Abstract
The Vav family of proteins are guanine nucleotide exchange factors (GEFs) for the Rho family of GTPases, which regulate various cellular functions, including T-cell activation. They contain a catalytic Dbl homology (DH) domain that is invariably followed by a pleckstrin homology (PH) domain, which is often required for catalytic activity. Vav proteins are the first GEFs for which an additional C1 domain is required for full biological activity. Here, we present the structure of a Vav1 fragment comprising the DH–PH–C1 domains bound to Rac1. This structure shows that the PH and C1 domains form a single structural unit that packs against the carboxy-terminal helix of the DH domain to stabilize its conformation and to promote nucleotide exchange. In contrast to previous reports, this structure shows that there are no direct contacts between the GTPase and C1 domain but instead suggests new mechanisms for the regulation of Vav1 activity.
Keywords: C1 domain, exchange factor, GTPase, Vav1, X-ray crystallography
Introduction
Vav1 is a guanine nucleotide exchange factor (GEF) for the Rho family of GTPases, and has been shown to be crucial in T-cell development and activation (Tybulewicz, 2005). Analysis of Vav1-deficient T cells has shown that Vav1 transduces T-cell antigen receptor signals to several downstream pathways and it has been suggested that some of these might depend on the GEF activity of Vav1, whereas others depend on the function of Vav1 as an adapter protein (Tybulewicz et al, 2003).
The primary structure of Vav1 shows that the protein contains eight domains (Fig 1A; Tybulewicz, 2005). Vav1, in common with many GEFs for the Rho family of GTPases (RhoGEFs), contains a conserved Dbl homology (DH) domain, which is responsible for catalysing nucleotide exchange (Crespo et al, 1997; Han et al, 1997). As seen in most RhoGEFs, the DH domain is flanked by a pleckstrin homology (PH) domain (Rossman et al, 2005). In addition, there is a calponin homology (CH) domain, and an acidic (Ac) region to the amino-terminal and a C1 domain to the carboxy-terminal side of the DH/PH module. Finally, the C terminus of the protein contains one SH2 and two SH3 domains. It has been proposed that the CH–C1 part of Vav1 is involved in regulating exchange activity, whereas the SH3/SH2/SH3 domains might have adapter function.
Figure 1.
Overall structure of the Rac1–Vav1 DH–PH–C1 complex. (A) Diagram showing the domain structure of Vav1. The DH–PH–C1 fragment is shown in the same colours as used throughout the manuscript. The domain boundaries as determined by Prosite (http://www.expasy.ch/prosite/) are indicated. (B) Ribbon diagram of the Rac1–Vav1 DH–PH–C1 structure, with Rac1 coloured in grey, the DH domain in cyan, the PH domain in yellow and the C1 domain in orange. The two Zn2+ ions bound to the C1 domain are shown as grey spheres. Ac, acidic; CH, calponin homology; DH, Dbl homology; PH, pleckstrin homology.
The GEF activity of Vav1 is regulated by several mechanisms. Phosphorylation of Tyr 174 within the Ac region results in an increase in GEF activity (Crespo et al, 1997; Han et al, 1997; Lopez-Lago et al, 2000). A solution structure of the DH domain of Vav1, including residues 170–189 of the Ac region, shows that Tyr 174 lies within an α-helix that binds to part of the GTPase interaction site, thereby occluding access to the GTPase (Aghazadeh et al, 2000). Phosphorylation of Tyr 174 causes dissociation of this helix from the DH domain, thereby relieving the autoinhibition. The CH domain has also been implicated in an autoinhibitory function, as deletion of this domain results in increased exchange activity (Zugaza et al, 2002). It has been proposed that the CH domain might bind directly to the C1 domain and thereby hold the Vav1 protein in an inactive ‘closed' conformation, in which the CH–C1 interaction helps to stabilize the inhibitory Tyr 174–DH interaction. Activation of Vav1 would then be achieved by a conformational change leading to an ‘open' conformation in which the DH domain is no longer occluded. Support for such a model has come from single-particle electron microscopy of Vav3 (Llorca et al, 2005).
The PH domains of DH-containing RhoGEFs seem to have diverse functions (Rossman et al, 2005). In some cases, they participate directly in binding to the GTPase, and in other cases they might bind to phospholipids and either regulate membrane targeting or allosterically activate GEF activity. In the case of Vav1, a direct effect of phosphoinositides on exchange activity in solution has been suggested, with activity enhanced in response to the binding of phosphatidylinositol (4,5)-bisphosphate (PtdIns(3,4)P2) or PtdIns(3,4,5)P3 and inhibited following the binding of PtdIns(4,5)P2 (Han et al, 1998; Das et al, 2000). It has been proposed that PtdIns(4,5)P2 might promote association between the PH and DH domains, resulting in occlusion of the GTPase-binding site, whereas binding of PtdIns(3,4)P2 or PtdIns(3,4,5)P3 would cause dissociation of the domains and hence increase GEF activity.
C1 domains are present in a wide range of signalling proteins and can be divided into typical C1 domains, which bind to lipids and regulate membrane association, and atypical C1 domains, which lack the features required for lipid binding and instead might be involved in protein–protein interactions (Colon-Gonzalez & Kazanietz, 2006). On the basis of its sequence, the C1 domain of Vav1 has been classified as atypical, which is supported by the observation that it does not interact with phorbol esters (Kazanietz et al, 1994). Instead, the C1 domain of the Vav family of GEFs has been proposed to be important for enzymatic activity. Mutations in the C1 domain reduce GEF activity, and this has been suggested to be due to direct interactions between the C1 domain and the GTPase (Booden et al, 2002; Zugaza et al, 2002; Heo et al, 2005). Finally, the inhibition of GEF activity by the CH domain might be dependent on its ability to interact with the C1 domain.
To understand the contribution of the PH and C1 domains to the GEF activity of Vav1, and to shed light on its substrate specificity, we determined a high-resolution X-ray structure of the DH–PH–C1 domains of Vav1 in complex with Rac1. We show that the PH and C1 domains contribute to efficient GEF activity by stabilizing the DH domain structure and not through direct contacts with the GTPase. We also report that Vav1 is a GEF for Rac1, RhoA and Cdc42, and interpret this promiscuity in the light of the three-dimensional structure.
Results And Discussion
Overall structure of Vav1 bound to Rac1
Vav1 (amino acids (aa) 170–575) and Rac1 were coexpressed and the nucleotide-free complex was purified by affinity and gel filtration chromatography. The complex crystallized readily and the structure was solved at 1.85 Å resolution by molecular replacement. The overall architecture of the GTPase/DH–PH portion of this complex is similar to that of other GTPase–GEF complexes except for the orientation of the PH domain, which differs extensively from structure to structure (Fig 1B). This is in part due to significant differences in the length and orientation of the C-terminal helix α6 of the DH domain. Interestingly, the orientation of this helix is similar between all structurally characterized DH domains up to a position equivalent to residue Arg 375 in Vav1, which marks the predicted C-terminal end of the DH domain. However, in a subset of GEFs, including Vav1, this helix is extended and often contains a slight kink around this position, which, in turn, determines the orientation of the PH domain. In contrast to all other RhoGEF structures reported, the Vav1 structure presented here is the first complex to contain another regulatory domain, the C1 domain that makes direct contacts with the DH domain. Strikingly, the interface between the DH and the PH–C1 cassette is extensive and buries 1,726 Å2 of solvent-accessible surface between the two domains (Fig 1B).
The Vav1–Rac1 interface
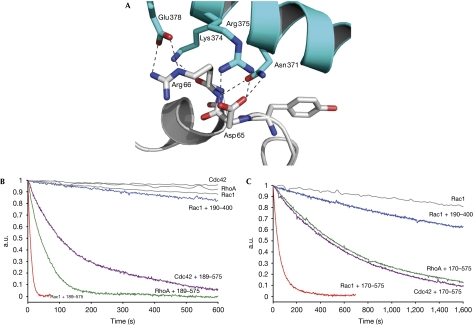
Complex formation between Rac1 and Vav1 buries about 2,600 Å2 of solvent-accessible surface between them. The interface is similar to that of other GTPase/DH–PH structures and, not surprisingly, the conformations of the two switch regions superimpose well with those of other GTPases, indicating that the mechanism of nucleotide exchange is conserved in Vav1 (Erickson & Cerione, 2004; Rossman et al, 2005). The structure of switch I is supported by interactions of Glu 201Vav1, a residue highly conserved in RhoGEFs, with the hydroxyl group of Tyr 32Rac1 and the backbone amides of Thr 35Rac1 and Val 36Rac1. Similarly, the backbone carbonyl of Ala 59Rac1 forms a hydrogen bond with the side chain of highly conserved Lys 335Vav1 to remodel the conformation of switch II. This is further supported by a hydrogen bond from the side chain of His 337Vav1 to the backbone oxygen of Gly 60Rac1. As seen in other GTPase–GEF complexes, the effect of the conformational changes induced in switch I and II is to disrupt binding to the nucleotide and Mg2+ ion, and thereby promote nucleotide release. One of the main differences to other structures is the lack of significant contacts between the DH domain and strands β2 and β3 of the GTPase, which are often present and are believed to contribute to GTPase specificity. Residues Asp 65Rac1 and Arg 66Rac1 in the switch II region are the most intimately involved residues in the Rac1–Vav1 interface and form a total of seven hydrogen bonds with helix α6 of the DH domain, including the side chains of Asn 371Vav1, Lys 374Vav1, Arg 375Vav1 and Glu 378Vav1 (Fig 2A). Some of these interactions, such as those made by Asn 371Vav1 and Glu 378Vav1, are also observed in many other GTPase–GEF structures, whereas others, especially those contributed by Lys 374Vav1, are absent in most complexes. Interestingly, in those GEFs in which the PH domain is in direct contact with the GTPase such as Dbs, Trio or LARG, some of the contacts with Asp 65Rac1 and Arg 66Rac1 are made by residues from the PH domain, and loss of these interactions often impairs nucleotide exchange, indicating that they are important for the stabilization of a catalytically active conformation (Rossman et al, 2002; Kristelly et al, 2004; Chhatriwala et al, 2007).
Figure 2.
The Rac1–Vav1 interaction and nucleotide exchange. (A) Detailed view of the interface between Asp 65 and Arg 66 in the switch II region of Rac1, and residues from helix α6 of the DH domain. Atoms are coloured by type except carbons, which are the same colour as in Fig 1B. The dashed lines indicate hydrogen bonds. Nucleotide exchange activity of (B) active (amino acids (aa) 189–575) and (C) autoinhibited (aa 170–575) Vav1 DH–PH–C1 and the isolated DH domain (aa 190–400) towards Rac1, Cdc42 and RhoA, as well as intrinsic exchange rates. DH, Dbl homology; PH, pleckstrin homology.
There have been conflicting reports on the substrate specificity of the Vav family of proteins. In particular, the ability of Vav proteins to be active on Cdc42 has been questioned, and it has been suggested that different Vav isoforms have different GTPase specificities (Schuebel et al, 1998; Aghazadeh et al, 2000; Movilla et al, 2001). By using two different DH–PH–C1 fragments of Vav1 (‘active' (aa 189–575) and ‘autoinhibited' (aa 170–575)), we found that although Rac1 is the preferred substrate of Vav1, nucleotide exchange on Cdc42 and RhoA is also enhanced, but to a lesser extent (Fig 2B,C; Table 1). A similar selectivity has been previously established for Vav2 (Abe et al, 2000; Heo et al, 2005). The structure shows that the residues of Vav1 that make contacts with Rac1 are conserved between all human and mouse Vav isoforms, raising the possibility that there are no major differences in their specificity, which is in contrast to previous suggestions (Movilla & Bustelo, 1999; Movilla et al, 2001). Inspection of the interface shows that there are no residues in Vav1 that would be expected to obviously discriminate against Cdc42 or RhoA through steric clashes. For some GEFs, discrimination between Rac1 and Cdc42 has been shown to depend on Trp 56Rac1 and the equivalent Phe 56Cdc42 (Karnoub et al, 2001; Snyder et al, 2002). In Rac1-specific GEFs such as Tiam1, conserved Ile 1187 allows binding of the bulky indole group of Trp 56Rac1. Conversely, the equivalent Leu 1376 in the Cdc42-specific GEF Intersectin (Itsn) will accommodate the smaller Phe 56Cdc42 but not Trp 56Rac1. In Vav1 the analogous residue is a methionine, Met 327, whereas in Vav2 and Vav3 it is a valine, and both the residues provide sufficient space to accommodate a bulky tryptophan.
Table 1.
Intrinsic and Vav1-stimulated nucleotide exchange rates
| Vav1 | GTPase | Exchange rate (s−1) | Enhancement over intrinsic rate |
|---|---|---|---|
| – | Rac1 | 0.00015 | – |
| DH | Rac1 | 0.0003 | 2 |
| DH–PH–C1 I | Rac1 | 0.0152 | 101 |
| DH–PH–C1 A | Rac1 | 0.117 | 780 |
| – | Cdc42 | 0.00013 | – |
| DH–PH–C1 I | Cdc42 | 0.0014 | 11 |
| DH–PH–C1 A | Cdc42 | 0.0072 | 55 |
| – | RhoA | 0.0003 | – |
| DH–PH–C1 I | RhoA | 0.0014 | 5 |
| DH–PH–C1 A | RhoA | 0.019 | 63 |
| The table lists rates of nucleotide exchange on Rac1, Cdc42 and RhoA (at 0.4 μM) either alone or in the presence of the following Vav1 fragments (at 1.6 μM): DH domain alone (amino acids (aa) 190–400) or DH–PH–C1 fragments containing aa 170–575 indicated by I for autoinhibited or aa 189–575 indicated by A for active. | |||
| DH, Dbl homology; PH, pleckstrin homology. | |||
Interactions between DH, PH and C1 domains
In principle, DH domains contain all the residues that are required for the remodelling of switch regions and nucleotide exchange. Nevertheless, there are many examples of GEFs that show enhanced exchange activity in the presence of a PH domain. In some GEFs, there are direct contacts between the PH domain and GTPase, and mutation of the residues involved can severely interfere with catalysis. In the case of Vav1, the C1 domain has been shown to be required for exchange activity and has been proposed to make direct contacts with the GTPase (Booden et al, 2002; Zugaza et al, 2002; Heo et al, 2005). To evaluate the importance of the PH and C1 domains in enzymatic activity, we compared the catalytic rates of DH–PH–C1 fragments with the isolated DH domain. Addition of the PH and C1 domains significantly increased the activity of the DH domain (Fig 2B,C; Table 1). However, our structure shows unequivocally that there are no direct contacts between the PH or C1 domain and the GTPase (Fig 1B), and thus these domains must contribute to activity by other means. We note that the PH and C1 domains make extensive contacts with the DH domain (Fig 3). Furthermore, the PH and C1 domains and the linker bridging the two domains form an extensive interface, explaining why it has not been possible to produce either an isolated domain or the DH–PH construct in a soluble form, as these would probably expose hydrophobic surfaces. The crucial residues in this interface are Asp365 and Asp376 located in helix α6 of the DH domain, Arg402 at the beginning of the PH domain and Phe540 in the C1 domain. Asp365 contacts the side chain of Arg537 and the backbone amide of Leu535. Asp376 and Arg402 are involved in an extensive hydrogen-bonding network that connects the PH and C1 domains, whereas Phe540 is at the centre of a hydrophobic pocket formed by all three domains (Fig 3). To examine the contribution of these interdomain interactions to catalytic activity, we introduced the following single amino-acid substitutions into both active (aa 189–575) and autoinhibited (aa 170–575) Vav1: D365A, D376A, R402A and F540A. However, introduction of these mutations severely decreased protein stability, making it impossible to produce sufficient quantities of pure and non-aggregated protein for exchange assays. This observation supports the idea that an intimate DH, PH and C1 domain interface is crucial for the structural integrity of the DH–PH–C1 cassette.
Figure 3.
The DH–PH–C1 interface. Detailed view of the interface between residues from helix α6 of the DH domain and the PH and C1 domains. The positions of residues Gln 542, Tyr 544 and Lys 555 within the C1 domain that have been discussed in the text are indicated. DH, Dbl homology; PH, pleckstrin homology.
On the basis of the structure presented here, we propose that the PH and C1 domains contribute to GEF activity by forming a single structural unit that binds to the critical helix α6 of the DH domain and thereby restricts its conformational flexibility. This helix, in turn, makes crucial contacts with the switch II region to remodel it and interfere with the binding of the Mg2+ ion. Similar stabilizing interactions in other GEFs are provided by direct PH–GTPase contacts, which might help to ‘lock down' and stabilize helix α6. The observation that in some GEFs such as Itsn there is no obvious beneficial effect of the PH domain on exchange activity (Pruitt et al, 2003) suggests that in those GEFs helix α6 is either intrinsically much more rigid or that the PH domain requires other factors such as membrane-bound lipids or other proteins to adopt the catalytically fully active conformation. In this respect, it is interesting to note that the DH–PH unit in RhoGEFs is often flexible and that for some GEFs the orientation of the PH domain in the unbound structure is different from that in the GTPase-bound structure. By contrast, we believe that the orientation of the PH–C1 unit observed in our structure might constitute the conformation that will be found in both the active and inactive states, as the interface between the DH, PH and C1 domains is so extensive that it might be energetically unfavourable to disrupt. This model of a structural unit formed by the PH and C1 domains that stabilizes and fixes the DH domain is supported by mutagenesis of the C1 domain, which identified three mutations that abolished Vav1 GEF activity: Q542A, Y544A and K555A (Zugaza et al, 2002). Gln 542 probably has a structural role but Tyr 544 and Lys 555 form hydrogen bonds across the PH–C1 interface to stabilize the observed domain arrangement, and thus explain why mutation of these residues abolishes GEF activity (Fig 3). By contrast, the structure presented here is not compatible with previous reports suggesting a direct interaction between Rac1 and the C1 domain, which were based on nuclear magnetic resonance (NMR) chemical shift mapping and glutathione-S-transferase (GST) pull-down experiments (Movilla & Bustelo, 1999; Heo et al, 2005). Instead, our structure shows that the C1 domain is a long way from Rac1, and is engaged in extensive contacts with the DH and PH domains. Our results are supported by a recent study that also failed to detect a direct GTPase–C1 interaction (Brooun et al, 2007). The discrepancy between these studies might be due to the tendency of the isolated C1 domain to aggregate, and thus the reported GTPase–C1 contacts might be nonspecific interactions.
Ligand-binding properties of the PH and C1 domains
Binding of phosphoinositides to the PH domain of Vav1 has been proposed to allosterically regulate GEF activity by modulating interactions between the PH and DH domains (Han et al, 1998; Das et al, 2000). However, our structure does not provide support for such a model, as the phosphoinositide-binding site is located away from the interface with the DH domain (supplementary Figs S1,S2 online). Furthermore, given the extensive interactions made between the DH domain and the PH–C1 module, it seems unlikely that this module would undergo major conformational changes before GTPase binding. In agreement with this, Bustelo and co-workers (Zugaza et al, 2002) could not detect an effect of phospholipids on Vav1 activity in vitro. Nonetheless, our structure does not preclude the possibility that phospholipid binding to the PH domain might regulate membrane targeting of Vav1.
The C1 domain of Vav1 belongs to the group of atypical C1 domains that do not bind to diacylglycerol (DAG) or phorbol ester (Kazanietz et al, 1994; Colon-Gonzalez & Kazanietz, 2006). In spite of this, its structure overlaps well with that of the typical protein kinase Cδ (PKCδ) C1 domain, bound to phorbol-13-acetate (46 atoms overlap with an r.m.s.d. of 0.9 Å; Fig 4A; Zhang et al, 1995). In particular, the loops making up the ligand-binding site in PKCδ are conserved in Vav1, in contrast to the structures of the atypical C1 domains of, for example, Raf1 (Mott et al, 1996; Fig 4A). Furthermore, Vav1 contains a solvent-exposed cavity in the same position in which phorbol-13-acetate is bound to PKCδ (Fig 4B) and that is flanked on one side by hydrophobic residues from helix α6 of the DH domain, indicating that it could accommodate a small molecule (Fig 4C). Intriguingly, this potential ligand-binding site is located next to the region in the α6 helix that makes contacts with switch II of Rac1, suggesting that ligand binding to the C1 domain could modulate Vav1 activity. Further studies are now required to identify such a putative ligand and to test this model.
Figure 4.
The C1 domain of Vav1. (A) Overlap of the C1 domain of Vav1 (orange) with that of the typical C1 domain of PKCδ bound to phorbol-13-acetate (1PTR, blue) and the atypical C1 domain of Raf1 (1FAR, green). The ligand bound to PKCδ is shown in a ball-and-stick representation. (B) Surface representation of the C1 domain of PKCδ with bound phorbol acetate. (C) Surface representation of Vav1 DH–PH–C1 shows a pocket in the C1 domain in the same position in which phorbol acetate is bound to PKCδ (shown for comparison). DH, Dbl homology; PH, pleckstrin homology; PKCδ, protein kinase Cδ.
Vav1 activation
Nucleotide exchange activity of Vav1 is regulated through an intricate interplay between reversible phosphorylation, autoinhibitory protein interactions and possibly lipid binding. The NMR structure of an autoinhibited DH fragment explained how the region containing Tyr 174 inhibits exchange activity by occluding part of the GTPase-binding site (Aghazadeh et al, 2000). However, the observation that we were able to purify a complex of Rac1 and Vav1 containing aa 170–190, and that such a fragment is active in exchange assays, indicates that autoinhibitory interactions made by the Tyr 174-containing helix are not sufficient on their own to maintain the inactive state. Instead, open and closed conformations seem to be in a dynamic equilibrium that can easily be shifted towards the open conformation in the presence of Rac1. Hence, other in vivo mechanisms for successful repression are crucial. These are believed to involve intramolecular interactions between the CH and C1 domains. So far, structural information that could confirm such a mechanism is limited to the low-resolution electron microscopic structure of Vav3 (Llorca et al, 2005). This study compared the structures of full-length inactive, full-length phosphorylated and N-terminally truncated Vav3. The structure most relevant to the complex presented here is that of the truncated protein. A distinguishing characteristic of the reconstructed model is the position of the DH and C1 domains, which are suggested to occupy opposite sides of the PH domain and do not form any contacts. The structure presented here clearly shows that this is not correct, but now provides the opportunity to reinterpret the electron microscopic data by fitting the structure presented here into the overall protein envelope.
Conclusion
Here, we have made a first step towards an understanding of how regulatory domains in RhoGEFs outside the DH–PH module can contribute to the regulation of enzymatic activity. In addition, our structure has highlighted the existence of a putative ligand-binding pocket located at the C1–DH domain interface in a location where ligand binding could possibly modulate the exchange activity of Vav1 and its isoforms.
Methods
The fragments of Vav1 used for nucleotide exchange assays were expressed in BL21-AI cells with an N-terminal His6 tag, and then purified by affinity and gel filtration chromatography. The Rac1–Vav1 complex used for crystallization was produced by coexpression of Vav1 with GST-Rac1 and purification by affinity chromatography, followed by thrombin cleavage of the GST tag and gel filtration.
The Rac1–Vav1 complex was crystallized in hanging drops containing equal amounts of complex at 12 mg/ml and well solution (100 mM Bicine pH 9.0, 10% PEG6000). Crystals were cryoprotected with PEG400 and data were collected at SRS Daresbury. The structure was solved by molecular replacement using the Vav1-DH domain and Rac1 as search models. Refinement was carried out in REFMAC/ARP and model building in COOT. The final model of the complex has an R-factor of 20.6% (Rfree 25.2%). The structural data have been deposited in the Protein Data Bank database, with the accession code 2vrw.pdb.
Guanine nucleotide exchange assays were carried out by fluorescence spectroscopy using N-methylanthraniloyl-GDP-labelled GTPases. Exchange was monitored by following fluorescence emission of the mant-labelled GTPase (400 nM) in the presence of 1.6 μM GEF together with 2 mM unlabelled GDP (using an ISS PC1 fluorimeter) at λex=360 nm and λem=440 nm.
For detailed descriptions, see the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank S. Gamblin for help with crystal freezing, P. Walker for data collection, SRS Daresbury beamline 10.1 for beam time and support, B. Stieglitz for mant-GDP, R. Morgan-Warren for the Vav1-DH plasmid, and S. Smerdon for assistance with crystallography and helpful discussions. This work was supported by the Medical Research Council.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ (2000) Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem 275: 10141–10149 [DOI] [PubMed] [Google Scholar]
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK (2000) Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102: 625–633 [DOI] [PubMed] [Google Scholar]
- Booden MA, Campbell SL, Der CJ (2002) Critical but distinct roles for the pleckstrin homology and cysteine-rich domains as positive modulators of Vav2 signaling and transformation. Mol Cell Biol 22: 2487–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooun A, Foster SA, Chrencik JE, Chien EY, Kolatkar AR, Streiff M, Ramage P, Widmer H, Weckbecker G, Kuhn P (2007) Remedial strategies in structural proteomics: expression, purification, and crystallization of the Vav1/Rac1 complex. Protein Expr Purif 53: 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatriwala MK, Betts L, Worthylake DK, Sondek J (2007) The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation. J Mol Biol 368: 1307–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Gonzalez F, Kazanietz MG (2006) C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochim Biophys Acta 1761: 827–837 [DOI] [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR (1997) Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385: 169–172 [DOI] [PubMed] [Google Scholar]
- Das B, Shu X, Day GJ, Han J, Krishna UM, Falck JR, Broek D (2000) Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J Biol Chem 275: 15074–15081 [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA (2004) Structural elements, mechanism, and evolutionary convergence of Rho protein-guanine nucleotide exchange factor complexes. Biochemistry 43: 837–842 [DOI] [PubMed] [Google Scholar]
- Han J, Das B, Wei W, Van Aelst L, Mosteller RD, Khosravi-Far R, Westwick JK, Der CJ, Broek D (1997) Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol 17: 1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D (1998) Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279: 558–560 [DOI] [PubMed] [Google Scholar]
- Heo J, Thapar R, Campbell SL (2005) Recognition and activation of Rho GTPases by Vav1 and Vav2 guanine nucleotide exchange factors. Biochemistry 44: 6573–6585 [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Worthylake DK, Rossman KL, Pruitt WM, Campbell SL, Sondek J, Der CJ (2001) Molecular basis for Rac1 recognition by guanine nucleotide exchange factors. Nat Struct Biol 8: 1037–1041 [DOI] [PubMed] [Google Scholar]
- Kazanietz MG, Bustelo XR, Barbacid M, Kolch W, Mischak H, Wong G, Pettit GR, Bruns JD, Blumberg PM (1994) Zinc finger domains and phorbol ester pharmacophore. Analysis of binding to mutated form of protein kinase Cζ and the vav and c-raf proto-oncogene products. J Biol Chem 269: 11590–11594 [PubMed] [Google Scholar]
- Kristelly R, Gao G, Tesmer JJ (2004) Structural determinants of RhoA binding and nucleotide exchange in leukemia-associated Rho guanine-nucleotide exchange factor. J Biol Chem 279: 47352–47362 [DOI] [PubMed] [Google Scholar]
- Llorca O, Arias-Palomo E, Zugaza JL, Bustelo XR (2005) Global conformational rearrangements during the activation of the GDP/GTP exchange factor Vav3. EMBO J 24: 1330–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lago M, Lee H, Cruz C, Movilla N, Bustelo XR (2000) Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol Cell Biol 20: 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott HR, Carpenter JW, Zhong S, Ghosh S, Bell RM, Campbell SL (1996) The solution structure of the Raf-1 cysteine-rich domain: a novel ras and phospholipid binding site. Proc Natl Acad Sci USA 93: 8312–8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movilla N, Bustelo XR (1999) Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol 19: 7870–7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movilla N, Dosil M, Zheng Y, Bustelo XR (2001) How Vav proteins discriminate the GTPases Rac1 and RhoA from Cdc42. Oncogene 20: 8057–8065 [DOI] [PubMed] [Google Scholar]
- Pruitt WM, Karnoub AE, Rakauskas AC, Guipponi M, Antonarakis SE, Kurakin A, Kay BK, Sondek J, Siderovski DP, Der CJ (2003) Role of the pleckstrin homology domain in intersectin-L Dbl homology domain activation of Cdc42 and signaling. Biochim Biophys Acta 1640: 61–68 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Worthylake DK, Snyder JT, Siderovski DP, Campbell SL, Sondek J (2002) A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. EMBO J 21: 1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on Rho GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180 [DOI] [PubMed] [Google Scholar]
- Schuebel KE, Movilla N, Rosa JL, Bustelo XR (1998) Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J 17: 6608–6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JT, Worthylake DK, Rossman KL, Betts L, Pruitt WM, Siderovski DP, Der CJ, Sondek J (2002) Structural basis for the selective activation of Rho GTPases by Dbl exchange factors. Nat Struct Biol 9: 468–475 [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL (2005) Vav-family proteins in T-cell signalling. Curr Opin Immunol 17: 267–274 [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Ardouin L, Prisco A, Reynolds LF (2003) Vav1: a key signal transducer downstream of the TCR. Immunol Rev 192: 42–52 [DOI] [PubMed] [Google Scholar]
- Zhang G, Kazanietz MG, Blumberg PM, Hurley JH (1995) Crystal structure of the cys2 activator-binding domain of protein kinase Cδ in complex with phorbol ester. Cell 81: 917–924 [DOI] [PubMed] [Google Scholar]
- Zugaza JL, Lopez-Lago MA, Caloca MJ, Dosil M, Movilla N, Bustelo XR (2002) Structural determinants for the biological activity of Vav proteins. J Biol Chem 277: 45377–45392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information