Abstract
Warfarin is a widely used anticoagulant that has a narrow therapeutic range because of both genetic and environmental factors. CYP2C9∗2 (p.R144C), CYP2C9∗3 (p.I359L), and the VKORC1 promoter (g.-1639G→A) polymorphisms occur frequently in patients who are warfarin “sensitive” and require lower doses, whereas patients with VKORC1 missense mutations are warfarin “resistant” and require higher doses. To compare the CYP2C9 and VKORC1 allele and genotype frequencies among 260 Ashkenazi (AJ) and 80 Sephardi Jewish (SJ) individuals, we genotyped six CYP2C9 and eight VKORC1 alleles by using the Tag-It Mutation Detection Kit and PCR-RFLP assays. The “sensitive” CYP2C9∗2 and ∗3 alleles had significantly higher frequencies in SJ than in AJ individuals, 0.194 and 0.144 versus 0.127 and 0.081, respectively (p ≤ 0.001). In contrast, the VKORC1 p.D36Y mutation, which predicts warfarin “resistance,” had a significantly higher frequency in AJ than in SJ individuals, 0.043 versus 0.006, respectively (p ≤ 0.025). Of note, 11.3% of AJ individuals predicted to be CYP2C9 extensive metabolizers and 8.7% of those predicted to be intermediate and poor metabolizers were VKORC1 p.D36Y carriers who require markedly higher warfarin doses. Thus, ∼10% of all AJ individuals would be misclassified when only genotyping CYP2C9∗2, ∗3, and VKORC1 g.-1639G→A, underscoring the importance of screening for p.D36Y prior to initiating warfarin anticoagulation in AJ individuals. Taken together, our findings show that ∼85% of AJ and ∼90% of SJ individuals have at least one “sensitive” (CYP2C9∗2, ∗3, VKORC1 g.-1639G→A) or “resistant” (VKORC1 p.D36Y) allele, indicating that each group has different warfarin pharmacogenetics and would benefit from genotype-based dose predictions.
Main Text
Warfarin is one of the most widely used anticoagulants, yet interindividual differences in drug response, a narrow therapeutic range, and a high risk of bleeding or stroke complicate its clinical use.1–3 The metabolism and anticoagulant action of warfarin presumably are moderated by many genes; however, the polymorphic cytochrome P450-2C9 (CYP2C9 [MIM 601130]) and vitamin K epoxide reductase complex subunit 1 (VKORC1 [MIM 608547]) genes alter warfarin pharmacokinetics and pharmacodynamics, respectively. In addition, environmental (e.g., vitamin K intake, comedications, age, and body-surface area) and other factors contribute to individual dose requirements, and together the currently identified genetic and environmental components account for approximately half of the interindividual warfarin dose variation.2,4–7 Importantly, dosing algorithms that incorporate several of these factors and the variant CYP2C9 and VKORC1 genotypes recently have been described.8,9
The variant CYP2C9∗2 (p.R114C) and ∗3 (p.I359L) alleles and the VKORC1 g.-1639G→A promoter polymorphism occur in patients who are drug “sensitive” and require a lower average warfarin dose,10,11 whereas VKORC1 missense mutations have been described in patients who are “resistant” and require higher warfarin doses.12–17 To facilitate the use of CYP2C9 and VKORC1 genotyping in routine anticoagulation practice, it is important to characterize the allele frequencies in various populations. Recently, we reported the CYP2C9, CYP2C19, and CYP2D6 allele and genotype frequencies in 250 healthy Ashkenazi Jewish (AJ) individuals.18 Here, we extend these studies to include the VKORC1 g.-1639G→A promoter polymorphism (rs9923231), previously reported missense mutations, p.V29L, p.A41S, p.V45A, p.R58G, p.V66M, and p.L128R, and the recently described warfarin-resistant p.D36Y missense mutation in a separate cohort of 260 AJ and 80 Sephardic Jewish (SJ) individuals.
Peripheral blood samples were obtained with informed consent from 592 unrelated healthy AJ and 80 SJ individuals from the greater New York metropolitan area. All personal identifiers were removed, the samples were tested anonymously, and genomic DNA was isolated with the Puregene DNA Purification kit (Gentra). Genotyping of six CYP2C9 alleles (∗1, ∗2, ∗3, ∗4, ∗5, and ∗6), and seven VKORC1 nucleotide variants (g.-1639G→A, g.85G→T [p.V29L], g.121G→T [p.A41S], g.134T→C [p.V45A], g.172A→G [p.R58G], g.1331G→A [p.V66M], and g.3487T→G [p.L128R]; Figure 1) was performed on 260 AJ and all 80 SJ individuals with the Tag-It Mutation Detection Kit (Luminex Molecular Diagnostics) according to the manufacturer's instructions. The CYP2C9 and VKORC1 genotypes for each sample were determined with Tag-It Data Analysis Software (Luminex Molecular Diagnostics), and the wild-type CYP2C9∗1 allele was assigned in the absence of other detectable variant alleles.7 The CYP2C9 allele designations refer to those defined by the Cytochrome P450 Allele Nomenclature Committee,19 and representative heterozygous samples for all identified alleles were confirmed by direct sequencing.
Figure 1.
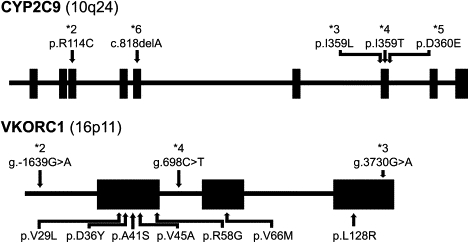
Schematic of the CYP2C9 and VKORC1 Genes
The locations of the nucleotide variations and missense mutations analyzed in this study are noted and are based on accession numbers M61857 and AY587020. The g.-1639G→A (rs9923231), g.3730G→A (rs7294), and g.698C→T (rs17708472) tag-SNPs for the VKORC1∗2,∗3, and ∗4 haplotypes correspond to 3673G→A, 9041G→A, and 6009C→T (accession number AY587020), respectively. Illustrations are not to scale.
The variant CYP2C9∗2, ∗3, ∗4, ∗5, and ∗6 alleles have been identified in individuals with impaired CYP2C9-mediated metabolism and lower warfarin dose requirements,4,20 yet only the ∗2 and ∗3 alleles were identified in our healthy AJ and SJ cohorts. When the CYP2C9 data from the 250 AJ individuals reported previously18 were combined with the results from the 260 AJ individuals studied here, the combined AJ allele frequencies (n = 510; Table 1) were distinct from those in Asian and African American populations and similar to those in other populations with European ancestry.21 In contrast, the SJ cohort had significantly higher (p ≤ 0.001) CYP2C9∗2 and ∗3 allele frequencies when compared to the AJ population, 0.194 and 0.144 versus 0.127 and 0.081, respectively (Table 1). On the basis of their genotypes, the assigned CYP2C9 metabolic phenotypes11 among the AJ and SJ were distributed as extensive, intermediate, and poor metabolizers (Table 2). Given the increased frequency of the deficient CYP2C9∗2 and ∗3 alleles in the SJ cohort, this population had significantly higher (p ≤ 0.002) frequencies of intermediate and poor metabolizers when compared to the AJ, 42.6% and 12.6% versus 32.4% and 4.7%, respectively (Table 2). Furthermore, the CYP2C9∗2 and ∗3 allele frequencies observed in our SJ cohort were considerably higher than those in other populations with European ancestry.20,22 Given that CYP2C9∗2 and ∗3 are associated with an increased risk of excessive anticoagulation and bleeding events among warfarin-treated patients,23,24 our results suggest that the SJ population is predisposed to warfarin sensitivity when compared to other populations with European ancestry.
Table 1.
CYP2C9 and VKORC1 Allele Frequencies
| AJa |
SJ |
|||||
|---|---|---|---|---|---|---|
| Allele | Alleles | Frequency | 95% CI | Alleles | Frequency | 95% CI |
| CYP2C9 | ||||||
| ∗1 | 806 | 0.790 | 0.765–0.815 | 106 | 0.663c | 0.589–0.763 |
| ∗2 | 130 | 0.127 | 0.107–0.148 | 31 | 0.194c | 0.133–0.255 |
| ∗3 | 83 | 0.081 | 0.065–0.098 | 23 | 0.144c | 0.089–0.198 |
| ∗5 | 1 | 0.001 | 0.000–0.003 | 0 | - | - |
| VKORC1b | ||||||
| g.-1639G | 277 | 0.533 | 0.490–0.576 | 80 | 0.500 | 0.423–0.577 |
| g.-1639A | 243 | 0.467 | 0.424–0.510 | 80 | 0.500 | 0.423–0.577 |
| g.106G | 1133 | 0.957 | 0.945–0.969 | 159 | 0.994d | 0.982–1.000 |
| g.106T (p.D36Y) | 51 | 0.043 | 0.031–0.055 | 1 | 0.006d | 0.000–0.018 |
Table 2.
CYP2C9 Genotype Frequencies
| AJa |
SJ |
|||
|---|---|---|---|---|
| Predicted Metabolizer Phenotype/Genotype | nb | Observed (Expectedc) Frequency (%) | nb | Observed (Expectedc) Frequency (%) |
| Extensive (EM) | ||||
| ∗1/∗1 | 321 | 62.8 (62.5) | 36 | 45.1d (43.9) |
| Intermediate (IM) | ||||
| ∗1/∗2 | 105 | 20.5 (20.1) | 23 | 28.8 (25.7) |
| ∗1/∗3 | 60 | 11.7 (12.8) | 11 | 13.8 (19.0) |
| ∗1/∗5 | 1 | 0.2 (0.2) | 0 | 0 (0.0) |
| Total | 166 | 32.4 (33.1) | 34 | 42.6d (44.7) |
| Poor (PM) | ||||
| ∗2/∗2 | 6 | 1.2 (1.6) | 1 | 1.3 (3.8) |
| ∗2/∗3 | 13 | 2.5 (2.1) | 6 | 7.5 (5.6) |
| ∗3/∗3 | 5 | 1.0 (0.7) | 3 | 3.8 (2.1) |
| Total | 24 | 4.7 (4.4) | 10 | 12.6d (11.5) |
Combined AJ CYP2C9 data with Scott et al.18
Number of subjects.
Predicted Hardy-Weinberg frequencies.
p ≤ 0.002.
Warfarin exerts its anticoagulant effect by inhibiting vitamin K epoxide reductase, the catalytic subunit of which is encoded by VKORC1, thereby preventing the regeneration of vitamin K from vitamin K epoxide. After the identification of VKORC1 in 2004,12,13 common VKORC1 polymorphisms and haplotypes that are strongly associated with warfarin response were reported.4,25,26 Although the molecular mechanisms by which VKORC1 polymorphisms modulate warfarin response remain unclear, recent evidence suggests that the g.-1639G→A promoter polymorphism reduces hepatic VKORC1 expression and, therefore, decreases warfarin dose requirements.10,26 The g.-1639G promoter allele is present in the VKORC1∗1, ∗3, and ∗4 haplotypes and is typically associated with a “normal” warfarin dose.27,28 In contrast, the g.-1639A promoter allele, which is in strong linkage disequilibrium with 6484C→T (rs9934438), 6853G→C (rs8050894), and 7566C→T (rs2359612; accession number AY587020),27 is present in the VKORC1∗2 haplotype17,27–29 and is consistently associated with warfarin-“sensitive” individuals who require lower warfarin doses.6,7,9,30,31 Unlike African Americans and similar to other individuals of European descent,27 the g.-1639A allele was present at high frequencies in both our AJ (0.467) and SJ (0.500) cohorts (Table 1). Similar G/G, G/A, and A/A genotype frequencies were observed in the two Jewish subpopulations (Table 3), and they were comparable to those reported in other individuals of European descent.26,27 Interestingly, haplotype analysis based on the PERLEGEN SNP public database indicates that the g.-1639A allele is predominant in the Chinese population (0.958),27 and such a finding is consistent with the lower warfarin dose requirements typically observed in this group.32
Table 3.
VKORC1 g.-1639G→A Genotype Frequencies
| AJ |
SJ |
|||
|---|---|---|---|---|
| Genotype | na | Observed (Expectedb) Frequency (%) | na | Observed (Expectedb) Frequency (%) |
| g.-1639G→A | ||||
| G/G | 76 | 29.2 (28.4) | 20 | 24.1 (26.2) |
| G/A | 125 | 48.1 (49.8) | 45 | 54.2 (50.0) |
| A/A | 59 | 22.7 (21.8) | 18 | 21.7 (23.8) |
Number of subjects.
Predicted Hardy-Weinberg frequencies.
Heterozygous VKORC1 missense mutations have been identified in individuals who are resistant to warfarin and homozygous mutations have been reported in families with combined deficiency of vitamin-K-dependent clotting factors type 2 (MIM 607473).13,14 Although other nongenetic factors can lead to increased warfarin doses (e.g., excessive vitamin K intake, drug interactions, etc.), heterozygous VKORC1 missense mutations alter vitamin K epoxide reductase activity and, therefore, play a considerable role in the warfarin-“resistant” phenotype.13,14,16 However, these mutations presumably are rare given that p.V29L, p.V45A, p.R58G, and p.L128R were not identified in 384 healthy non-Jewish control chromosomes.13 Moreover, none of these and other (p.A41S and p.V66M) missense mutations were identified among the healthy AJ and SJ individuals in our study.
The p.D36Y VKORC1 mutation was recently identified in individuals who required an average warfarin dose greater than 10 mg/day.17 This missense mutation is located in the conserved lumenal loop (L1) VKORC1 region that also contains p.V29L, p.A41S, p.V45A, p.R58G, and p.V66M (Figure 1).13,33 For an analysis of VKORC1 p.D36Y in our AJ and SJ cohorts, a PCR-RFLP assay was employed17 with sense (5′-AACCTGGAGATAATGGGCAGCA-3′) and antisense (5′-ACACCGATCCCAGACTCCAGAATA-3′) PCR primers and RsaI (New England BioLabs). The frequency of p.D36Y in 100 AJ and 100 East African individuals was 0.040 and 0.150, respectively,17 and it had a comparable allele frequency of 0.043 in our larger AJ cohort (n = 592), and this was significantly higher than that observed among the SJ individuals (0.006; Table 1). The high carrier frequency of p.D36Y in the AJ (1 in 12) indicates that this population may be predisposed to warfarin resistance.
Table 4 summarizes the combined CYP2C9 and VKORC1 genotype frequencies for the 260 AJ and 80 SJ cohorts, which were in Hardy-Weinberg equilibrium. Predicted CYP2C9 metabolizer phenotypes11 for both the AJ and SJ were subdivided on the basis of VKORC1 g.-1639G→A status and the presence of the p.D36Y warfarin-resistant mutation. Although the VKORC1 phase could not be determined in our study, it is notable that no p.D36Y carriers were identified in g.-1639A/A (VKORC1∗2/∗2) individuals, suggesting that p.D36Y occurs on a g.-1639G background (Table 4). Given that the VKORC1∗3 and ∗4 haplotypes, which are associated with “normal” warfarin dose requirements,28 also occur on a g.-1639G background,27 additional PCR-RFLP genotyping to identify these alleles was performed on all p.D36Y carriers. Tag-SNPs 9041G→A (rs7294; g.3730G→A) and 6009C→T (rs17708472; g.698C→T; accession number AY587020; Figure 1)27 were interrogated for identification of VKORC1∗3 and ∗4 with sense (VKORC1∗3: 5′-TTTGCTTTGGCATGTGAGCCTTGC-3′; VKORC1∗4: 5′-GCATAATGACGGAATACAGAGGAGGC-3′) and antisense (VKORC1∗3: 5′-ACAGTCCATGGCAGACACATGGTT-3′; VKORC1∗4: 5′-GGTAGAGACAGGCTTTCACCATGT-3′) PCR primers and AciI and BfaI (New England BioLabs), respectively. The VKORC1∗1 haplotype was assigned in the absence of other detectable alleles, and representative p.D36Y, VKORC1∗3, and ∗4 positive samples were confirmed by direct sequencing. Interestingly, all the p.D36Y heterozygous individuals evaluated in our AJ and SJ cohorts were heterozygotes for VKORC1∗1 and ∗2, ∗3, or ∗4, and importantly, one AJ individual was identified who was homozygous for both p.D36Y and VKORC1∗1 (data not shown). Thus, our findings strongly support the hypothesis that p.D36Y tags a unique haplotype found on a VKORC1∗1 background.17
Table 4.
CYP2C9 and VKORC1 Combined Genotype Frequencies
| AJ |
SJ |
||||||
|---|---|---|---|---|---|---|---|
| CYP2C9 Genotype (Predicted Metabolizer Phenotypea) | VKORC1 g.-1639G→A | nb | Frequency (%) | No. of p.D36Y Carriers | nb | Frequency (%) | No. of p.D36Y Carriers |
| ∗1/∗1 (EM) | |||||||
| G/G | 49 | 18.8 | 9c | 9 | 11.3 | 1 | |
| G/A | 81 | 31.2 | 10 | 14 | 17.5 | 0 | |
| A/A | 38 | 14.6 | 0 | 13 | 16.3 | 0 | |
| ∗1/∗2 or ∗1/∗3 (IM) | |||||||
| G/G | 26 | 10.0 | 2 | 11 | 13.8 | 0 | |
| G/A | 38 | 14.6 | 3 | 17 | 21.3 | 0 | |
| A/A | 20 | 7.7 | 0 | 6 | 7.5 | 0 | |
| ∗2/∗2, ∗2/∗3, or ∗3/∗3 (PM) | |||||||
| G/G | 1 | 0.4 | 1 | 2 | 2.5 | 0 | |
| G/A | 6 | 2.3 | 2 | 5 | 6.3 | 0 | |
| A/A | 1 | 0.4 | 0 | 3 | 3.8 | 0 | |
EM, extensive metabolizer; IM, intermediate metabolizer; and PM, poor metabolizer.
Number of subjects.
One p.D36Y homozygote was found in this group.
Notably, patients with p.D36Y who have dose-reducing variant CYP2C9∗2, ∗3, or VKORC1∗2 alleles still require higher warfarin doses (>10 mg/day), indicating that this missense mutation is “dominant” when present in individuals with warfarin-“sensitive” CYP2C9 and VKORC1 alleles.17 Importantly, 11.3% of AJ individuals predicted to be CYP2C9 extensive metabolizers and 8.7% of those predicted to be intermediate and poor metabolizers were VKORC1 p.D36Y carriers who would require markedly higher warfarin doses (Table 4). Thus, ∼10% of all AJ individuals would be misclassified when only genotyping CYP2C9∗2, ∗3 and VKORC1 g.-1639G→A, thereby underscoring the importance of screening for p.D36Y prior to initiating warfarin anticoagulation in AJ individuals.
The recently reported warfarin dosing algorithms that incorporate genotype information only include CYP2C9∗2, ∗3, and VKORC1 g.-1639G→A.8,9 On the basis of these dosing guidelines and given that 211 AJ and 71 SJ individuals had at least one variant CYP2C9 (∗2 and ∗3) or VKORC1 (g.-1639G→A) allele, 81.2% of AJ and 88.8% of SJ individuals would be candidates for genotype-based dose adjustments (Table 4). The high frequency of variant CYP2C9 and VKORC1 alleles in AJ and SJ individuals indicates that clinical testing of these genes is warranted among individuals initiating warfarin therapy, as recently recommended by a Food and Drug Administration Advisory Committee (see Web Resources). Moreover, warfarin-“resistant” VKORC1 missense mutations are currently not incorporated into published dosing algorithms, yet their identification prior to warfarin administration may reduce complications because of ineffective anticoagulation. By including p.D36Y in our analysis, 84.6% of AJ and 90.0% of SJ individuals would benefit from CYP2C9 and VKORC1 genotyping prior to initiating warfarin-based anticoagulation. Further studies are necessary to correlate warfarin dose requirements in patients with the p.D36Y allele and various other CYP2C9 and VKORC1 alleles. Such information is required to accurately model the contribution of p.D36Y to warfarin dose for inclusion in warfarin dosing algorithms for the AJ population.
Acknowledgments
This research was supported in part by a research grant (5 R01 DK26824) and a grant (5 M01 RR00071) from the Division of Research Resources for the Mount Sinai General Clinical Research Center, both from the National Institutes of Health, and by a research grant from Luminex Molecular Diagnostics. S.A.S. is the recipient of a Biochemical/Molecular Genetics Fellowship from the Genzyme Corporation.
Web Resources
The URLs for data presented herein are as follows:
Online Mendelian Inheritance in Man (OMIM), http://ncbi.nlm.nih.gov/Omim/ (for CYP2C9, VKORC1, and combined deficiency of vitamin-K-dependent clotting factors type 2)
U.S. Food and Drug Administration, http://www.fda.gov/cder/drug/infopage/warfarin/
WarfarinDosing.org, http://warfarindosing.org
References
- 1.El Rouby S., Mestres C.A., LaDuca F.M., Zucker M.L. Racial and ethnic differences in warfarin response. J. Heart Valve Dis. 2004;13:15–21. [PubMed] [Google Scholar]
- 2.Kamali F. Genetic influences on the response to warfarin. Curr. Opin. Hematol. 2006;13:357–361. doi: 10.1097/01.moh.0000239708.70792.4f. [DOI] [PubMed] [Google Scholar]
- 3.Sconce E.A., Kamali F. Appraisal of current vitamin K dosing algorithms for the reversal of over-anticoagulation with warfarin: The need for a more tailored dosing regimen. Eur. J. Haematol. 2006;77:457–462. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2957.x. [DOI] [PubMed] [Google Scholar]
- 4.Wadelius M., Pirmohamed M. Pharmacogenetics of warfarin: Current status and future challenges. Pharmacogenomics J. 2007;7:99–111. doi: 10.1038/sj.tpj.6500417. [DOI] [PubMed] [Google Scholar]
- 5.Carlquist J.F., Horne B.D., Muhlestein J.B., Lappe D.L., Whiting B.M., Kolek M.J., Clarke J.L., James B.C., Anderson J.L. Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: A prospective study. J. Thromb. Thrombolysis. 2006;22:191–197. doi: 10.1007/s11239-006-9030-7. [DOI] [PubMed] [Google Scholar]
- 6.Yin T., Miyata T. Warfarin dose and the pharmacogenomics of CYP2C9 and VKORC1 - Rationale and perspectives. Thromb. Res. 2007;120:1–10. doi: 10.1016/j.thromres.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y., Shennan M., Reynolds K.K., Johnson N.A., Herrnberger M.R., Valdes R., Jr., Linder M.W. Estimation of warfarin maintenance dose based on VKORC1 (−1639 G→A) and CYP2C9 genotypes. Clin. Chem. 2007;53:1199–1205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 8.Tham L.S., Goh B.C., Nafziger A., Guo J.Y., Wang L.Z., Soong R., Lee S.C. A warfarin-dosing model in Asians that uses single-nucleotide polymorphisms in vitamin K epoxide reductase complex and cytochrome P450 2C9. Clin. Pharmacol. Ther. 2006;80:346–355. doi: 10.1016/j.clpt.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Sconce E.A., Khan T.I., Wynne H.A., Avery P., Monkhouse L., King B.P., Wood P., Kesteven P., Daly A.K., Kamali F. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: Proposal for a new dosing regimen. Blood. 2005;106:2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 10.Yuan H.Y., Chen J.J., Lee M.T., Wung J.C., Chen Y.F., Charng M.J., Lu M.J., Hung C.R., Wei C.Y., Chen C.H. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum. Mol. Genet. 2005;14:1745–1751. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 11.Kirchheiner J., Brockmoller J. Clinical consequences of cytochrome P450 2C9 polymorphisms. Clin. Pharmacol. Ther. 2005;77:1–16. doi: 10.1016/j.clpt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Li T., Chang C.Y., Jin D.Y., Lin P.J., Khvorova A., Stafford D.W. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427:541–544. doi: 10.1038/nature02254. [DOI] [PubMed] [Google Scholar]
- 13.Rost S., Fregin A., Ivaskevicius V., Conzelmann E., Hortnagel K., Pelz H.J., Lappegard K., Seifried E., Scharrer I., Tuddenham E.G. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 14.Harrington D.J., Underwood S., Morse C., Shearer M.J., Tuddenham E.G., Mumford A.D. Pharmacodynamic resistance to warfarin associated with a Val66Met substitution in vitamin K epoxide reductase complex subunit 1. Thromb. Haemost. 2005;93:23–26. doi: 10.1160/TH04-08-0540. [DOI] [PubMed] [Google Scholar]
- 15.D'Ambrosio R.L., D'Andrea G., Cafolla A., Faillace F., Margaglione M. A new vitamin K epoxide reductase complex subunit-1 (VKORC1) mutation in a patient with decreased stability of CYP2C9 enzyme. J. Thromb. Haemost. 2007;5:191–193. doi: 10.1111/j.1538-7836.2006.02261.x. [DOI] [PubMed] [Google Scholar]
- 16.Bodin L., Horellou M.H., Flaujac C., Loriot M.A., Samama M.M. A vitamin K epoxide reductase complex subunit-1 (VKORC1) mutation in a patient with vitamin K antagonist resistance. J. Thromb. Haemost. 2005;3:1533–1535. doi: 10.1111/j.1538-7836.2005.01449.x. [DOI] [PubMed] [Google Scholar]
- 17.Loebstein R., Dvoskin I., Halkin H., Vecsler M., Lubetsky A., Rechavi G., Amariglio N., Cohen Y., Ken-Dror G., Almog S. A coding VKORC1 Asp36Tyr polymorphism predisposes to warfarin resistance. Blood. 2007;109:2477–2480. doi: 10.1182/blood-2006-08-038984. [DOI] [PubMed] [Google Scholar]
- 18.Scott S.A., Edelmann L., Kornreich R., Erazo M., Desnick R.J. CYP2C9, CYP2C19 and CYP2D6 allele frequencies in the Ashkenazi Jewish population. Pharmacogenomics. 2007;8:721–730. doi: 10.2217/14622416.8.7.721. [DOI] [PubMed] [Google Scholar]
- 19.Sim S.C., Ingelman-Sundberg M. The human cytochrome P450 Allele Nomenclature Committee Web site: Submission criteria, procedures, and objectives. Methods Mol. Biol. 2006;320:183–191. doi: 10.1385/1-59259-998-2:183. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz U.I. Clinical relevance of genetic polymorphisms in the human CYP2C9 gene. Eur. J. Clin. Invest. 2003;33(Suppl 2):23–30. doi: 10.1046/j.1365-2362.33.s2.6.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee C.R., Goldstein J.A., Pieper J.A. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12:251–263. doi: 10.1097/00008571-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Xie H.G., Prasad H.C., Kim R.B., Stein C.M. CYP2C9 allelic variants: Ethnic distribution and functional significance. Adv. Drug Deliv. Rev. 2002;54:1257–1270. doi: 10.1016/s0169-409x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 23.Visser L.E., van Schaik R.H., van Vliet M., Trienekens P.H., De Smet P.A., Vulto A.G., Hofman A., van Duijn C.M., Stricker B.H. The risk of bleeding complications in patients with cytochrome P450 CYP2C9∗2 or CYP2C9∗3 alleles on acenocoumarol or phenprocoumon. Thromb. Haemost. 2004;92:61–66. doi: 10.1160/TH03-12-0741. [DOI] [PubMed] [Google Scholar]
- 24.Higashi M.K., Veenstra D.L., Kondo L.M., Wittkowsky A.K., Srinouanprachanh S.L., Farin F.M., Rettie A.E. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–1698. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 25.D'Andrea G., D'Ambrosio R.L., Di Perna P., Chetta M., Santacroce R., Brancaccio V., Grandone E., Margaglione M. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–649. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 26.Rieder M.J., Reiner A.P., Gage B.F., Nickerson D.A., Eby C.S., McLeod H.L., Blough D.K., Thummel K.E., Veenstra D.L., Rettie A.E. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 27.Geisen C., Watzka M., Sittinger K., Steffens M., Daugela L., Seifried E., Muller C.R., Wienker T.F., Oldenburg J. VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb. Haemost. 2005;94:773–779. doi: 10.1160/TH05-04-0290. [DOI] [PubMed] [Google Scholar]
- 28.Osman A., Enstrom C., Arbring K., Soderkvist P., Lindahl T.L. Main haplotypes and mutational analysis of vitamin K epoxide reductase (VKORC1) in a Swedish population: A retrospective analysis of case records. J. Thromb. Haemost. 2006;4:1723–1729. doi: 10.1111/j.1538-7836.2006.02039.x. [DOI] [PubMed] [Google Scholar]
- 29.Gage B.F. Pharmacogenetics-based coumarin therapy. Hematology (Am Soc Hematol Educ Program) 2006:467–473. doi: 10.1182/asheducation-2006.1.467. [DOI] [PubMed] [Google Scholar]
- 30.Montes R., Ruiz de Gaona E., Martinez-Gonzalez M.A., Alberca I., Hermida J. The c.-1639G→A polymorphism of the VKORC1 gene is a major determinant of the response to acenocoumarol in anticoagulated patients. Br. J. Haematol. 2006;133:183–187. doi: 10.1111/j.1365-2141.2006.06007.x. [DOI] [PubMed] [Google Scholar]
- 31.Aquilante C.L., Langaee T.Y., Lopez L.M., Yarandi H.N., Tromberg J.S., Mohuczy D., Gaston K.L., Waddell C.D., Chirico M.J., Johnson J.A. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin. Pharmacol. Ther. 2006;79:291–302. doi: 10.1016/j.clpt.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Gan G.G., Teh A., Goh K.Y., Chong H.T., Pang K.W. Racial background is a determinant factor in the maintenance dosage of warfarin. Int. J. Hematol. 2003;78:84–86. doi: 10.1007/BF02983247. [DOI] [PubMed] [Google Scholar]
- 33.Goodstadt L., Ponting C.P. Vitamin K epoxide reductase: Homology, active site and catalytic mechanism. Trends Biochem. Sci. 2004;29:289–292. doi: 10.1016/j.tibs.2004.04.004. [DOI] [PubMed] [Google Scholar]


