Abstract
The chi63 promoter directs glucose-sensitive, chitin-dependent transcription of a gene involved in the utilization of chitin as carbon source. Analysis of 5′ and 3′ deletions of the promoter region revealed that a 350-bp segment is sufficient for wild-type levels of expression and regulation. The analysis of single base changes throughout the promoter region, introduced by random and site-directed mutagenesis, identified several sequences to be important for activity and regulation. Single base changes at −10, −12, −32, −33, −35, and −37 upstream of the transcription start site resulted in loss of activity from the promoter, suggesting that bases in these positions are important for RNA polymerase interaction. The sequences centered around −10 (TATTCT) and −35 (TTGACC) in this promoter are, in fact, prototypical of eubacterial promoters. Overlapping the RNA polymerase binding site is a perfect 12-bp direct repeat sequence. Some base changes within this direct repeat resulted in constitutive expression, suggesting that this sequence is an operator for negative regulation. Other base changes resulted in loss of glucose repression while retaining the requirement for chitin induction, suggesting that this sequence is also involved in glucose repression. The fact that cis-acting mutations resulted in glucose resistance but not inducer independence rules out the possibility that glucose repression acts exclusively by inducer exclusion. The fact that mutations that affect glucose repression and chitin induction fall within the same direct repeat sequence module suggests that the direct repeat sequence facilitates both chitin induction and glucose repression.
Keywords: transcriptional regulation, catabolite control, carbohydrate utilization
The regulation of carbon source utilization is an important aspect of the physiology of all microorganisms. The ability to recognize the presence of rapidly metabolizable carbon sources and regulate the synthesis of enzymes involved in the utilization of more complex carbohydrates requires the coordinated activation and repression of sets of genes. Although catabolite control of gene expression is common to most bacteria, the mechanisms employed to accomplish gene regulation at the molecular level are fundamentally different.
In Streptomyces, the regulation of carbon utilization is central to the most interesting and important aspects of its biology. It is clear from the study of mutants blocked in the initiation of development that there is a direct connection between carbon-source catabolite repression, activation of antibiotic biosynthesis, and morphological development, especially those aspects of morphological development that involve cell–cell signaling (1). Although relatively little is known about the mechanism of catabolite repression in Streptomyces, there are important differences in the regulation of carbon utilization between Streptomyces and other bacteria. In contrast to Escherichia coli (2, 3), cAMP does not play a regulatory role in catabolite repression in Streptomyces coelicolor. Levels of cAMP do not change with changes in carbon source utilization in either S. coelicolor (4) or Streptomyces venezuelae (5), and null mutations in adenylate cyclase have no apparent effect on glucose repression (Charles Thompson, personal communication). In contrast to Bacillus, there is no detectable serine phosphorylation of HPr in Streptomyces (6, 7), so if the phosphotransferase system is involved in catabolite control in Streptomyces it may be by a novel mechanism. Instead, glucose kinase is required for the control of most (8–12) but not all (13) catabolite-controlled genes. Glucose repression of the gyl (11, 14), dag (15, 16), and aml (12, 17) promoters has been shown to be dependent on glucose kinase activity. Mutations in the glucose kinase gene of S. coelicolor result in the inability to utilize glucose as carbon source and in the loss of catabolite repression of some genes but have no effect on glucose transport (4). Furthermore, glucose kinase genes from other organisms or a normally cryptic glucose kinase gene of S. coelicolor are able to restore glucose utilization but not glucose repression to an S. coelicolor glkA mutant (9). Angell et al. (8) and Kwakman and Postma (18) have suggested that glucose kinase in Streptomyces may, in fact, function in a way that is similar to that of Hexokinase II in Saccharomyces cerevisiae (19–22). The involvement of glucose kinase in glucose repression has also recently been reported in Staphylococcus xylosus (23, 24). Taken together, these observations suggest that glucose kinase may be involved in a mechanism of catabolite control that exists in all Gram-positive bacteria.
Relatively few catabolite-controlled genes have been cloned and characterized from Streptomyces, and even fewer have been examined at the level of transcription initiation. The agarase gene, dagA, has four promoters (15) and is transcribed by at least three different RNA polymerase holoenzymes (16). The galP1 promoter, which directs glucose-sensitive, galactose-dependent transcription of the Streptomyces galactose utilization operon, contains an unusual RNA polymerase binding site and is apparently transcribed by a new form of RNA polymerase holoenzyme (25). Mutational analysis of the promoter region of galP1 also revealed a complex operator that consists of hexamer and direct repeat sequences that overlap the RNA polymerase binding site (26).
Of particular interest to this study is the analysis of the glycerol and maltose utilization operons of S. coelicolor. The gylCABX operon is transcribed from two promoters, both of which are glucose-sensitive and glycerol-dependent (11, 14, 27, 28) although to different extents (28). A repressor gene, gylR, has been identified and partially characterized (27–29). Hindle and Smith (29) showed, somewhat surprisingly, that null mutations in the gylR gene relieved glucose repression of both gylR and the gylCABX operon. Their data suggested that both substrate induction and glucose repression are mediated through the GylR protein. In recent work, Van Wezel et al. (30) have shown that transcription of the malE gene, which is part of the malEFG operon required for maltose utilization, is repressed by the product of the malR gene. Disruption or deletion of malR resulted in glucose-resistant expression of malE, indicating that this repressor plays a role in glucose repression as well as maltose induction. Both of these studies have relied on mutations in the repressor genes to suggest a role for the repressor proteins in glucose repression.
In this study we report an analysis of cis-acting mutations in an operator that define it as the site of glucose repression as well as substrate induction. Transcription from chi63 is chitin-dependent and glucose-sensitive (13, 31, 32). In previous work we identified a transcription start site for this gene and showed that a direct repeat sequence upstream of the transcription start site is involved in regulation (13). A point mutation within the direct repeat sequence resulted in glucose-resistant, chitin-independent expression from chi63 in vivo (13). In electrophoretic gel mobility shift assays, we detected a protein in crude extracts from S. coelicolor that binds the direct repeat sequences specifically (13). In the analysis reported here, deletions of the promoter region were constructed, and a 350-bp region containing the transcription start site and the direct repeat sequence was shown to be sufficient for wild-type expression and regulation of chi63. The introduction of single base changes throughout the promoter region by random and site-directed mutagenesis identified an apparent RNA polymerase binding site prototypical of eubacterial promoters (TTGACC at −35 and TATTCT at −10) and a simple operator that apparently directs both glucose repression and chitin induction. We suggest that our work and that of Hindle and Smith (29) and Wezel et al. (30) provide evidence that glucose repression of some catabolite-controlled genes in Streptomyces may act through the same repressor proteins and operator sequences that are involved in substrate induction.
MATERIALS AND METHODS
Bacterial Strains.
Streptomyces lividans 1326 (33) was used for analysis of wild-type and mutant chi63 derivatives. RNA for primer extension analysis was prepared from S. lividans containing wild-type and mutant derivatives of the chi63 promoter. E. coli strain DH5αF′ (34) was used for propagation of plasmids and M13 phage. E. coli BW313 (34) was used to prepare uracil-substituted single-stranded DNA for mutagenesis. Manipulations of Streptomyces (33) and E. coli were as described (34).
Construction of Deletion Derivatives of the chi63 Promoter.
A 850-bp HindIII–BamHI fragment from pXE60 (13) containing the chi63 promoter was cloned into M13mp18. Oligonucleotide-directed mutagenesis was used to engineer restriction sites at various positions within the promoter fragment to generate deletions. For upstream deletions with respect to the transcription start site, a HindIII site was introduced, and for downstream deletions a BamHI site was introduced to allow directional subcloning of the promoter-containing fragments to generate transcriptional fusions to the xylE reporter gene. M13mp18 recombinant phages containing mutant derivatives of chi63 were sequenced using the Sequenase kit from United States Biochemical to verify constructions.
Oligonucleotide-Directed Mutagenesis.
A 560-bp HindIII–BamHI fragment containing the chi63 promoter was used as a substrate for mutagenesis. For random mutagenesis, two oligonucleotides were synthesized: a 37-bp fragment corresponding to the sequence from −50 to −14 and a 33-bp fragment corresponding to the sequence from −37 to −5 with respect to the transcription start site. Oligonucleotides were synthesized with 1.7% contamination of each of the other nucleotides at each position. For oligonucleotide-directed, site-specific mutagenesis, oligonucleotides were synthesized with specific base changes. Incorporation of mutagenized oligonucleotides was performed according to the method of Kunkel and colleagues (35, 36). The pool of mutagenized promoter-containing fragments was subcloned into pXE3 upstream of the xylE reporter gene. Base changes that resulted in altered expression of chi63 were detected using catechol dioxygenase plate assays.
Catechol Dioxygenase Assays.
Both qualitative and quantitative catechol dioxygenase assays were performed as previously described (13, 37). For plate assays, S. lividans strains were grown in modified minimal medium containing either 1% glucose, chitin, or chitin plus glucose as sole carbon source for 48 hr at 30°C and then sprayed with a 1% (wt/vol) catechol solution. Plates were inspected within 5–10 min for color change. For quantitative assays, S. lividans strains were grown in minimal medium containing 1% glycerol for 28 hr at 30°C. Mycelia were collected by centrifugation, washed twice with 10 ml minimal medium containing no carbon source, then resuspended in 5 ml minimal medium, and aliquots were distributed into minimal medium containing 1% glycerol, glucose, chitin, or glucose plus chitin and incubated another 16 hr at 30°C.
Primer Extension Analysis.
RNA for primer extension analysis was isolated from cultures grown as for catechol dioxygenase assays. RNA isolation from Streptomyces (33) for primer extension analysis (13) was done as previously described. The primer used for the extension reaction, 5′-GTCAGGCTAGGACCAGGTCC-3′, was complementary to the 5′ end of the xylE-containing fragment and did not hybridize with chromosomal DNA. Transcripts detected in this way were specific for RNA generated from the chi63-xylE fusion.
RESULTS
A 350-bp Region of the chi63 Promoter Is Sufficient for Activity and Regulation.
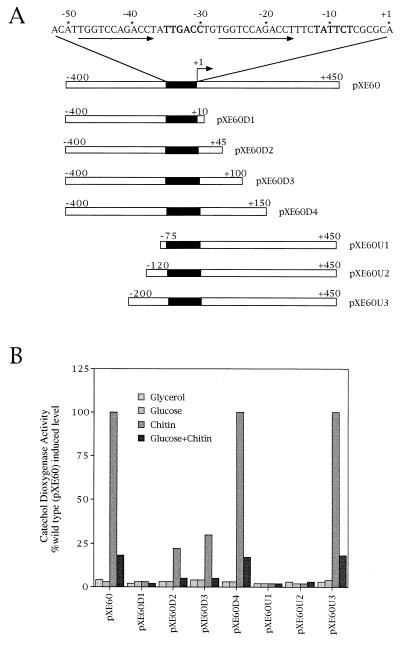
To identify the DNA region of chi63 required for expression and regulation, a series of deletions 5′ and 3′ of the transcription start site was constructed (Fig. 1A). The modified promoter-containing fragments were cloned upstream of the xylE reporter gene, and quantitative catechol dioxygenase assays were performed (Fig. 1B). Deletions to +150 or −200 had no effect on expression or regulation of chi63. Deletions to +45 showed decreased expression but retained glucose repression and chitin induction. Deletions to +10 or −120 resulted in the complete loss of expression from chi63. The results of this analysis indicate that the DNA sequence from −200 to +150, with respect to the transcription start site, is sufficient for regulation and wild-type levels of expression from the promoter.
Figure 1.
Analysis of deletions of the chi63 promoter region. (A) DNA sequence of the promoter region containing the RNA polymerase binding site (bold letters) and direct repeat sequences (underlined with arrows) and diagrams of deletions of an 850-bp region containing the transcription start site (indicated as +1). (B) Histogram showing the results of quantitative catechol dioxygenase assays plotted as a percentage of the fully induced wild-type level from S. lividans-containing plasmids bearing chi63-xylE transcriptional fusions. pXE60D1, pXE60D2, pXE60D3, pXE60D4, pXE60U1, pXE60U2, and pXE60U3 are pXE60 derivatives that contain different truncated versions of chi63. Promoter-containing plasmids indicated in B refer to the deletions shown in A. Each plasmid-containing strain was assayed after growth on the carbon source indicated.
Uninducible Mutations Define Sequences Important for Promoter Activity.
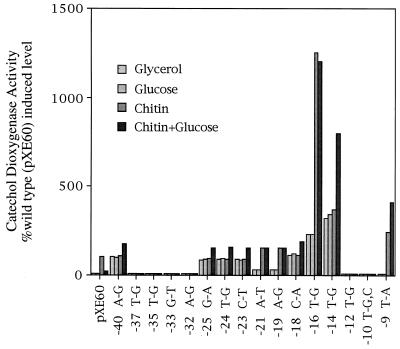
Single base changes that result in loss of promoter activity most likely identify bases important for RNA polymerase recognition and interaction. As shown in Fig. 2, a T-to-G transversion or T-to-C transition at −10, a T-to-G transversion at −12, an A-to-G transition at −32, a G-to-T transversion at −33, a T-to-G transversion at −35, or a T-to-G transversion at −37 resulted in complete loss of promoter activity. All of these changes are centered around −10 and −35 base pairs upstream of the transcription start site, positions where RNA polymerase would be expected to make contact.
Figure 2.
Analysis of point mutations in the chi63 promoter region. This histogram shows the results of catechol dioxygenase assays plotted as a percentage of fully induced wild type from S. lividans containing plasmids with chi63-xylE transcriptional fusions. Promoter mutations are labeled as to the position upstream of the transcription start site and the base change observed. Each plasmid-containing strain was assayed after growth on the carbon source indicated.
Mutations Within a 12-bp Direct Repeat Sequence Affect both Chitin Induction and Glucose Repression.
In previous work (13), a C-to-A transversion 18 bp upstream of the transcription start site and within the 12-bp direct repeat sequence resulted in glucose-resistant, chitin-independent expression, suggesting that the direct repeat sequences serve to bind a negative regulator of transcription in the way classical operators function to modulate repression. As shown in Fig. 2, base changes at −16, −18, −23, −24, −25, and −40 resulted in a constitutive phenotype. These mutations identify the direct repeat sequences as a site for negative regulation.
Surprisingly, base changes at −19 and −21, also within the direct repeat sequences (Fig. 2), resulted in glucose-resistant expression but retained the requirement for chitin induction. These mutations did not affect the level of expression of the promoter. A T-to-G transversion at −16 also relieved glucose repression without affecting the requirement for chitin induction, but this change also had a dramatic effect on expression. The existence of these mutations suggests that the direct repeat sequences are also directly involved in glucose repression. The fact that mutations that give rise to these two different phenotypes are dispersed throughout the same direct repeat sequence suggests that the same sequences facilitate both chitin induction and glucose repression.
Of special interest is a base change located outside the direct repeat sequence and within the −10 region that relieves glucose repression. A T-to-A transversion at −9 resulted in glucose-resistant, chitin-dependent expression. Two additional base changes, a T-to-G transversion at −14, which resulted in elevated expression, and a T-to-G transversion at −16, which affected expression and regulation, are located at the 3′ end of the direct repeat sequence and immediately adjacent to or within the −10 region of the promoter. Their dual effect on regulation and promoter activity suggests the possibility of an interaction between RNA polymerase and the transcription factor that interacts with the direct repeat sequences.
Deregulated Expression from chi63 Originates at the Same Transcription Start Site as That of the Wild Type.
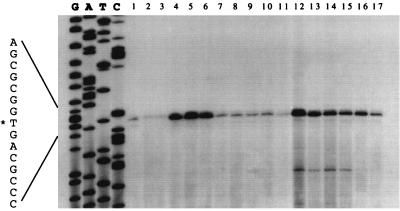
Because point mutations within the promoter region that affect regulation may also affect a variety of interactions, including RNA polymerase recognition and binding, it was important to establish the site of transcription initiation in these mutants. It was particularly important to examine the promoter mutations that resulted in dramatic overexpression, such as the changes at −16 or −14. As shown in Fig. 3, in all cases, transcription from the mutant chi63 promoters originated from the wild-type start site. We conclude from these data that the base changes that affected regulation of chi63 do not grossly alter the way in which RNA polymerase recognizes the promoter region to initiate transcription.
Figure 3.
Primer extension analysis of mutant chi63-directed transcripts. The 5′ termini of transcripts were identified using a radiolabeled primer complementary to the RNA sequence 150 bp downstream of the transcription start site, extended with reverse transcriptase. RNA was isolated from S. lividans containing the wild-type chi63 after growth on minimal media with chitin as carbon source (lane 1); with an A-to-G substitution at −40, grown on glycerol (lane 2) or glucose (lane 3); with a T-to-G substitution at −14, grown on glycerol (lane 4), glucose plus chitin (lane 5), or glucose (lane 6); with a G-to-A substitution at −25, grown on glucose (lane 7); with a T-to-G substitution at −24, grown on glucose (lane 8); with a C-to-T substitution at 23, grown on glucose (lane 9); with a T-to-A substitution at −21, grown on glucose (lane 10); with an A-to-G substitution at −19, grown on glucose (lane 11); with a T-to-G substitution at −16, grown on glucose (lane 12), glucose plus chitin (lane 13), or glycerol (lane 14); with a T-to-A substitution at −9, grown on glucose plus chitin (lane 15); with a C-to-A substitution at −18, grown on glucose plus chitin (lane 16); with an A-to-G substitution at −19, grown on glucose plus chitin (lane 17). The same primer was used to prime dideoxynucleotide sequencing reactions from a single-stranded DNA template, M13mp19, containing the corresponding fragment of chi63. The letters above the sequence ladder indicate the dideoxynucleotide used to terminate each reaction. The asterisk indicates the nucleotide at the apparent transcription start site.
DISCUSSION
We report the isolation and characterization of base substitution mutations introduced throughout the chi63 promoter region. Base changes located at −10, −12, −32, −33, −35, and −37 resulted in loss of promoter activity. The location of these mutations and the fact that they lie within sequences that are similar to those typical of eubacterial RNA polymerase recognition sequences, TATTCT at −10 and TTGACC at −35, suggest that bases in these positions are important for RNA polymerase interaction. There are at least five sigma factors in Streptomyces (38, 39) known to recognize these sequences or variations of these sequences. Overlapping the RNA polymerase binding site is a perfect 12-bp direct repeat sequence. Base changes within this direct repeat sequence at −16, −18, −23, −24, −25, and −40 resulted in glucose-resistant, chitin-independent expression. The constitutive phenotype of these mutations suggests that the direct repeat sequence is an operator that binds a negative regulator of transcription. Base changes at −19 and −21 resulted in glucose-resistant, chitin-dependent expression, suggesting that the direct repeat sequence also facilitates glucose repression. The fact that mutations that give rise to these two different phenotypes are dispersed throughout the same direct repeat sequence module suggests that the same sequence facilitates both chitin induction and glucose repression.
Several lines of evidence suggest that the chi63 repressor may mediate glucose repression as well as chitin induction. The fact that cis-acting mutations resulted in glucose resistance while retaining the requirement for chitin induction rules out the possibility that glucose repression acts exclusively by inducer exclusion. Because mutations outside the transcript coding region of chi63 resulted in deregulated expression, regulation is at least in part at the level of transcription initiation. The existence of cis-acting mutations in the chi63 operator that result in constitutive expression or relieve glucose repression indicate that the operator and, therefore, the repressor may mediate both chitin induction and glucose repression. The fact that some mutations lie in the putative RNA polymerase binding site indicated that regulation may involve the promoter, the repressor, and RNA polymerase acting in concert. These observations support and extend the work of Hindle and Smith (29) and Van Wezel et al. (30), who have shown that null mutations in the gylR and malR genes, which encode repressors of the glycerol (gylCABX) and maltose (malE) utilization operons, respectively, of S. coelicolor, relieve glucose repression of the entire operon. Their observations argue strongly that both substrate induction and catabolite repression are mediated through the gylR and malR proteins.
However, we have not rigorously ruled out the possibility that two different proteins, one that responds to chitin induction and another that responds to glucose repression, bind to overlapping targets within the direct repeat sequence. We also have not ruled out the existence of a positive activator of transcription. The replacement of A-T base pairs with G-C base pairs at −9, −14, and −16 might be expected to reduce the melting efficiency of RNA polymerase during open complex formation. Somewhat surprisingly, these base changes resulted in a dramatic increase in expression from the promoter and may indicate the existence of a positive activator of transcription. In addition, the T-to-A substitution at −9 changes the −10 hexamer from TATTCT to TATACT, which is closer to the TATAAT consensus sequence. This could be interpreted to indicate that an activator that is required for promoter activity and is absent in the presence of glucose is not required in the −9 T-to-A mutant. This would be similar to the lacUV5 promoter, which functions independently of cAMP/CRP complex. It is also important to point out that bases upstream of the direct repeat sequences to −120 are required for full wild-type activity of the promoter and may include a binding site for such an activator.
Hindle and Smith suggested (29) a possible mechanism for catabolite repression of glycerol catabolism. They suggested that because glucose kinase is required for glucose repression of the gyl operon, the metabolism of glucose via glucose kinase may modulate the intracellular level of glycerol-3-phosphate, which might in turn determine the degree of repression by GylR. This model includes a role for glycerol kinase in regulating glycerol uptake and the possible involvement of the phosphotransferase system. Glucose repression of chi63 transcription does not require glucose kinase, and only insoluble polymers of chitin act as inducer (refs. 31 and 32; Phillips Robbins, personal communication). Hindle and Smith (29) also showed that null mutations in GylR do not relieve glucose repression of agarase expression, suggesting that whatever mechanism is exerted through the repressor/operator may involve some specificity for given operons. For both chi63 and gylCABX, evidence to date suggests that there is no general activator of transcription. We point out, however, that our analysis of chi63 is not exhaustive and that our attempts to introduce random base changes throughout the promoter region were only partially successful. Furthermore, bases at positions shown to be important do not represent all possible changes. This is perhaps a limitation of the method of mutagenesis used to introduce base changes, because contaminated synthesis of oligonucleotides discriminates against the incorporation of some bases. The genetic and biochemical analysis of the regulation of operons such as gylCABX, which requires glucose kinase for glucose repression, and genes such as chi63, which does not, should contribute to our understanding of the mechanisms of catabolite control in this complex organism.
Several Streptomyces chitinase genes have been cloned and sequenced. chi63 and chi35 encode chitinases from S. plicatus (32). chiC and chiA encode chitinases from S. lividans (40), and chi40 encodes a thermostable chitinase from S. thermoviolaceus (41). For chi63, chi35, chiC, and chiA, evidence that expression of these genes is glucose-sensitive and chitin-dependent has been reported. Transcription start sites have been identified for chi63 (13), chiA (42), and chiC (40). chi63 is the only gene for which cis-acting mutations define regulatory sequences. Although these chitinases genes are from different organisms and encode different enzyme activities, a comparison of the 5′ regulatory regions of these genes revealed striking similarities. As shown in Fig. 4, each contains a putative RNA polymerase binding site prototypical of eubacteria and each contains a similar pair of direct repeat sequences. Interestingly, bases identified in our analysis as important for activity and regulation of chi63 are highly conserved in all of these promoters. There are only two possible inconsistencies. The presence of a C in the third position of the putative −10 of chi35 or an A in fourth position of the putative −10 region of chi40 would not be consistent with our analysis. We point out that no transcription start site has been reported for either of these promoters and putative −10 region is deduced from sequence analysis. Also of interest is that in all cases, the direct repeat sequences in these promoters are positioned on the same face of the double helix. The spacing between the direct repeats is 19 bp in chiA, 9 bp in chi63 and chiC, and 10 bp in chi40 and chi35. It is also noteworthy that the direct repeat sequences lie in close proximity to or overlapping the RNA polymerase recognition sequences of these promoters. The existence of the same direct repeat sequence in the promoters of these genes suggests that they may be coordinately regulated by the same repressor and have similar mechanisms of transcriptional regulation. Because only insoluble polymers of chitin serve to induce chi63, it is possible that the inducer is sensed extracellularly and initiation of transcription of chitinase genes involves a signal transduction cascade.
Figure 4.
Comparison of the promoter regions of different chitinase genes. Underlined sequences indicate the direct repeat sequences. Bold letters indicate the putative RNA polymerase binding sites. Bold and underlined letters indicate the transcription start sites.
The regulation of carbon utilization plays a central role in the most interesting aspects of the biology of Streptomyces. In response to nutrient depletion, including starvation for carbon, Streptomyces coelicolor initiates an elaborate process of morphogenesis and produces a wide variety of secondary metabolites, including four chemically distinct antibiotics (43, 44). Mutants blocked at the earliest stages of differentiation, known as bld (bald) mutants, are unable to erect aerial hyphae and are also defective to varying degrees in antibiotic production (45, 46). Interestingly, the morphological defects, and often the antibiotic deficiencies of most classes of bld mutants characterized to date, bldA, bldC, bldD, bldG, and bldH, are carbon-source-dependent. All of these mutant classes are defective in their ability to initiate differentiation and are blocked for antibiotic production when glucose is provided as the sole carbon source. When these mutants are grown on a poor carbon source such as mannitol, the ability to sporulate is partially restored, and, depending on the mutant class, so is the ability to produce antibiotics. We have recently shown that all classes of bld mutants [except for the very recently described bldK mutant (47), which has not been tested] are defective in the regulation of the galP1 promoter and that the bldB mutant is globally deregulated for catabolite control (1). We have suggested that the bld genes may not be involved in morphogenesis per se, but instead play a central role in the ability of the organism to sense and/or signal starvation. The use of promoters such as chi63 as tools may identify mutations and genes that elucidate the connection between the regulation of catabolite control and the initiation of morphogenesis.
Acknowledgments
We thank Charlie Moran, Brian Green, Mary Brawner, and Meg Pope for helpful discussions during the course of this work, and we thank Charlie Moran and Jai Behari for their critical reading of the manuscript. This work was supported by National Science Foundation Grant DMB-9196067 to J.W.
References
- 1.Pope M K, Green B D, Westpheling J. Mol Microbiol. 1996;19:747–756. doi: 10.1046/j.1365-2958.1996.414933.x. [DOI] [PubMed] [Google Scholar]
- 2.Pastan I, Perlman R. Science. 1970;169:339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- 3.Aiba H. J Biol Chem. 1985;260:3063–3070. [PubMed] [Google Scholar]
- 4.Hodgson D A. J Gen Microbiol. 1982;128:2417–2430. [Google Scholar]
- 5.Chatterjee S, Vining L C. Can J Microbiol. 1982;28:311–317. doi: 10.1139/m82-046. [DOI] [PubMed] [Google Scholar]
- 6.Deutscher J, Kuster E, Bergstedt U, Charrier V, Hillen W. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 7.Henkin T M. FEMS Microbiol Lett. 1996;135:9–15. doi: 10.1111/j.1574-6968.1996.tb07959.x. [DOI] [PubMed] [Google Scholar]
- 8.Angell S, Schwartz E, Bibb M J. Mol Microbiol. 1992;6:2833–2844. doi: 10.1111/j.1365-2958.1992.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 9.Angell S, Lewis C G, Buttner M J, Bibb M J. Mol Gen Genet. 1994;244:135–143. doi: 10.1007/BF00283514. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda H, Seno E T, Bruton C J, Chater K F. Mol Gen Genet. 1984;196:501–507. doi: 10.1007/BF00436199. [DOI] [PubMed] [Google Scholar]
- 11.Seno E T, Chater K F. J Gen Microbiol. 1983;129:1403–1413. doi: 10.1099/00221287-129-5-1403. [DOI] [PubMed] [Google Scholar]
- 12.Virolle M J, Bibb M J. Mol Microbiol. 1988;2:197–208. doi: 10.1111/j.1365-2958.1988.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 13.Delic I, Robbins P, Westpheling J. Proc Natl Acad Sci USA. 1992;89:1885–1889. doi: 10.1073/pnas.89.5.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seno E T, Bruton C J, Chater K F. Mol Gen Genet. 1984;193:119–128. doi: 10.1007/BF00327424. [DOI] [PubMed] [Google Scholar]
- 15.Buttner M J, Fearnley I M, Bibb M J. Mol Gen Genet. 1987;209:101–109. doi: 10.1007/BF00329843. [DOI] [PubMed] [Google Scholar]
- 16.Buttner M J, Smith A M, Bibb M J. Cell. 1988;52:599–609. doi: 10.1016/0092-8674(88)90472-2. [DOI] [PubMed] [Google Scholar]
- 17.Flores M E, Ponce E, Rubio M, Huitron C. Biotechnol Lett. 1993;15:595–600. [Google Scholar]
- 18.Kwakman J H, Postma P W. J Bacteriol. 1994;176:2694–2698. doi: 10.1128/jb.176.9.2694-2698.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Entain K D. Mol Gen Genet. 1980;178:633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- 20.Bennett W S, Jr, Steitz T A. Proc Natl Acad Sci USA. 1978;75:4848–4852. doi: 10.1073/pnas.75.10.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma H, Botstein D. Mol Cell Biol. 1986;6:4046–4052. doi: 10.1128/mcb.6.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose M, Albig Q, Entian K D. Eur J Biochem. 1991;199:511–518. doi: 10.1111/j.1432-1033.1991.tb16149.x. [DOI] [PubMed] [Google Scholar]
- 23.Egeter O, Brückner R. Mol Microbiol. 1996;21:739–749. doi: 10.1046/j.1365-2958.1996.301398.x. [DOI] [PubMed] [Google Scholar]
- 24.Wagner E, Marcandier S, Egeter O, Deustscher J, Gotz F, Bruckner R. J Bacteriol. 1995;177:6144–6152. doi: 10.1128/jb.177.21.6144-6152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brawner M, Mattern S G, Babcock M J, Westpheling J. J Bacteriol. 1997;179:3222–3231. doi: 10.1128/jb.179.10.3222-3231.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattern S G, Brawner M E, Westpheling J. J Bacteriol. 1993;175:1213–1220. doi: 10.1128/jb.175.5.1213-1220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith C P, Chater K F. J Mol Biol. 1988;204:569–580. doi: 10.1016/0022-2836(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 28.Smith C P, Chater K F. Mol Gen Genet. 1988;211:129–137. doi: 10.1007/BF00338403. [DOI] [PubMed] [Google Scholar]
- 29.Hindle Z, Smith C P. Mol Microbiol. 1994;12:737–745. doi: 10.1111/j.1365-2958.1994.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Wezel G P, White J, Young P, Postma P W, Bibb M J. Mol Microbiol. 1997;23:537–549. doi: 10.1046/j.1365-2958.1997.d01-1878.x. [DOI] [PubMed] [Google Scholar]
- 31.Robbins P W, Albright C, Benfield B. J Biol Chem. 1988;263:443–447. [PubMed] [Google Scholar]
- 32.Robbins P W, Overbye K, Albright C, Benfield B, Pero J. Gene. 1992;111:69–76. doi: 10.1016/0378-1119(92)90604-n. [DOI] [PubMed] [Google Scholar]
- 33.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic Manipulation Of Streptomyces: A Laboratory Manual. 1st Ed. Norwich, U.K.: The John Innes Foundation; 1985. [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1982. [Google Scholar]
- 35.Kunkel T A. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 37.Ingram C, Brawner M, Youngman P, Westpheling J. J Bacteriol. 1989;171:6617–6624. doi: 10.1128/jb.171.12.6617-6624.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westpheling J, Ranes M, Losick R. Nature (London) 1985;313:22–27. doi: 10.1038/313022a0. [DOI] [PubMed] [Google Scholar]
- 39.Buttner M J. Mol Microbiol. 1989;3:1653–1659. doi: 10.1111/j.1365-2958.1989.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 40.Fujii T, Miyashita K. J Gen Microbiol. 1993;139:677–686. doi: 10.1099/00221287-139-4-677. [DOI] [PubMed] [Google Scholar]
- 41.Tsujibo H, Endo H, Minoura K, Miyamoto K, Inamori Y. Gene. 1993;134:113–117. doi: 10.1016/0378-1119(93)90183-4. [DOI] [PubMed] [Google Scholar]
- 42.Miyashita K, Fujii T. Biosci Biotech Biochem. 1993;57:1691–1698. doi: 10.1271/bbb.57.1691. [DOI] [PubMed] [Google Scholar]
- 43.Chater K F. In: Microbial Development. Losick R, Shapiro L, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1984. pp. 89–115. [Google Scholar]
- 44.Champness W C, Chater K F. In: Regulation of Bacterial Development. Piggot P J, Moran C P, Youngman P, editors. Washington, DC: Am. Soc. Microbiol.; 1994. pp. 61–93. [Google Scholar]
- 45.Champness W. J Bacteriol. 1988;170:1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merrick M J. J Gen Microbiol. 1976;96:299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- 47.Nodwell J R, McGovern K, Losick R. Mol Microbiol. 1996;22:881–893. doi: 10.1046/j.1365-2958.1996.01540.x. [DOI] [PubMed] [Google Scholar]