Abstract
Rationale: It is hypothesized that the affective dimension of dyspnea (unpleasantness, emotional response) is not strictly dependent on the intensity of dyspnea.
Objectives: We tested the hypothesis that the ratio of immediate unpleasantness (A1) to sensory intensity (SI) varies depending on the type of dyspnea.
Methods: Twelve healthy subjects experienced three stimuli: stimulus 1: maximal eucapnic voluntary hyperpnea against inspiratory resistance, requiring 15 times the work of resting breathing; stimulus 2: PetCO2 6.1 mm Hg above resting with ventilation restricted to less than spontaneous breathing; stimulus 3: PetCO2 7.7 mm Hg above resting with ventilation further restricted. After each trial, subjects rated SI, A1, and qualities of dyspnea on the Multidimensional Dyspnea Profile (MDP), a comprehensive instrument tested here for the first time.
Measurements and Main Results: Stimulus 1 was always limited by subjects failing to meet a higher ventilation target; none signaled severe discomfort. This evoked work and effort sensations, with relatively low unpleasantness (mean A1/SI = 0.64). Stimulus 2, titrated to produce dyspnea ratings similar to those subjects gave during stimulus 1, evoked air hunger and produced significantly greater unpleasantness (mean A1/SI = 0.95). Stimulus 3, increased until air hunger was intolerable, evoked the highest intensity and unpleasantness ratings and high unpleasantness ratio (mean A1/SI = 1.09). When asked which they would prefer to repeat, all subjects chose stimulus 1.
Conclusions: (1) Maximal respiratory work is less unpleasant than moderately intense air hunger in this brief test; (2) unpleasantness of dyspnea can vary independently from perceived intensity, consistent with the prevailing model of pain; (3) separate dimensions of dyspnea can be measured with the MDP.
Keywords: dyspnea; signs and symptoms, respiratory; pain; psychophysiology
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Pain includes both sensory and affective dimensions. Studies have shown similar brain activations in dyspnea and pain, suggesting that the perceptual model of pain may be appropriate for dyspnea; this hypothesis has not been thoroughly tested.
What This Study Adds to the Field
We show that laboratory-induced air hunger is more potent in causing discomfort than maximal respiratory work/effort. This may be important in evaluating causes of patient discomfort and validates the multidimensional model of dyspnea.
Two of the most common and troubling symptoms experienced by patients are dyspnea, defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” (1), and pain, defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (2). Progress in understanding and treatment of pain leads progress in dyspnea by two decades (3). In part, this is the result of more complete knowledge of how pain is perceived in both laboratory and clinical situations. The recognition that different qualities and dimensions of pain exist, and the development over the past three decades of measuring instruments that can reveal these dimensions, has led to a better understanding of the neurophysiological mechanisms and treatment of pain. Pain is understood as having a “sensory dimension” and an “affective dimension” (4, 5). The sensory dimension comprises components such as intensity (SI), quality (SQ), and time course (ST); the affective dimension comprises stages of immediate unpleasantness (A1) and evaluative and emotional response (A2). These components of pain are indeed linked, but they also have substantial independence, and are processed at different sites in the brain. Although SI is a strong predictor of A1, it is not the only factor that determines A1, nor is A1 the only determinant of A2. Different kinds of pain vary widely in magnitude of A1 at similar SI. For instance, the ratio of A1 to SI is much higher in esophageal pain than in cutaneous pain (6). Likewise, analgesic drugs, hypnosis, and some brain lesions can reduce the A1/SI ratio (e.g., References 7–9). Thus, comprehensive measurement of pain requires more than a single measure of intensity; we hypothesized that the same is true for dyspnea.
Recent functional imaging studies of dyspnea (e.g., Reference 10) have shown that dyspnea activates many of the same limbic brain structures involved in the affective dimension of pain, providing biological evidence that the perceptual model of pain may be appropriate for dyspnea. A few prior studies (reviewed in Discussion) have examined some aspects of affective responses to dyspnea, but none has tested a comprehensive measurement model. We propose a model of dyspnea perception incorporating all major aspects of the multidimensional pain model and present an instrument, the Multidimensional Dyspnea Profile (MDP), that can be used in both laboratory and clinical settings to measure the qualitative, sensory, and affective dimensions of dyspnea.
The present study was designed (1) to determine whether different forms of dyspnea differ in provoking an affective response and (2) to test the MDP. We tested the following null hypothesis: The ratio of unpleasantness, A1, to sensory intensity, SI, is the same for all dyspnea stimuli. This is the key aspect of the multidimensional dyspnea model that remains unsettled. To test the hypothesis, we subjected healthy volunteers to different combinations of laboratory dyspnea stimuli as follows: (1) maximal hyperpnea against a moderate inspiratory resistance was designed to evoke predominantly a sense of excessive respiratory work and effort; (2) mildly elevated end-tidal carbon dioxide partial pressure (PetCO2) with an enforced limit to ventilation was designed to evoke predominantly air hunger. We show that, at similar SI, laboratory-induced perception of air hunger has a significantly greater A1 than the induced sense of respiratory work/effort. This finding disproves the null hypothesis under test, demonstrating the face validity of the measurement concept. It also shows the greater potency of air hunger in causing discomfort, which may be important in evaluating causes of patient discomfort. This study has been reported in abstract form (11).
METHODS
Subjects
We studied 12 healthy subjects (Table 1); none was familiar with dyspnea research or the hypothesis under study. The study protocol was approved by internal review boards at the University of Massachusetts Medical Center (Worcester, MA; performance site) and Harvard School of Public Health (Boston, MA; primary grantee institution). All subjects read and signed consent forms that informed them that we were studying shortness of breath, that they would be uncomfortable for periods during the study, and that they could interrupt or stop procedures at any time without penalty.
TABLE 1.
SUBJECT CHARACTERISTICS
| Subject No. | Age (yr) | Sex | HT (cm) | WT (kg) | Resting PetCO2 (mm Hg) | Education | Relevant Experience |
|---|---|---|---|---|---|---|---|
| BN14 | 34 | M | 191 | 109 | 40 | PhD, medical physics | Yoga |
| BN18 | 29 | F | 163 | 63 | 36 | MS Biol | Yoga breathing |
| BN19 | 49 | M | 178 | 84 | 34 | DVM | Snorkeling |
| BN21 | 32 | F | 165 | 59 | 41 | MS Biol | None |
| BN22 | 26 | F | 178 | 83 | 35 | Medical student | None |
| BN23 | 28 | F | 152 | 42 | 34 | MA, non-Biol | None |
| BN24 | 28 | F | 152 | 61 | 32 | BS Biol | None |
| BN25 | 22 | F | 157 | 57 | 37 | Grad student, Biol | None |
| BN26 | 55 | F | 165 | 68 | 32 | MS Biol | Asthma |
| BN27 | 29 | F | 165 | 56 | 36 | BS Biol | Wind instruments, asthma |
| BN28 | 33 | F | 155 | 67 | 33 | Physician | None |
| BN29 | 23 | M | 168 | 56 | 40 | BS Biol | Wind instruments |
| Median | 29 | 165 | 62 | 35 |
Definition of abbreviations: Biol = biology; F = female; HT = height; M = male; PetCO2 = end-tidal carbon dioxide partial pressure; WT = weight.
Measurement of Dyspnea
Our primary measure of dyspnea was the MDP, an instrument under development in our laboratory. The MDP incorporates standard measuring techniques of rating scales and descriptor selection and is patterned after a validated multidimensional pain instrument (12, 13) and previous work on the quality of dyspnea sensation (14–17). This instrument is designed to measure sensory intensity (SI), immediate unpleasantness (A1), sensory quality (SQ), and emotional response (A2). Details of this questionnaire are being refined before final validation (persons interested in the current form may contact the authors).
We used a scripted “radio analogy” developed by Price and colleagues to explain the difference between SI (“how strong the breathing sensation feels”) and A1 (“how uncomfortable or bad it feels”) (18). Briefly, we told subjects that SI is analogous to how loud a sound is and A1 is analogous to how unpleasant the sound is, which depends on what sound is heard, and that a sound can be unpleasant even if it's not loud. All subjects averred they understood the concept. Scales comprising all integers from 0 to 10, equally spaced, were presented for rating SI and A1. In addition to the numbers, words descriptive of magnitude and dimension were ranged along each scale to help subjects to distinguish between the dimensions to be rated and to improve consistency among subjects; the words were placed according to their semantic magnitude as determined in published studies (e.g., Reference 19). The upper end of the SI scale was labeled “maximum,” whereas the upper end of the A1 scale was labeled “unbearable.”
Subjects reported SQ using a list of terms derived from previous work (14, 15, 20, 21), narrowed to five categories using information on the internal correlations within longer lists (16). The five categories were as follows: “smothering, suffocating”; “breathing requires work or effort”; “cannot get enough air, hunger for air”; “chest and lungs feel tight, constricted”; “breathing a lot; rapidly, deeply, heavily”. A descriptor category not expected to describe dyspnea was added to assess the individual's tendency to agree with every statement; in this instance, we used “crushing or heavy sensation in chest,” a symptom of myocardial infarction, but seldom chosen as a dyspnea descriptor. Subjects rated how much of each sensation quality they felt (0 to 10), and chose the most apt single descriptor.
Finally, subjects were asked to rate a list of five negative emotions: depression, anxiety, frustration, anger, and fear. Subjects rated how much of each emotion they experienced on a scale ranging from 0 to 10 (“most severe I can imagine”). Subjects were also asked if they had any current pain, and if so, whether it was related to breathing.
Physiological Measurements
Before each experiment, we measured resting PetCO2 via a fine nasal catheter while the subject sat comfortably reading. During the experiment, tidal Pco2 and mask pressure were sampled at the common line between mask and rehumidifier (Capstar 100; CWE, Inc., Ardmore, PA; Omega PX138-001D5V; Omega, Stamford, CT). Inspiratory and expiratory flows were measured with separate pneumotachometers (no. 2 Fleisch with Omega PX163PC01D75, Omega). Pulse rate, SpO2, and noninvasive arterial pressure were monitored (Criticare 506DXNP2; Criticare Systems, Waukesha, WI). Data were digitized and recorded for later analysis (Powerlab/16s with Chart 4.2.3 software; AD Instruments, Colorado Springs, CO; and Macintosh G3 Powerbook; Apple, Inc., Cupertino, CA).
Dyspnea Stimuli
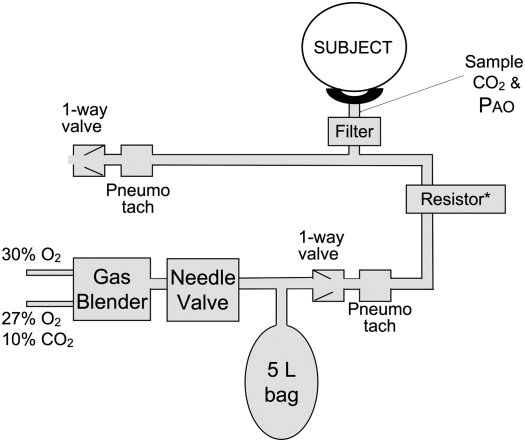
To provide different mixes of dyspnea qualities, different stimuli were effected by independently controlling the amount of minute ventilation, inspiratory resistance, and PetCO2. The design of breathing apparatus is shown in Figure 1.
Figure 1.
Breathing circuit. During stimulus administration, subjects breathed via a tight-fitting facemask connected to a non-rebreathing valve system via a viral filter/rehumidifier (Airlife HEPA; Cardinal Health, McGaw Park, IL). Inspired gas was supplied from a 5-L rubber anesthesia bag; expired gas exited to the room. The subject's minute ventilation was set by the flow rate of gas into the bag (see stimulus descriptions in text). *During stimulus 1, an inspiratory resistance was imposed (14 cm H2O at 1.0 L · s−1). The resistor was not present during stimuli 2 and 3. We controlled gas flow to the bag and CO2 concentration to meet the needs of each stimulus.
Stimulus 1: hyperpnea (maximal).
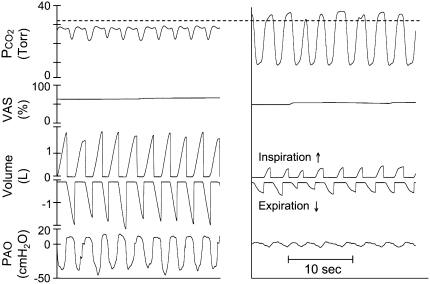
During the hyperpnea stimulus, a moderate resistance was imposed (14 cm H2O at 1 L/s). The subject viewed the anesthesia bag, and was instructed to prevent it from becoming fully distended or collapsed—thus, the amount of gas flowing into the bag determined the target minute ventilation. This target flow began at resting levels and was gradually increased until the subject could no longer keep pace, then decreased slightly to obtain a stimulus sustainable for 30 seconds. Fraction of inspired carbon dioxide (FiCO2) was manipulated to hold PetCO2 0 to 7 mm Hg below resting Pco2 throughout the trial. The left side of Figure 2 shows a typical recording of the key variables during the stimulus 1 rating focus period.
Figure 2.
Time traces of physiological data and online ratings (visual analog scale [VAS]) during rating focus periods for stimulus 1 (left) and stimulus 2 (right). Data are from subject BN28. Pao = pressure measured in the mask. Horizontal dashed line represents resting PetCO2 in this subject. Breaths 3, 4, and 6 in the right panel are examples of inadequate end-tidal samples that were dropped from analysis. Volume = separate inspiratory and expiratory volumes obtained by integrating flow from the two pneumotachometers, reset for each breath.
Stimulus 2: hypopnea (matched).
At the outset of this period, the FiCO2 was raised to elevate PetCO2 approximately 6 mm Hg above resting, with ample flow to supply the increased spontaneous breathing. (The bag was not visible to the subject during this task.) The experimenter then began to decrease flow to the bag to limit ventilation, holding PetCO2 constant by simultaneously reducing FiCO2. Ventilation was gradually decreased until online ratings of breathing discomfort increased to approximately match the maximum online ratings given near the end of stimulus 1; this required only modest reduction from spontaneous ventilation. The right side of Figure 2 shows a typical recording of the key variables during the stimulus 2 rating focus period.
Stimulus 3: hypopnea (maximal).
In eight subjects, we performed a third trial in which the hypopnea stimulus was further increased (by further decreasing minute ventilation) until the subject signaled intolerable discomfort. (With one exception: one trial included in the analysis was stopped due to technical failure at a point when the subject was rating 85% scale.) This required ventilation about half that as in stimulus 2.
Stimulus order and time course.
Each subject visited the laboratory twice. Day 1 was designed to familiarize the subject with the stimuli and the rating scales; primary data were collected during Day 2. On Day 1, the order in which the stimuli were presented was alternated between subjects, and the intensity of stimuli was varied in an unpredictable fashion.
To help us guide the experiment, subjects gave continuous online single-dimension ratings of overall breathing discomfort using an electronic visual analog scale (VAS). We denoted the upper end of this VAS as “intolerable,” and informed the subject that the stimulus would be immediately reduced if she or he rated 100% scale. This rating was used only to approximately match the magnitude of sensation produced by stimuli 1 and 2, and to terminate the stimulus if discomfort was intolerable; it was not used as an outcome measure. We administered the MDP immediately after each stimulus, instructing subjects to attend to a “focus period” near the end of the stimulus period during which online ratings had been constant for at least 10 seconds (median, 30 s).
In pilot studies, we found it impossible to drive most subjects above midscale ratings using the hyperpnea stimulus; in contrast, all but one subject could be driven to the top of the scale with the hypopnea stimulus. Because of this limitation, stimulus 1 was administered first on Day 2 and the maximal online rating was noted; we then adjusted stimulus 2 to match this rating. After obtaining MDP responses for stimulus 2, we obtained a behavioral measure of the relative unpleasantness experienced by asking whether the subject would rather repeat stimulus 1 or 2, and why. In the third trial, we administered stimulus 3.
RESULTS
We found that air hunger was distinctly more unpleasant than work/effort sensation. The MDP was capable of measuring this difference, and subjects' ratings were consistent with their behavioral choices and qualitative comments. In Characterization of Stimuli below, we characterize the stimuli (physiological changes and resultant qualities of sensation). In Affective Dimension, we present evidence that the ratio of unpleasantness (A1) to SI is greater for air hunger than for work/effort, supporting the hypothesis that sensory and affective dimensions are separate, and can be measured. In Evaluation of MDP, we present further information on use of the MDP.
Characterization of Stimuli
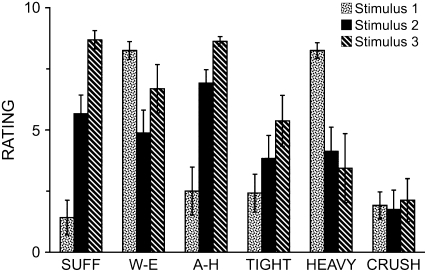
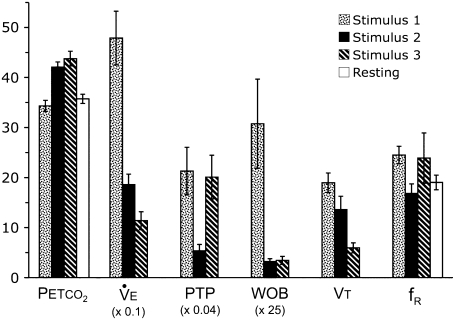
Measurements of SQ using the MDP (Figure 3) confirmed that the two maneuvers produced the expected sensations, and that they felt quite different from each other. This is not a fundamentally new finding; it confirms that our interventions were effective and that subjects can distinguish different kinds of dyspnea. Figure 4 depicts the key physiological variables corresponding to the MDP ratings. To account for perceptual response time (10, 22), mechanical values (e.g., Vt) were averaged over the focus period plus the prior 20 seconds and PetCO2 was averaged over 2 minutes.
Figure 3.
Ratings of qualities of respiratory sensation (mean ± SE) during the rating focus periods for stimulus 1, normocapnic hyperpnea with inspiratory resistance; stimulus 2, hypercapnia with moderate restriction of ventilation; stimulus 3, hypercapnia with severe restriction of ventilation. SUFF = “I am smothering, suffocating”; W-E = “My breathing requires work or effort”; A-H = “I cannot get enough air. I feel hunger for air”; TIGHT = “My chest and lungs feel tight, constricted”; HEAVY = “I am breathing a lot; breathing rapidly, deeply or heavily”; CRUSH = “I feel a crushing, heavy sensation in my chest”. Scale maximum definition: “As intense as I can imagine”. Eight of the 12 subjects completed stimulus 3; all completed stimuli 1 and 2.
Figure 4.
Physiological variables during rating focus periods plus the preceding 20 seconds, as well as PetCO2 and fR during resting breathing without mask. Values shown are the mean and SE for all subjects. Rates are normalized to 1 minute. Extensive variables (V̇e, WOB, Vt) were normalized to body weight. Several variables were multiplied by a scaling factor for the figure; factor is noted for each. fR = breathing frequency (breaths · min−1); PetCO2 = end-tidal Pco2 (mm Hg); PTP = pressure time product (cm H2O · s · min−1; scaling factor, 0.04); V̇e = minute ventilation (ml · min−1 · kg−1; scaling factor, 0.1); Vt = expiratory tidal volume (ml · kg−1); WOB = external work of breathing (joules · min−1 · kg−1; scaling factor, 25). PetCO2 for each time point was calculated based on published air hunger response dynamics (22).
Stimulus 1.
As intended, stimulus 1 entailed much higher V̇e (0.48 L · min−1 · kg−1 ± 0.19 SD) and lower PetCO2 (1.7 mm Hg ± 2.2 SD below resting) than stimuli 2 and 3. The median external work of breathing at this condition was 1.23 cm H2O · L · s−1 · kg−1 (i.e., more than 10 times the internal respiratory work rate at rest for a typical healthy subject). Although we did not measure pleural pressure to calculate internal work, a conservative estimate is that internal work increased proportionally to ventilation, or about fivefold; thus, total work would have been about 15 times the work of resting breathing. Work of breathing was likely underestimated in some subjects (see Discussion). In no case did the subject terminate stimulus 1 due to discomfort; the stimulus limit was always determined by the subject's failure to meet the target flow.
As expected during stimulus 1, ratings of respiratory work/effort and the sense of rapid deep breathing were substantial (mean rating for both was 83% scale). Seven subjects chose “rapid deep breathing,” three subjects chose “work/effort,” and one chose “chest tightness” as the best descriptor for stimulus 1 (the subject who chose tightness did not have asthma; one subject was not asked for best descriptor).
Stimulus 2.
PetCO2 during stimulus 2 was 6.1 mm Hg ± 1.2 SD above resting; ratings comparable to stimulus 1 were achieved at a mean V̇e = 0.19 L · min−1 · kg−1 (±0.07 SD), only modestly below the expected minute ventilation at the prevailing PetCO2. Work of breathing was about one-tenth that in stimulus 1.
During stimulus 2, ratings of suffocation and air hunger were substantial (mean: 57% and 69% scale, respectively), much higher than during stimulus 1, whereas work/effort and rapid/deep ratings were much lower than during stimulus 1. One subject added the descriptor “unsatisfied inspiration.” Seven subjects chose “air hunger” as the best descriptor for this stimulus, two chose “suffocating,” and two chose “work/effort.”
Stimulus 3.
Stimulus 3, the more intense iteration of stimulus 2, entailed slightly higher PetCO2 (7.7 mm Hg ± 2.5 SD above resting) and slightly lower ventilation (0.11 L · min−1 · kg−1 ± 0.05 SD). Work of breathing was about one-tenth that in stimulus 1, but due to static efforts against the collapsed bag, the pressure time product (PTP) was nearly equal to that in stimulus 1. Again, ratings of suffocation and air hunger were much higher than during stimulus 1. Subjects selected slightly different descriptors than for stimulus 2: four subjects now chose “suffocation” and four chose “air hunger” as the best descriptor, and suffocation ratings equaled air hunger ratings.
Other sensations.
Chest tightness and crushing sensations were not prominent SQ qualities in any maneuver, tightness was chosen only once as the best descriptor, and crushing was never chosen as best descriptor. Crushing was not expected to be chosen as best descriptor, nor to be rated high, providing confirmation that the subject discriminated carefully, and that the descriptor list discriminated well between symptoms.
Subjects BN22 and BN29 reported pain (“side stitch”) related to stimulus 1, but both achieved greater than average ventilation; there were no other reports of pain. The presence of pain may have contributed to the A1 ratings of stimulus 1 for these subjects; nonetheless, these subjects rated low A1 for stimulus 1.
Affective Dimension
Separation of unpleasantness (A1) from SI.
The a priori prediction for this study was that the ratio of unpleasantness (A1) to SI would vary systematically within subject with the type of dyspnea experienced. The statistical null hypothesis for the primary hypothesis under test was “the ratio A1/SI is the same for both forms of dyspnea.”
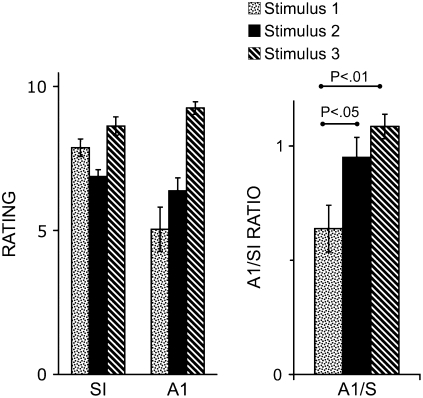
Mean A1/SI ratio was 0.95 during stimulus 2 (air hunger), significantly greater than the mean of 0.64 during the matched magnitude stimulus 1 (work) (P = 0.039, two-tailed paired t test [Microsoft Excel 2004; Microsoft Corp., Redmond, WA]; Bonferroni corrected for two comparisons, as described by M. Bland, University of York, http://www-users.york.ac.uk/∼mb55/intro/bonf.htm). The A1/SI ratio of stimulus 3 was 1.09, significantly higher (P = 0.003) than the 0.54 A1/SI for stimulus 1 in the subset of eight subjects undergoing stimulus 3 (see Figure 5, right).
Figure 5.
Sensory intensity (SI) and immediate unpleasantness (A1) of each stimulus (mean ± SE; left panel). A 10 on the SI scale was defined as “maximum”; 10 on the A1 scale was defined as “unbearable.” The relative unpleasantness (A1/SI) ratios (right panel) were significantly greater for those stimuli evoking predominantly air hunger (stimuli 2 and 3). Although 4 of the 12 subjects were not tested with stimulus 3, A1/SI for the 8 subjects tested was not significantly different from the group of 12 for the other stimuli (0.53 for stimulus 1 and 0.91 for stimulus 2).
When asked whether they would prefer to repeat stimulus 1 or stimulus 2, all subjects immediately and emphatically chose stimulus 1 (work/effort), and all gave explanations referring to the greater unpleasantness of air hunger (see quotations in Table 2). Another way to match the stimuli is to raise the intensity of each stimulus to the subject's limit. In stimulus 1 and stimulus 3, the two kinds of stimuli were increased until the subject could no longer perform the stimulus task. All subjects rated unpleasantness of stimulus 3 (maximal air hunger) greater than 80% scale (group mean, 93% of scale). No subject rated unpleasantness of stimulus 1 (maximal work of breathing) greater than 80% scale (group mean, 50% of scale).
TABLE 2.
TYPICAL VERBATIM COMMENTS AFTER VOLUNTARY MAXIMAL HYPERPNEA (STIMULUS 1) AND MATCHED HYPOPNEA (STIMULUS 2)*
| Subject No. | Comment |
|---|---|
| BN21 | Stimulus 1: “I wasn't short of breath, it wasn't as unpleasant” |
| BN21 | Stimulus 2: “I wanted to breathe more” |
| BN23 | Stimulus 2: “If I didn't trust the experimenters, I would have rated a 10 for fear” |
| BN28 | Stimulus 2: “felt like I wanted to take more breath, but it wasn't there, it was scary” |
| BN29 | Stimulus 1 “Breathing a lot doesn't worry me; however, not breathing enough does” |
| BN29 | Stimulus 2: “I couldn't expand my chest enough, like an unsatisfied inspiration” |
| BN14 | Stimulus 1: “was the lesser of two evils” |
| BN18 | Stimulus 1: “No unpleasant feelings associated” |
Most comments were volunteered in the course of explaining why one task was preferable.
Emotional response (A2) to laboratory stimuli.
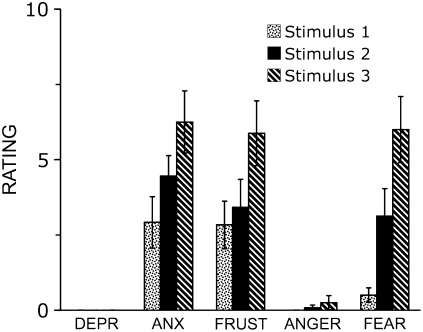
Ratings of anxiety and frustration were greater than zero for all stimuli, and appreciable fear emotion was rated during stimuli 2 and 3, even though all subjects intellectually understood that they were safe (see BN23's comment in Table 2). The sample size of the current experiment was not powered for multiple comparisons of all outcome measures, but the data shown in Figure 6 strongly suggest that the emotional response to air hunger is substantially greater than the response to work/effort. These emotions were greatest during stimulus 3. Subjects commented that the frustration in both stimuli arose from their inability to do the task at higher levels. Depression and anger were not evoked by these brief laboratory tests in normal subjects.
Figure 6.
Ratings of A2 (emotional/evaluative response: depression [DEPR], anxiety [ANX], frustration [FRUST], anger, and fear; 10 defined as “Most severe I can imagine”; mean ± SE).
Evaluation of MDP
Subjects rated the MDP as understandable (mean = 8.3 ± 1.8 SD, where 0 = not clear, 10 = extremely clear), helpful in expressing their experience (mean = 8.4 ± 2.0 SD, where 0 = did not help, 10 = extremely helpful), and easy to complete (mean = 0.7 ± 1.1 SD, where 0 = not at all difficult, 10 = extremely difficult).
DISCUSSION
We conclude the following: (1) moderate air hunger is more unpleasant than maximal respiratory work, and maximal air hunger is far more unpleasant; (2) unpleasantness of dyspnea (A1) can vary independently from perceived intensity (SI), suggesting that dyspnea conforms to the multidimensional pain model; (3) the MDP instrument is convenient to use, and can distinguish differences in affective responses among kinds of dyspnea.
Affective Dimension of Dyspnea
SI versus unpleasantness.
The ratio of unpleasantness to the strength of sensation (A1/SI) during air hunger was 50–70% greater than during the sensation of work/effort. The higher A1/SI ratio was due to a difference in stimulus quality, not to nonlinearity in the relationship of A1 to SI, because the greater ratio was observed regardless of whether SI during work/effort was more than, or less than, SI during air hunger (stimulus 2 vs. stimulus 1 and stimulus 3 vs. stimulus 1, respectively).
Emotional response.
Pain and dyspnea are often accompanied by negative emotional responses in patients (e.g., References 12, 23, and 24). Some aspects of patients' strong emotional response to dyspnea may be missing in the laboratory, but our subjects did report fear, anxiety, and frustration (depression and anger were absent). Emotions would likely have been stronger if the experience had been inescapable: for example, “If I didn't know I could pull the mask off I would have been very fearful” (subject BN14, Table 1). Our measurement approach can quantify how well a particular laboratory intervention simulates dyspnea in a particular group of patients.
Relationship of present findings to prior studies.
A number of reviews have mentioned the idea that dyspnea has separate affective and SI components (e.g., References 25–27). There have been few studies to support this contention, and none that incorporated the multiple component model used here.
In several studies of dyspnea, subjects were asked to rate both the intensity of dyspnea and some aspect of affective response, described in terms such as “distress” or “anxiety” (28–31). Subjects gave separate ratings, and different subjects assigned different relative values to intensity and affect. There were, however, no interventions designed to alter the relationship between intensity and affective response, so it could not be concluded that the dimensions are independent.
The first series of experiments to strongly suggest that affective response can be independent of dyspnea intensity examined the effect of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease (29, 32). Dyspnea-related anxiety (a component of A2 in our model) fell by 25% relative to dyspnea intensity. We calculated an A/SI ratio using published mean data; statistical testing was not possible without paired individual data.
More recent studies have measured SI and unpleasantness (A1) while attempting to alter their relationship by acutely altering psychological state (33, 34). These studies were performed on healthy subjects in whom moderate respiratory discomfort was produced with inspiratory resistive loads. The results obtained support the multidimensional model proposed here: modest (15–25%) changes in A1/SI were seen with interventions altering attentional state or emotional state. The strength of this support is limited because the investigators did not measure or control the physiological variables pertinent to respiratory sensation (Vt, V̇e, Pco2, or Po2), and because these psychological interventions could cause change in general affect, not specific to dyspnea.
Implications of Air Hunger Unpleasantness
The greater affective potency of air hunger suggests that it is likely to be a key component of severe dyspnea in patients, highlighting the need to keep air hunger stimuli in mind when evaluating the cause and treatment of dyspnea. Indeed, several studies show the increasing importance of air hunger as dyspnea approaches the tolerable limit during exercise or chemostimulation in obstructive lung disease (e.g., Reference 35). Nonetheless, dyspnea in patients is of mixed origin, and work/effort sensation frequently figures in their descriptions (e.g., References 15, 35, and 36).
Critique of Physiological Methods
There was some overlap in SQ between stimuli; notably, subjects often reported some work or effort accompanying air hunger during stimuli 2 and 3. Although some subjects made (futile) inspiratory efforts against the collapsed bag, work effort ratings were not correlated with inspiratory PTP during air hunger stimuli (r2 = 0.05). We suggest that the work/effort ratings largely reflected the mental effort of suppressing involuntary respiratory muscle contractions in the face of a strong drive to breathe, as reported previously (17).
We chose to use a mask rather than a mouthpiece, and pilot studies showed no difference in air hunger sensation stimulus response with a mask compared with a mouthpiece. Although mask fit was carefully tested before each experiment, examination of inspiratory and expiratory flow tracings suggested leaks at the mask face seal in several subjects during the strenuous high ventilation of stimulus 1. These leaks would cause our ventilation and work measures to underestimate those actually achieved by those subjects; nonetheless, subjects breathed hard enough to produce work ratings of at least 60% of scale and SI ratings of at least 60% of scale in every subject (PetCO2 measures would be unaffected by the leak).
MDP Measurement Performance
The MDP was sufficiently sensitive and specific to show clear differences in sensory qualities with different stimuli. Most prior studies have assessed SQ by asking subjects to choose best descriptors from a list of 10 or more terms. The present study differs in asking subjects to scale the contribution of each sensation, as suggested by Parshall and colleagues (37). This has several advantages: (1) Quality scales can more readily detect the presence and magnitude of secondary sensations. For instance, work/effort was not detected by yes/no choice of descriptors during similar interventions in earlier studies (38, 39). (2) The scaling format was also more subject friendly. Many subjects have difficulty and vacillate over yes/no answers; in contrast, subjects typically worked through the scaled descriptor list quickly. The simplified list of terms based on Parshall and colleagues' analysis (16) is easier for subjects, and reduces ambiguity in interpretation arising from redundant descriptors. It was very uncommon for subjects to add additional descriptors. Suffocation ratings were highly correlated with air hunger ratings (r2 = 0.78); thus, these descriptors seem to be essentially synonymous.
Our impression from verbal debriefings is that the true difference in unpleasantness may be even greater than our quantitative results indicate. This is not surprising, because 5 to 10% of normal subjects cannot give ratings that correlate with changes in respiratory stimuli (40, 41), and the semantic distinction between intensity and unpleasantness is more subtle than subjects are ordinarily called on to make. We did not attempt to exclude such subjects from this study, as they will occur in a clinical population, but such subjects can be screened using pretests of rating correlation with repeated known stimuli (40, 42). Even with these limitations, the MDP was capable of showing a clear difference in affective response between kinds of dyspnea.
Subjects rated the MDP highly for clarity, helpfulness, and ease of use. Initial use of the questionnaire, including explanations, was usually accomplished in less than 5 minutes, and subsequent use of the questionnaire required 1 to 2 minutes for most subjects. We spent only 1 to 2 minutes explaining the concept of separate scales for intensity and unpleasantness, and all subjects professed to understand the explanation, although three said the distinction was difficult to make in practice.
Comparison with established dyspnea instruments.
The multidimensional nature of dyspnea is seldom recognized in measurement methods. The commonly used clinical dyspnea scales ask about the frequency, severity, or behavioral impact of dyspnea in everyday activities (reviewed in Reference 43). These instruments, although useful in obtaining a clinical history, cannot be applied to dyspnea evoked in the laboratory or to acute testing of patients (e.g., during exercise testing, during mechanical ventilation). One-dimensional rating scales (VAS or Borg scales) have sometimes been used in clinical studies (43), but have not been standardized—for example, the quality of sensation to be rated and the end markers of the scales vary widely among studies. Lists of SQ descriptors have been used in several studies and have proven useful in the clinic and laboratory (14, 15, 44). The MDP is the first instrument proposed that provides comprehensive measures of sensory intensity and quality, and multiple components of the affective dimensions of dyspnea.
Conclusions
We present here the first quantitative data showing that the sensation of air hunger is far more unpleasant than the sensation of excessive respiratory work. This is the strongest evidence to date that multiple dimensions of dyspnea exist and can be measured. Failure to measure the salient dimensions of dyspnea makes it difficult to translate between laboratory experiments and clinical experience. Incomplete measurement hampers understanding of treatment outcomes.
If the global rating of dyspnea comprises both sensory and affective components, a multidimensional measurement such as the MDP may help the clinician. Assessment of SQ can help distinguish disease states that cause dyspnea (44, 45). Determining whether a change in dyspnea primarily reflects a change in the primary sensation or the affective response may inform us about the role of the psychological state of the patient in ratings of respiratory discomfort and guide therapies such as psychological interventions or psychoactive drugs, that reduce the A1/SI ratio to reduce discomfort and enhance function.
Existing measurement instruments have not been adequate to address these problems. Although the individual concepts underlying the MDP have appeared in other instruments, the MDP integrates these concepts into one instrument. We believe that the MDP will be of value in studies of dyspnea mechanisms, as well as in clinical trials evaluating treatment.
Acknowledgments
The authors thank Richard Gracely for many enlightening discussions that were fundamental in helping us to understand pain, and to develop this line of inquiry. Paula Meek and Mark Parshall were instrumental in developing the MDP. Jeanette Hoit was extremely helpful in designing and performing preliminary experiments. The authors are deeply indebted to David Paydarfar of the University of Massachusetts Medical School for generous logistical support as well as probing discussion. Lily Nguyen provided valuable logistical help. The authors thank members of the community at UMass Medical Center for volunteering for these studies.
Supported by National Institutes of Health grants HL46690 and NR10006 to R.B.B.
Originally Published in Press as DOI: 10.1164/rccm.200711-1675OC on March 27, 2008
Conflict of Interest Statement: R.B.B. received $30,000 from Boehringer Ingelheim as an unrestricted grant in support of a 2005 symposium. S.H.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.W.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society. Dyspnea: mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med 1999;159:321–340. [DOI] [PubMed] [Google Scholar]
- 2.International Association for the Study of Pain Task Force on Taxonomy. Classification of chronic pain. In: Merskey H, Bogduk N, editors. Classification of chronic pain, 2nd ed. Seattle, WA: IASP Press; 1994. p. 210.
- 3.Dudgeon DJ. Managing dyspnea and cough. Hematol Oncol Clin North Am 2002;16:557–577. [DOI] [PubMed] [Google Scholar]
- 4.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;288:1769–1772. [DOI] [PubMed] [Google Scholar]
- 5.Price DD, Harkins SW. The affective-motivational dimension of pain: a two-stage model. APS Journal 1992;1:229–239. [Google Scholar]
- 6.Strigo IA, Bushnell MC, Boivin M, Duncan GH. Psychophysical analysis of visceral and cutaneous pain in human subjects. Pain 2002;97:235–246. [DOI] [PubMed] [Google Scholar]
- 7.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain 1985;22:261–269. [DOI] [PubMed] [Google Scholar]
- 8.Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 1999;82:159–171. [DOI] [PubMed] [Google Scholar]
- 9.Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol 1988;24:41–49. [DOI] [PubMed] [Google Scholar]
- 10.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 2002;88:1500–1511. [DOI] [PubMed] [Google Scholar]
- 11.Banzett R, Pedersen S, Lansing R. Dyspnea unpleasantness can vary independently from dyspnea intensity [abstract]. Am J Respir Crit Care Med 2007;175:A343. [Google Scholar]
- 12.Wade JB, Dougherty LM, Archer CR, Price DD. Assessing the stages of pain processing: a multivariate analytical approach. Pain 1996;68:157–167. [DOI] [PubMed] [Google Scholar]
- 13.Harkins SW, Price DD, Braith J. Effects of extraversion and neuroticism on experimental pain, clinical pain, and illness behavior. Pain 1989;36:209–218. [DOI] [PubMed] [Google Scholar]
- 14.Simon PM, Schwartzstein RM, Weiss JW, Lahive K, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable sensations of breathlessness induced in normal volunteers. Am Rev Respir Dis 1989;140:1021–1027. [DOI] [PubMed] [Google Scholar]
- 15.Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis 1990;142:1009–1014. [DOI] [PubMed] [Google Scholar]
- 16.Parshall MB. Psychometric characteristics of dyspnea descriptor ratings in emergency department patients with exacerbated chronic obstructive pulmonary disease. Res Nurs Health 2002;25:331–344. [DOI] [PubMed] [Google Scholar]
- 17.Lansing RW, Im BS, Thwing JI, Legedza AT, Banzett RB. The perception of respiratory work and effort can be independent of the perception of air hunger. Am J Respir Crit Care Med 2000;162:1690–1696. [DOI] [PubMed] [Google Scholar]
- 18.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- 19.Gracely RH, McGrath P, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain 1978;5:19–29. [DOI] [PubMed] [Google Scholar]
- 20.Mahler DA, Harver A, Lentine T, Scott JA, Beck K, Schwartzstein RM. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med 1996;154:1357–1363. [DOI] [PubMed] [Google Scholar]
- 21.Elliott MW, Adams L, Cockcroft A, MacRae KD, Murphy K, Guz A. The language of breathlessness: use of verbal descriptors by patients with cardiopulmonary disease. Am Rev Respir Dis 1991;144:826–832. [DOI] [PubMed] [Google Scholar]
- 22.Banzett RB. Dynamic response characteristics of CO2-induced air hunger. Respir Physiol 1996;105:47–55. [DOI] [PubMed] [Google Scholar]
- 23.Price D. Psychological mechanisms of pain and analgesia, progress in pain research and management. Seattle, WA: IASP Press; 1999.
- 24.Gift AG. Psychologic and physiologic aspects of acute dyspnea in asthmatics. Nurs Res 1991;40:196–199. [PubMed] [Google Scholar]
- 25.Steele B, Shaver J. The dyspnea experience: nociceptive properties and a model for research and practice. ANS Adv Nurs Sci 1992;15:64–76. [DOI] [PubMed] [Google Scholar]
- 26.Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med 1995;333:1547–1553. [DOI] [PubMed] [Google Scholar]
- 27.Banzett RB, Dempsey JA, O'Donnell DE, Wamboldt MZ. Symptom perception and respiratory sensation in asthma. Am J Respir Crit Care Med 2000;162:1178–1182. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RC, Jones PW. Differentiation between the intensity of breathlessness and the distress it evokes in normal subjects during exercise. Clin Sci 1991;80:65–70. [DOI] [PubMed] [Google Scholar]
- 29.Carrieri-Kohlman V, Gormley JM, Douglas MK, Paul SM, Stulbarg MS. Differentiation between dyspnea and its affective components. West J Nurs Res 1996;18:626–642. [DOI] [PubMed] [Google Scholar]
- 30.Isenberg S, Lehrer P, Hochron S. Defensiveness and perception of external inspiratory resistive loads in asthma. J Behav Med 1997;20:461–472. [DOI] [PubMed] [Google Scholar]
- 31.von Leupoldt A, Dahme B. Differentiation between the sensory and affective dimension of dyspnea during resistive load breathing in normal subjects. Chest 2005;128:3345–3349. [DOI] [PubMed] [Google Scholar]
- 32.Carrieri-Kohlman V, Gormley JM, Eiser S, Demir-Deviren S, Nguyen H, Paul SM, Stulbarg MS. Dyspnea and the affective response during exercise training in obstructive pulmonary disease. Nurs Res 2001;50:136–146. [DOI] [PubMed] [Google Scholar]
- 33.von Leupoldt A, Seemann N, Gugleva T, Dahme B. Attentional distraction reduces the affective but not the sensory dimension of perceived dyspnea. Respir Med 2007;101:839–844. [DOI] [PubMed] [Google Scholar]
- 34.von Leupoldt A, Mertz C, Kegat S, Burmester S, Dahme B. The impact of emotions on the sensory and affective dimension of perceived dyspnea. Psychophysiology 2006;43:382–386. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997;155:109–115. [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell DE, Chau LK, Webb KA. Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol 1998;84:2000–2009. [DOI] [PubMed] [Google Scholar]
- 37.Parshall MB, Welsh JD, Brockopp DY, Heiser RM, Schooler MP, Cassidy KB. Reliability and validity of dyspnea sensory quality descriptors in heart failure patients treated in an emergency department. Heart Lung 2001;30:57–65. [DOI] [PubMed] [Google Scholar]
- 38.Moosavi SH, Binks AP, Lansing RW, Topulos GP, Banzett RB, Schwartzstein RM. Effect of inhaled furosemide on air hunger induced in healthy humans. Respir Physiolo Neurobiol 2007;156:1–8. [DOI] [PubMed] [Google Scholar]
- 39.Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol 2003;94:141–154. [DOI] [PubMed] [Google Scholar]
- 40.Revelette WR, Zechman FW Jr, Parker DE, Wiley RL. Effect of background loading on perception of inspiratory loads. J Appl Physiol 1984;56:404–410. [DOI] [PubMed] [Google Scholar]
- 41.Lansing R, Banzett R. Psychophysical methods in the study of respiratory sensation. In: Adams L, Guz A, editors. Respiratory sensation, lung biology in health and disease. New York: Marcel Dekker; 1996. pp. 69–100.
- 42.Teghtsoonian R. The study of individuals in psychophysical measurement. In: Ljunggren G, Dornic S, editors. Psychophysics in action. Berlin: Springer-Verlag; 1989. pp. 95–102.
- 43.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med 2007;21:177–191. [DOI] [PubMed] [Google Scholar]
- 44.Schwartzstein R. The language of dyspnea: using verbal clues to diagnose. J Crit Illn 1999;14:435–441. [Google Scholar]
- 45.Flaherty KR, Wald J, Weisman IM, Zeballos RJ, Schork MA, Blaivas M, Rubenfire M, Martinez FJ. Unexplained exertional limitation: characterization of patients with a mitochondrial myopathy. Am J Respir Crit Care Med 2001;164:425–432. [DOI] [PubMed] [Google Scholar]