) for normonatremic and hyponatremic subjects, respectively (log-rank χ2 = 25.19, P < 0.001). The unadjusted risk of death (hazard ratio) in hyponatremic compared with normonatremic subjects was 10.16 (95% CI, 3.42–30.10, P < 0.001). Hyponatremia predicted outcome after adjusting for WHO class, diuretic use, as well as right atrial pressure and cardiac index.
Conclusions: Hyponatremia is strongly associated with right heart failure and poor survival in PAH.
Keywords: hyponatremia, pulmonary heart disease, pulmonary hypertension, heart failure
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Hyponatremia is a powerful predictor of advanced heart failure and poor prognosis in patients with left ventricular systolic dysfunction. The relationship between hyponatremia, right heart failure, and prognosis in pulmonary arterial hypertension is not known.
What This Study Adds to the Field
This study shows that hyponatremia, found on a routine chemistry panel, is strongly associated with more advanced right heart failure, right ventricular dysfunction, and very poor prognosis in pulmonary arterial hypertension.
Hyponatremia (HN) is a well-established marker of advanced left heart failure (LHF), and has been shown to predict poor outcome independent of left ventricular (LV) ejection fraction, pulmonary capillary wedge pressure (PCWP), cardiac index, and other hemodynamic parameters (1–3). The prognostic significance of HN in chronic LHF reflects the strong correlation between serum sodium and plasma neurohormone concentrations, such as norepinephrine, renin, and angiotensin II, all of which are linked to poor outcome in advanced LHF (1, 4–6). Neurohormone-mediated, nonosmotic release of vasopressin accounts for the fall in sodium concentration in these patients (7–9).
Pulmonary arterial hypertension (PAH) is a clinical syndrome through which vasoconstriction and remodeling of the pulmonary vascular bed lead to marked increases in pulmonary vascular resistance, pulmonary arterial pressure, exercise intolerance, and in many cases, premature death (10). Prognosis in PAH is strongly linked to the ability of the right heart to adapt to the increased vascular load. Clinical right heart failure (RHF) as well as invasive and noninvasive indices of right heart dysfunction indicate right heart maladaptation, and thus have been shown to predict poor outcome in PAH (11). Given the prevalence of RHF in this population and its prognostic significance, we sought to determine whether HN is associated with more advanced RHF as well as worse prognosis in patients with PAH. Such an association may be of particular value, given that serum sodium is routinely available on a standard chemistry panel. Some of the results in the current study have been reported in abstract form (12).
METHODS
This study was approved by the Johns Hopkins Institutional Review Board, and informed consent was obtained from all subjects before enrollment.
Patients
From March to September of 2004, 72 patients with known or suspected pulmonary hypertension underwent a clinically indicated right heart catheterization (RHC) and within 1 hour of RHC, had a comprehensive two-dimensional echocardiographic/Doppler protocol as described previously (13). Forty-seven patients had PAH (World Health Organization [WHO] group I pulmonary hypertension); 40 patients with PAH also had a serum sodium measured before their catheterization and comprised the study population. These patients were followed in a prospective observational study (median, 28.6 mo); this cohort represents a subset of a cohort we reported on previously (13).
Methods for hemodynamic and echocardiographic measures of right heart function are further detailed in the online supplement.
Serum Measurements
Serum sodium and other chemistries were obtained before RHC using standard clinical laboratory assays. The coefficient of variation for the sodium assay ranges from 0.8 to 1.0%. Glomerular filtration rate was estimated (eGFR) using the Modification of Diet in Renal Disease (MDRD) study equation (14). HN was defined as a serum sodium of 136 mEq/L or less and normonatremia (NN) as a sodium concentration of greater than 136 mEq/L, based on prior published reports demonstrating that HN at this level predicts poor outcome in patients with LHF (1, 18, 24). A follow-up serum sodium was obtained at the time nearest to death in nonsurvivors (median, 0 d [0 d indicating sodium measured on day of death]; interquartile range, 0–70 d; range, 0–176 d) to determine if their HN had persisted or worsened over time.
Clinical Follow-up
At study completion, the vital status was confirmed by review of medical records, phone contact, and Social Security Death Index. The primary endpoint was all-cause mortality.
Data Analysis
Continuous variables were summarized by mean ± SD or median (interquartile range). Differences between and within groups were detected using unpaired and paired Student t tests, respectively. A P value of less than 0.05 was considered significant.
Sodium levels were dichotomized (⩽136 mEq/L or >136 mEq/L), and their prognostic significance tested using the Kaplan-Meier method. Survival differences were tested using the log-rank statistic to compare the time to event (death) between patients with HN (⩽136 mEq/L) and NN (sodium > 136 mEq/L).
Univariable and bivariable survival analyses were performed using Cox proportional hazards methods (15). Models used sodium as a continuous or dichotomous variable (⩽136 mEq/L or >136 mEq/L). Variables found to be significant in univariable analyses (P value < 0.15) and variables previously shown to have prognostic significance were included in bivariable analyses (11). Potential effect modification was examined in each bivariable model by using an interaction term. The proportional hazards assumption was examined for all covariates using a continuous time-varying predictor and Schoenfeld residuals.
RESULTS
Table 1 summarizes the demographics and clinical characteristics of the overall study cohort, and for NN patients (Na+ > 136 mEq/L) and HN subjects (Na+ ⩽ 136 mEq/L). Overall, the majority of patients were white women. Most of the patients had PAH related to connective tissue disease (PAH-CTD; 26/40, 65%). The majority of the patients were New York Heart Association functional class II or III, with a mean six-minute-walk distance (6MWD) of 345 ± 120 m, suggesting moderate functional impairment. The mean eGFR indicated stage II kidney disease by MDRD classification (mean eGFR, 60–89 ml/min/1.73 m2). Spirometry and lung volumes were near normal; however, single-breath diffusion capacity of carbon monoxide was moderately reduced. Most patients were receiving diuretic therapy at the time of enrollment (37/40, 93%), most commonly a loop diuretic (33/40, 83%). More than 40% of patients were taking spironolactone (17/40, 43%); fewer were taking hydrochlorothiazide (4/40, 10%). Twenty of the subjects were receiving specific PAH therapy at enrollment: intravenous or subcutaneous prostanoid (n = 9), endothelin receptor antagonist (n = 9), combination of prostanoid and endothelin receptor antagonist (n = 1), or combination of prostanoid and phosphodiesterase inhibitor (n = 1). During the follow-up period, 35 patients were receiving specific PAH therapy: intravenous or subcutaneous prostanoid (7), endothelin receptor antagonist or phosphodiesterase inhibitor alone or in combination with each other (18), or a combination of a prostanoid and endothelin receptor antagonist or phosphodiesterase inhibitor (10).
TABLE 1.
PATIENT DEMOGRAPHICS AND CLINICAL CHARACTERISTICS
| Overall (n = 40) | Sodium > 136 mEq/L (n = 27) | Sodium ⩽ 136 mEq/L (n = 13) | P Value | |
|---|---|---|---|---|
| Age, yr | 56 ± 15 | 55 ± 16 | 56 ± 13 | 0.83 |
| Female sex, n (%) | 33 (83) | 22 (81) | 11 (85) | 0.81 |
| White, n (%) | 33 (83) | 23 (85) | 10 (77) | 0.52 |
| African American, n (%) | 6 (15) | 4 (15) | 2 (15) | 0.78 |
| Other, n (%) | 1 (2) | 0 (0) | 1 (7) | 0.84 |
| Diagnosis, n (%) | ||||
| IPAH | 14 (35) | 13 (48) | 1 (8) | |
| PAH-CTD | 26 (65) | 14 (52) | 12 (92) | |
| Body mass index, kg/m2 | 27 ± 6 | 28 ± 6 | 26 ± 5 | 0.32 |
| WHO class, n (%) | ||||
| I/II | 17 (43) | 15 (56) | 2 (15) | |
| III/IV | 23 (57) | 12 (44) | 11 (85) | |
| 6MWD, m | 345 ± 120 | 361 ± 114 | 267 ± 134 | 0.11 |
| Estimated GFR, ml/min/1.73 m2 | 65 ± 26 | 74 ± 23 | 45 ± 21 | 0.001 |
| Serum creatinine, mg/dl | 1.2 ± 0.5 | 1.0 ± 0.3 | 1.5 ± 0.7 | 0.003 |
| Serum BUN, mg/dl | 27 ± 17 | 22 ± 14 | 38 ± 18 | 0.003 |
| JVP, cm H2O | 9 ± 5 | 7 ± 3 | 14 ± 6 | 0.0001 |
| Lower extremity edema, n (%) | 16 (40) | 7 (26) | 9 (69) | 0.009 |
| Furosemide, n (%) | ||||
| Total daily dose ⩽ 80 mg | 21 (53) | 14 (52) | 7 (54) | 0.91 |
| Total daily dose > 80 mg | 11 (27) | 5 (18) | 6 (46) | 0.07 |
| Patients hospitalized 1 yr before enrollment, n (%) | 18 (45) | 10 (37) | 8 (62) | 0.15 |
| Patients hospitalized during follow-up, n (%) | 22 (55) | 11 (41) | 11 (85) | 0.009 |
| Treatment status at enrollment, n (%) | 20 (50) | 16 (59) | 4 (31) | 0.09 |
| Treatment status during 2-year follow-up, n (%) | 35 (88) | 26 (96) | 9 (69) | 0.81 |
Definition of abbreviations: BUN = blood urea nitrogen; GFR = glomerular filtration rate; IPAH = idiopathic pulmonary arterial hypertension; JVP = jugular venous pressure; PAH-CTD = pulmonary arterial hypertension related to connective tissue disease; 6MWD = six-minute-walk distance; WHO = World Health Organization.
Patient demographics and clinical characteristics for the overall cohort, and subgroups with a serum sodium >136 mEq/L and ⩽136 mEq/L. Values are expressed as n (%) or mean ± SD.
Thirteen of the 40 subjects had HN (mean, 132.4 ± 4.4 mEq/L; range, 119–136 mEq/L), whereas the remainder had a sodium concentration of more than 136 mEq/L (mean, 140.3 ± 1.6 mEq/L; range, 138–145 mEq/L). Comparing the HN and NN subjects, there were no significant differences in age, sex, ethnicity, or body mass index. More patients in the HN group had PAH-CTD (12/13 vs. 14/27, P = 0.01). Subjects with HN also had worse WHO functional class (P = 0.02), and tended to have shorter baseline 6MWD. There were no significant differences in pulmonary function test parameters between the two groups. Renal function was more impaired in the HN group (mean eGFR, 45 ± 21 vs. 74 ± 23 ml/min/1.73 m2; P = 0.001) with higher mean blood urea nitrogen (BUN) and serum creatinine concentrations compared with the NN group. Subjects in the HN group were more likely to receive loop diuretics, whereas the NN group was more likely to receive thiazide diuretics. A small proportion of patients in the NN (4/27) and HN (3/13) groups were receiving either an angiotensin converting enzyme inhibitor or angiotensin receptor antagonist during the study.
Despite the higher use of loop diuretics in the HN group, these patients had a higher estimated jugular venous pressure, and were nearly three times as likely to have lower extremity edema. The NN group tended to be receiving PAH-specific therapy (prostanoids, endothelin receptor antagonists, and/or phosphodiesterase inhibitors) at enrollment compared with the low sodium group, but this difference was not statistically significant (16/27 [59%] vs. 4/13 [31%], P = 0.09). During the follow-up period, 26 of 27 patients in the NN group and 9 of 13 patients in the HN group received specific PAH therapy (P = 0.81). Importantly, two of the four patients with HN who were not on specific therapy died within 30 days of enrollment and thus before initiation of specific therapy. One patient was maintained on diuretic therapy and another was lost to follow-up and died 18 months after enrollment, according to the Social Security Death Index.
Table 2 summarizes the baseline hemodynamics and echocardiographic findings for the overall cohort, and for patients with NN and HN. In the overall cohort, the hemodynamics revealed elevated right atrial pressure (RAP), mean pulmonary arterial pressure (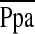 ), and pulmonary vascular resistance index (PVRI) with depressed cardiac and stroke volume indices compared with normal reference values (16). Although there were no differences in the
), and pulmonary vascular resistance index (PVRI) with depressed cardiac and stroke volume indices compared with normal reference values (16). Although there were no differences in the 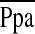 or PCWP between groups, the cardiac index was lower in the HN group despite significantly higher heart rates, reflecting marked differences in the stroke volume index between the two groups. It was noteworthy that the HN patients had a depressed stroke volume index in the context of an average RAP that was nearly double that of the NN group, reflecting a state of right ventricular (RV) decompensation in these subjects (Table 2). The PVRI tended to be higher in the HN group, whereas the ratio of stroke volume to pulmonary artery pulse pressure, a measure of large pulmonary artery compliance, was significantly lower in the HN group.
or PCWP between groups, the cardiac index was lower in the HN group despite significantly higher heart rates, reflecting marked differences in the stroke volume index between the two groups. It was noteworthy that the HN patients had a depressed stroke volume index in the context of an average RAP that was nearly double that of the NN group, reflecting a state of right ventricular (RV) decompensation in these subjects (Table 2). The PVRI tended to be higher in the HN group, whereas the ratio of stroke volume to pulmonary artery pulse pressure, a measure of large pulmonary artery compliance, was significantly lower in the HN group.
TABLE 2.
HEMODYNAMICS AND ECHOCARDIOGRAPHIC MEASUREMENTS
| Parameter | Overall (n = 40) | Sodium >136 mEq/L (n = 27) | Sodium ⩽ 136 mEq/L (n = 13) | P Value |
|---|---|---|---|---|
| Hemodynamics | ||||
| Heart rate, beats/min | 83 (15) | 77 (11) | 94 (14) | 0.0002 |
| SBP, mm Hg | 121 (18) | 124 (20) | 113 (15) | 0.07 |
| DBP, mm Hg | 70 (10) | 70 (10) | 71 (11) | 0.82 |
| RAP, mm Hg | 11 (5) | 9 (3) | 14 (6) | 0.0004 |
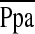 , mm Hg , mm Hg |
48 (11) | 47 (12) | 49 (10) | 0.60 |
| Cardiac index, L/min/m2 | 2.2 (0.6) | 2.4 (0.6) | 2.0 (0.6) | 0.05 |
| SVI, ml/m2 | 28 (10) | 32 (10) | 21 (7) | 0.002 |
| RVSWI, g-m/m2 | 14.3 (6.5) | 16.4 (6.4) | 10.0 (4.2) | 0.002 |
| PVRI | 1,463 (700) | 1,327 (597) | 1,744 (832) | 0.08 |
| SV/PP, ml/mm Hg | 1.10 (0.49) | 1.21 (0.56) | 0.85 (0.29) | 0.02 |
| PCWP, mm Hg | 11 (3) | 11 (3) | 11 (5) | 0.74 |
| SvO2, % | 63 (9) | 67 (7) | 54 (9) | 0.000 |
| Echocardiography | ||||
| TAPSE, cm | 1.8 (0.3) | 2.0 (0.6) | 1.4 (0.3) | 0.001 |
| RVFAC, % | 27.8 (9.8) | 30.1 (10.2) | 22.8 (6.4) | 0.03 |
| RAAI, cm2/m | 14.8 (5.1) | 13.3 (4.6) | 18.3 (4.8) | 0.003 |
| RVAI, cm2/m | 15.5 (3.6) | 14.4 (3.0) | 18.1 (3.7) | 0.003 |
| TR, grade 0–4 | 1.97 (0.93) | 1.67 (0.16) | 2.67 (0.22) | 0.001 |
| RA:LA dimension | 1.47 (0.41) | 1.37 (0.35) | 1.68 (0.47) | 0.03 |
| RV:LV area | 1.45 (0.48) | 1.29 (0.42) | 1.81 (0.42) | 0.0008 |
| LV dimension, cm | 3.9 (0.8) | 4.2 (0.6) | 3.3 (0.8) | 0.0002 |
| RVSP, mm Hg | 78 (18) | 79 (20) | 75 (14) | 0.60 |
| Pericardial effusion, n (%) | 12 (41) | 6 (22) | 6 (50) | 0.03 |
Definition of abbreviations: DBP = diastolic blood pressure; PCWP = pulmonary capillary wedge pressure; 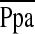 = mean pulmonary artery pressure; PVRI = pulmonary vascular resistance index; RAAI = right atrial area index; RA:LA dimension = ratio of right atrial to left atrial dimensions; RAP = right atrial pressure; RVAI = right ventricular area index; RVFAC = right ventricular fractional area change; RV:LV area = ratio of right ventricular to left ventricular area; ; RVSP = right ventricular systolic pressure; RVSWI = right ventricular stroke work index; SBP = systolic blood pressure; SVI = stroke volume index; SvO2 = mixed venous oxygen saturation; SV/PP = ratio of stroke volume to pulmonary artery pulse pressure; TAPSE = triscuspid annular plane systolic excursion; TR = tricuspid regurgitation.
= mean pulmonary artery pressure; PVRI = pulmonary vascular resistance index; RAAI = right atrial area index; RA:LA dimension = ratio of right atrial to left atrial dimensions; RAP = right atrial pressure; RVAI = right ventricular area index; RVFAC = right ventricular fractional area change; RV:LV area = ratio of right ventricular to left ventricular area; ; RVSP = right ventricular systolic pressure; RVSWI = right ventricular stroke work index; SBP = systolic blood pressure; SVI = stroke volume index; SvO2 = mixed venous oxygen saturation; SV/PP = ratio of stroke volume to pulmonary artery pulse pressure; TAPSE = triscuspid annular plane systolic excursion; TR = tricuspid regurgitation.
Hemodynamic and echocardiographic data for patients in the overall cohort, and subgroups with a serum sodium >136 mEq/L or ⩽136 mEq/L. Values are expressed as mean (SD).
Patients with HN also had significantly more advanced RV systolic dysfunction by echocardiography, including marked differences in triscuspid annular plane systolic excursion between the two groups. Patients with HN also had significantly larger right heart dimensions, greater right to left heart disproportion, and more severe tricuspid regurgitation, with 62% of these patients having grade 3 or greater tricuspid regurgitation, versus only 15% of the NN patients. Moreover, the HN patients had smaller left heart dimensions, suggesting that the larger right heart size in the low sodium group led to greater encroachment on LV size. In keeping with these findings, the HN group had lower systolic blood pressure and higher heart rates, suggesting greater systemic hemodynamic compromise in these patients.
In the year before enrollment in the study, there was no difference in hospitalization rates between the HN and NN groups. In the 2 years after enrollment, the HN patients had more than double the rate of hospitalization compared with the NN subjects (11/13 [85%] vs. 11/27 [41%], P = 0.009; Table 1).
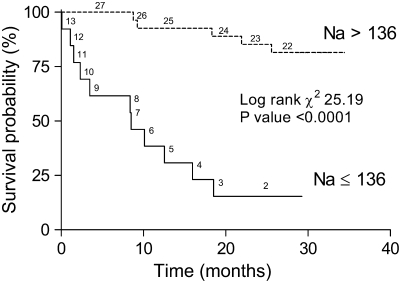
Overall, 16 of the subjects died during follow-up. Nine patients had progressive RHF, three had sudden cardiac death, one had pneumonia and adult respiratory distress syndrome, and three had unknown causes of death. Thus, at least 12 of the 16 subjects died of a cardiovascular cause. Eleven of the 13 patients (85%) in the HN group died compared with 5 of the 27 patients (19%) in the NN group (P < 0.001). Time-to-event analysis revealed markedly worse survival in HN patients versus NN subjects (Figure 1). The 1- and 2-year survival estimates were 93% (95% confidence interval [CI], 73–98%) and 85% (95% CI, 65–94%), respectively, for patients with NN, and 38% (95% CI, 14–63%) and 15% (95% CI, 2–39%), respectively, for the HN group (log-rank χ2 = 25.19, P < 0.001). The time to death was significantly shorter (225 ± 57 d) in the HN patients as compared with NN subjects (559 ± 108 d, P = 0.01). The median survival in the HN patients was only 8.5 months. All 10 patients with a serum Na less than 135 mEq/L died, whereas all 16 subjects with an Na concentration of 140 mEq/L or greater survived.
Figure 1.
Kaplan and Meier estimates of survival (all-cause mortality) in patients stratified by serum sodium.
Fifteen patients who ultimately died had a serum sodium measured at enrollment and on follow-up; 11 of the 15 patients had a low serum sodium at enrollment that persisted over time, and in fact showed a small but significant decline at or near the time of death (baseline Na, 134 ± 5 vs. 132 ± 6 mEq/L; P = 0.04).
The unadjusted risk of death during the study period for patients in the HN group compared with those in the NN group was 10.16 (95% CI, 3.42–30.10; P < 0.001). Sodium also predicted death when analyzed as a continuous variable (hazard ratio [HR], 1.33; 95% CI, 1.19–1.49; P < 0.001; Table 3). Bivariable models using Cox proportional hazards were constructed that consisted of sodium as a dichotomous variable (⩽136 mEq/L or >136 mEq/L) and variables found to be significant in univariable analyses or variables previously shown to have prognostic significance in PAH (Table 4). Sodium, both as a continuous and dichotomous variable, remained a strong predictor of mortality in this cohort. Furthermore, when stratified by treatment status at baseline, serum sodium remained highly predictive of death, suggesting that sodium levels predicted outcome regardless of whether patients were being treated for pulmonary hypertension at the time of enrollment. Evaluation for interaction between variables included in the bivariable analyses (e.g., diuretic use and RAP, eGFR and RAP, diuretic use and cardiac index, among others) did not reveal significant effect modification.
TABLE 3.
UNIVARIABLE COX PROPORTIONAL HAZARD MODEL
| Variable | Unadjusted HR (95% CI) | P Value |
|---|---|---|
| Diagnosis | 5.29 (1.20–23.37) | 0.03 |
| WHO class at baseline | 5.39 (1.99–14.59) | 0.001 |
| Sodium (continuous) | 0.75 (0.67–0.84) | <0.001 |
| Sodium (dichotomous) | 10.16 (3.42–30.10) | <0.001 |
| eGFR (categorical) | 2.36 (1.15–4.79) | 0.02 |
| RAP | 1.22 (1.11–1.34) | 0.001 |
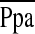 |
1.02 (0.98–1.07) | 0.27 |
| Cardiac index | 0.54 (0.24–1.22) | 0.14 |
| PVRI | 1.00 (0.99–1.00) | 0.10 |
Definition of abbreviations: CI = confidence interval; eGFR = estimated glomerular filtration rate; HR = hazard ratio; 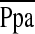 = mean pulmonary artery pressure; PVRI = pulmonary vascular resistance index; RAP = right atrial pressure; WHO = World Health Organization.
= mean pulmonary artery pressure; PVRI = pulmonary vascular resistance index; RAP = right atrial pressure; WHO = World Health Organization.
Values represent unadjusted HRs for all-cause mortality for serum sodium (continuous and dichotomized), and other clinical, hemodynamic, and echocardiographic variables.
TABLE 4.
BIVARIABLE COX PROPORTIONAL HAZARDS MODEL
| Variables | HR for Sodium ⩽ 136 mEq/L vs. Sodium > 136 mEq/L | 95% CI | P Value |
|---|---|---|---|
| Unadjusted | 10.2 | 3.4-30.2 | <0.001 |
| Adjusted for | |||
| Diagnosis | 8.0 | 2.6-24.8 | <0.001 |
| WHO class at baseline | 6.2 | 2.1-18.6 | 0.001 |
| Diuretic Use | 15.0 | 4.1-53.3 | <0.001 |
| RAP | 6.2 | 1.7-22.3 | 0.006 |
| Cardiac index | 10.1 | 3.2-32.3 | <0.001 |
Definition of abbreviations: CI = confidence interval; RAP = right atrial pressure; WHO = World Health Organization.
Values represent HRs for all-cause mortality, stratified by serum sodium, and adjusted for various clinical, echocardiographic, and hemodynamic parameters.
DISCUSSION
The current study demonstrates that HN is associated with advanced RHF and dramatically reduced survival in patients with PAH as compared with subjects with a normal serum sodium concentration. HN predicted death even after adjusting for hemodynamic, echocardiographic, and clinical variables of known prognostic importance in PAH. Thus, our results suggest that HN is a powerful, yet simple and readily available marker of advanced RHF and poor prognosis in patients with PAH.
HN and Heart Failure
HN is an established marker of more clinically advanced LHF. Dzau and colleagues demonstrated that patients hospitalized for heart failure had lower serum sodium concentrations, and were more often edematous than patients with chronic stable LHF (2). Similarly, others have found that HN is associated with higher hospitalization, rehospitalization, and increased inotrope use as compared with patients with a normal serum sodium concentration (3, 17, 18). Taken together, these results suggest that LHF patients with HN are more often in a state of circulatory decompensation than LHF patients with normal serum sodium concentrations.
In our study of patients with PAH, 85% of the subjects with HN were WHO functional class III or IV at enrollment, whereas the NN group had similar proportions of class I–II and III–IV patients. In keeping with these findings, the HN patients had more prominent physical exam findings for RHF and were twice as likely to be hospitalized as NN subjects. The average 6MWD in the HN group was quite low versus the NN subjects (267 vs. 361 m) but fell short of significance, likely in part due to the far lower proportion of HN subjects walked within 6 months of RHC. The HN subjects were also more likely to be azotemic despite an average right atrial pressure of 14 mm Hg, making volume depletion an unlikely unifying cause. The constellation of hypervolemia, azotemia, and clinical decompensation observed in our HN patients with PAH has been previously reported in HN patients with LHF (2), suggesting that low serum sodium is a global marker of circulatory maladaptation in heart failure, regardless of whether left or right ventricular function is compromised. The higher proportion of patients with PAH-CTD in the HN group also raises the possibility that intrinsic renal disease may have contributed to the differences in renal function reported between the two groups.
The mechanism of HN was not established in the current study. Clinically, the HN patients in our cohort had more edema and nearly twofold higher RAP versus the NN subjects, which is consistent with the hypervolemic phenotype of HN, as seen in LHF (7, 19). Although diuretics could have contributed to the HN in our cohort, diuretic-induced HN is invoked more commonly in the hypovolemic patient, most commonly with thiazide but not loop diuretics (20). HN in LHF reflects nonosmotic release of vasopressin consequent to activation of the sympathetic and renin–angiotensin–aldosterone system axes (7, 8), and thus a low serum sodium reflects neurohormonal axis activation in these patients, as well as in other hypervolemic hyponatremic states such as cirrhosis and nephrotic syndrome (6, 7, 21). Importantly, neurohormonal activation has also been demonstrated in patients with PAH, and occurs in proportion to the degree of RV dysfunction (22, 23). Thus, it is tempting to speculate that the HN observed in our patients with PAH resulted from neurohormonal activation in response to their more advanced RV dysfunction and that HN is not simply a marker but in fact a consequence of more advanced RV function and hemodynamics in patients with PAH. Future studies are needed to establish a possible link between HN, neurohormonal activation, and RV dysfunction in PAH, and may provide further insight into the pathogenesis and possible treatment strategies of RHF in PAH.
HN and Prognosis
In the current study, patients with serum sodium of 136 mEq/L or less had a 10-fold increased risk of death as compared with the patients with more normal serum sodium, and had a median survival of only 8.5 months. These findings parallel those of Lee and Packer, who showed dramatically reduced survival in patients with LHF using a similar cut point to define HN (Na ⩽ 137 mEq/L) (1). All 10 patients in our study with a sodium concentration of less than 135 mEq/L died, whereas all 16 subjects with a sodium concentration of 140 mEq/L or greater survived. Diuretic use had no bearing on the relationship between HN and outcome in these subjects. The use of angiotensin converting enzyme inhibitor or angiotensin receptor antagonist therapy was uncommon in our cohort and thus is unlikely to have affected our results. The prognostic significance of serum sodium was observed when examined as a dichotomous variable or as a continuous variable, deemphasizing the importance of the exact cut point for defining HN, and suggesting that a pathobiologic continuum exists across a range of serum sodium values, which correlates with patient survival. The prognostic significance of HN persisted after adjusting for WHO functional class, treatment status, renal function, as well as several previously established hemodynamic predictors of survival in PAH (11).
Our findings are consistent with prior studies showing that the prognostic significance of HN in patients with LHF occurs independent of hemodynamics and other measures of ventricular dysfunction. Taken together, our data suggest that a low serum sodium concentration in PAH is an integrative measure that signifies a syndrome of right heart dysfunction and ensuing circulatory maladaptation, analogous to the pathophysiologic paradigm in LHF.
Our study represents the first to demonstrate the prognostic significance of HN in patients with PAH. Eleven of the 13 patients with baseline HN who died in the current study had a sodium between 131 and 136 mEq/L, reflecting the observations in patients with LHF that severe HN (i.e., <130 mEq/L) is not required for patients to be at dramatically increased risk of death (1, 24). Also, of the 15 patients in our study who died and had a follow-up sodium measurement at or near the time of death, 87% were hyponatremic, consistent with previous work showing that over 80% of patients with LHF have a serum sodium concentration of less than 135 mEq/L in the 6 months before death (25).
Limitations
There are limitations to our study. In this cohort, more than half of the patients had PAH-CTD. This population has been shown by our group and others to have worse survival when compared with patients with idiopathic PAH (26, 27). Thus, our survival and hazard analyses may be biased by inclusion of a population of patients who were more likely to die, regardless of serum sodium, and thus our findings may be less applicable to other PAH populations. Nevertheless, when controlling for diagnosis, the risk of death remained significantly greater for those patients with HN (HR, 8.0; 95% CI, 2.6–24.8; P < 0.001). Alternatively, patients with PAH-CTD may be more subject to the biology leading to HN, perhaps providing insight into why these patients have poorer survival despite similar degrees of pulmonary vascular disease and PAH.
Survival analyses may have also been skewed by lead-time bias because patients with established disease may have been more likely to die in the follow-up period than newly diagnosed patients. Importantly, because our center is a tertiary referral center for pulmonary hypertension, it is likely that our population comprises patients who are at greater risk of death (referral bias). Thus, although serum sodium is clearly related to higher mortality, the magnitude of this relationship must be interpreted with caution given the small number of events in this cohort and the potentially limited generalizability of these results. Larger scale prospective studies are needed to confirm the prognostic ability of serum sodium in PAH.
Conclusions
The present study demonstrates that HN is associated with advanced RHF and markedly reduced survival in patients with PAH, independent of established hemodynamic, echocardiographic, and clinical markers of poor outcome. Thus, serum sodium has important implications regarding right heart dysfunction, clinical right heart failure, and patient outcome, and should not be overlooked in the clinical assessment of patients with PAH.
Supplementary Material
Supported by the Johns Hopkins General Clinical Research Center and NIH/NHLBI Specialized Center of Clinically Oriented Research in Pulmonary Vascular Disease P50 HL084946 (P.M.H.) and National Research Service Award grant F32HL083714 (S.C.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200712-1876OC on March 20, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lee WH, Packer M. Prognostic importance of serum sodium concentration and its modification by converting-enzyme inhibition in patients with severe chronic heart failure. Circulation 1986;73:257–267. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation 1981;63:645–651. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Abraham WT, Albert NM, Gattis SW, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J 2007;28:980–988. [DOI] [PubMed] [Google Scholar]
- 4.Packer M, Lee WH, Kessler PD, Gottlieb SS, Bernstein JL, Kukin ML. Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation 1987;75:IV80–IV92. [PubMed] [Google Scholar]
- 5.Levine TB, Franciosa JA, Vrobel T, Cohn JN. Hyponatraemia as a marker for high renin heart failure. Br Heart J 1982;47:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilly LS, Dzau VJ, Williams GH, Rydstedt L, Hollenberg NK. Hyponatremia in congestive heart failure: implications for neurohumoral activation and responses to orthostasis. J Clin Endocrinol Metab 1984;59:924–930. [DOI] [PubMed] [Google Scholar]
- 7.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999;341:577–585. [DOI] [PubMed] [Google Scholar]
- 8.Levine TB, Francis GS, Goldsmith SR, Simon AB, Cohn JN. Activity of the sympathetic nervous system and renin-angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am J Cardiol 1982;49:1659–1666. [DOI] [PubMed] [Google Scholar]
- 9.Schrier RW, Berl T, Anderson RJ. Osmotic and nonosmotic control of vasopressin release. Am J Physiol 1979;236:F321–F332. [DOI] [PubMed] [Google Scholar]
- 10.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655–1665. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, Ahearn G. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004;126(1, Suppl):78S–92S. [DOI] [PubMed] [Google Scholar]
- 12.Forfia PR, Mathai SC, M, Hassoun PM. Hyponatremia is associated with severe RV dysfunction and poor prognosis in pulmonary arterial hypertension [abstract]. Am J Respir Crit Care Med 2007;175:A714. [Google Scholar]
- 13.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–1041. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol 1972;34:187–220. [Google Scholar]
- 16.Lange RA, Hillis LD. Cardiac catheterization and hemodynamic assessment. In: Topol EJ, editor. Textbook of cardiovascular medicine, 1st ed. Philadelphia: Lippincott-Raven; 1998. pp. 1957–1976.
- 17.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190–1195. [DOI] [PubMed] [Google Scholar]
- 18.Klein L, O'Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF Jr, Califf RM, Gheorghiade M. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 2005;111:2454–2460. [DOI] [PubMed] [Google Scholar]
- 19.De Luca L, Klein L, Udelson JE, Orlandi C, Sardella G, Fedele F, Gheorghiade M. Hyponatremia in patients with heart failure. Am J Cardiol 2005;96:19L–23L. [DOI] [PubMed] [Google Scholar]
- 20.Spital A. Diuretic-induced hyponatremia. Am J Nephrol 1999;19:447–452. [DOI] [PubMed] [Google Scholar]
- 21.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med 2006;119(7, Suppl 1):S47–S53. [DOI] [PubMed] [Google Scholar]
- 22.Velez-Roa S, Ciarka A, Najem B, Vachiery JL, Naeije R, van de Borne P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004;110:1308–1312. [DOI] [PubMed] [Google Scholar]
- 23.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, Rich S. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol 1995;26:1581–1585. [DOI] [PubMed] [Google Scholar]
- 24.Ghali JK. Hyponatraemia in heart failure: a call for redefinition. Eur Heart J 2007;28:920–921. [DOI] [PubMed] [Google Scholar]
- 25.Teuteberg JJ, Lewis EF, Nohria A, Tsang SW, Fang JC, Givertz MM, Jarcho JA, Mudge GH, Baughman KL, Stevenson LW. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail 2006;12:47–53. [DOI] [PubMed] [Google Scholar]
- 26.Kawut SM, Taichman DB, Archer-Chicko CL, Palevsky HI, Kimmel SE. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003;123:344–350. [DOI] [PubMed] [Google Scholar]
- 27.Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, Krishnan JA, Wigley F, Hassoun PM. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum 2006;54:3043–3050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.