Abstract
A new study reveals that extracellular signals can activate a signal-transduction cascade that simultaneously alters alternative splicing and translation of the same target. These concerted efforts probably serve to increase the speed and strength of the cellular response to changes in the extracellular environment.
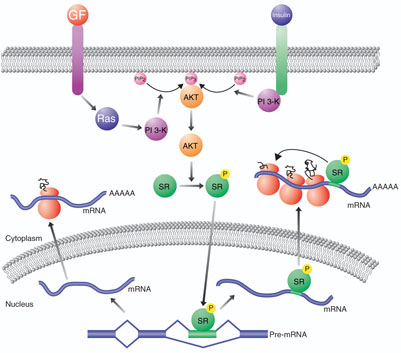
Alternative splicing is a powerful mechanism by which multiple proteins can be synthesized from a single gene. This is important both in increasing protein diversity while allowing for genome compaction and in regulating gene expression1. The way in which the information encoded by specific alternative exons is accessed and extracted at the correct time and in the correct cells is an active area of investigation2. It has become increasingly clear that certain alternative splicing choices can be modulated by the activation of signal-transduction pathways3. However, in most cases the mechanisms by which signaling pathways lead to specific changes in alternative splicing are not known. On page 1037 of this issue, Blaustein et al.4 show that growth factor–induced activation of a signaling pathway involving Ras, phosphatidyl inositol (PI) 3-kinase and AKT results in phosphorylation of the serine/arginine-rich (SR) proteins 9G8 and SF2/ASF by AKT (Fig. 1). They find that this, in turn, modulates alternative splicing in the nucleus and enhances translation in the cytoplasm. This work not only sheds light on the mechanisms by which extracellular events can signal changes in gene expression in the nucleus, but also demonstrates that multiple steps in the gene expression pathway can be coordinately regulated by a common set of proteins.
Figure 1.
A model connecting signal transduction, alternative splicing and translation regulation. Extracellular ligands such as growth factors (GF) or insulin bind receptors that activate signaltransduction pathways involving Ras, PI 3-kinase (PI 3-K) and AKT. PI 3-kinase catalyzes the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), which binds and activates AKT. AKT, in turn, can phosphorylate SR proteins, which enhance translation in the cytoplasm and translocate to the nucleus, where they enhance the inclusion of alternatively spliced exons they bind to in precursor mRNA (pre-mRNA).
The SR protein family consists of a group of structurally and functionally related splicing factors with a diverse portfolio of activities5. Though they were originally isolated as proteins required for both constitutive and alternative splicing, it is now known that they also have important roles in mRNA export6,7, mRNA stability8, nonsensemediated decay9, genome stability10 and even translation11. In fact, one must seriously wonder whether there are any RNA processing events that do not involve SR proteins in one way or another.
SR proteins contain an N-terminal RNA-binding domain and a C-terminal domain rich in arginine and serine residues called the RS domain5. The RS domain has been shown to engage in both protein-protein interactions and protein-RNA interactions12. Moreover, the RS domain is extensively phosphorylated by a number of protein kinases, including SR protein kinases 1 and 2 (SRPK1 and SRPK2), Clk (also called Sty) and topoisomerase I (ref. 5). This phosphorylation can change both the protein- and RNA-interaction properties of the RS domain12.
In the new work, Blaustein et al.4 have examined the effect of cell signaling on alternative splicing using a reporter gene derived from fibronectin precursor mRNA. Fibronectin contains an exon called EDA that is included at low levels in the adult, but at high levels during embryogenesis as well as during wound healing and in certain tumors. Blaustein et al.4 performed a series of overexpression and siRNA knockdown experiments to demonstrate that growth factors activate the Ras–PI 3-kinase–AKT pathway and that this results in the inclusion of the EDA exon (Fig. 1). The authors also demonstrate that AKT directly phosphorylates the SR proteins 9G8 and SF2/ASF, which in turn bind the EDA exon and stimulate its inclusion. Recently, a separate group found that insulin activates PI 3-kinase and AKT, that AKT directly phosphorylates the SR protein SRp40 and that SRp40 binds and enhances the inclusion of an alternative exon in the PKCβII precursor mRNA13 (Fig. 1). Blaustein et al.4 also show that the signal-induced changes in alternative splicing can be transferred simply by placing a 20-nucleotide binding site for 9G8 in a heterologous alternative exon. Thus, this is a modular system, suggesting that there are likely to be many alternative exons in the genome that are responsive to this signaling pathway through 9G8, SRp40 and perhaps other SR proteins. Identifying these exons and determining whether they encode a set of proteins that function cooperatively to alter the physiology of a cell in accordance with the biological role of the signaling molecule will be of tremendous interest.
What sets the results of Blaustein et al.4 apart from previous studies is the discovery that phosphorylation of 9G8 and SF2/ASF by AKT not only enhances inclusion of the EDA exon but also enhances the translation of mRNAs containing the EDA exon (Fig. 1). This alone is not new because SR proteins have previously been shown to enhance translation: the ever promiscuous SR proteins have been found associated with translating ribosomes and can increase the translation of an mRNA when artificially tethered to it11. Rather, what is so striking is the finding that activating a single signal-transduction pathway acts at (at least) two different points to enhance the production of a specific protein. This will serve to increase both the speed and strength of the signaling response as measured by production of the induced protein. The response will be rapid because the translation of any preexisting mRNAs containing the alternative exon will be preferentially and immediately upregulated; it will be strong because more mRNAs containing the alternative exon will be produced and these will be translated more efficiently. This two-pronged strategy is reminiscent of signal-transduction cascades that use multiple sequentially activated kinases to amplify the speed and strength of a signal initiated by a ligand14. Coupling alternative splicing and translation would have a similar effect; regulating only one of these two steps alone would not be nearly as effective.
Though intriguing, these studies do raise several issues. First and foremost, the experiments reported by Blaustein et al.4 were all conducted with artificial reporters. Thus, it is imperative to determine whether these findings apply to endogenously expressed genes. If these results turn out to be generally true, many mechanistic questions should be addressed. Does AKT phosphorylation modulate the protein- or RNA-interaction properties of the SR proteins? Are the SR proteins that enhance translation in the cytoplasm the very same ones that activate splicing in the nucleus, or are there two pools of SR proteins that independently regulate translation and splicing? Also, are the SR proteins that activate splicing in the nucleus transported to the cytoplasm bound to the RNA? Despite the fact that these and other questions remain unanswered, these results provide evidence that the coupling between the different steps of the gene expression pathway is even more complicated than previously thought15.
References
- 1.Graveley BR. Trends Genet. 2001;17:100–107. doi: 10.1016/s0168-9525(00)02176-4. [DOI] [PubMed] [Google Scholar]
- 2.Matlin AJ, Clark F, Smith CW. Nat. Rev. Mol. Cell Biol. 2005;6:386–398. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- 3.Shin C, Manley JL. Nat. Rev. Mol. Cell Biol. 2004;5:727–738. doi: 10.1038/nrm1467. [DOI] [PubMed] [Google Scholar]
- 4.Blaustein, et al. Nat. Struct. Mol. Biol. 2005;12:1037–1044. doi: 10.1038/nsmb1020. [DOI] [PubMed] [Google Scholar]
- 5.Graveley BR. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Gattoni R, Stevenin J, Steitz JA. Mol. Cell. 2003;11:837–843. doi: 10.1016/s1097-2765(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Steitz JA. Mol. Cell. 2001;7:899–905. doi: 10.1016/s1097-2765(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 8.Lemaire R, et al. Genes Dev. 2002;16:594–607. doi: 10.1101/gad.939502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Krainer AR. Mol. Cell. 2004;16:597–607. doi: 10.1016/j.molcel.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Manley JL. Cell. 2005;122:365–378. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Sanford JR, Gray NK, Beckmann K, Caceres JF. Genes Dev. 2004;18:755–768. doi: 10.1101/gad.286404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hertel KJ, Graveley BR. Trends Biochem. Sci. 2005;30:115–118. doi: 10.1016/j.tibs.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Patel NA, et al. J. Biol. Chem. 2005;280:14302–14309. doi: 10.1074/jbc.M411485200. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich R, Neel BG, Rapoport TA. Mol. Cell. 2002;9:957–970. doi: 10.1016/s1097-2765(02)00528-2. [DOI] [PubMed] [Google Scholar]
- 15.Maniatis T, Reed R. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]