Abstract
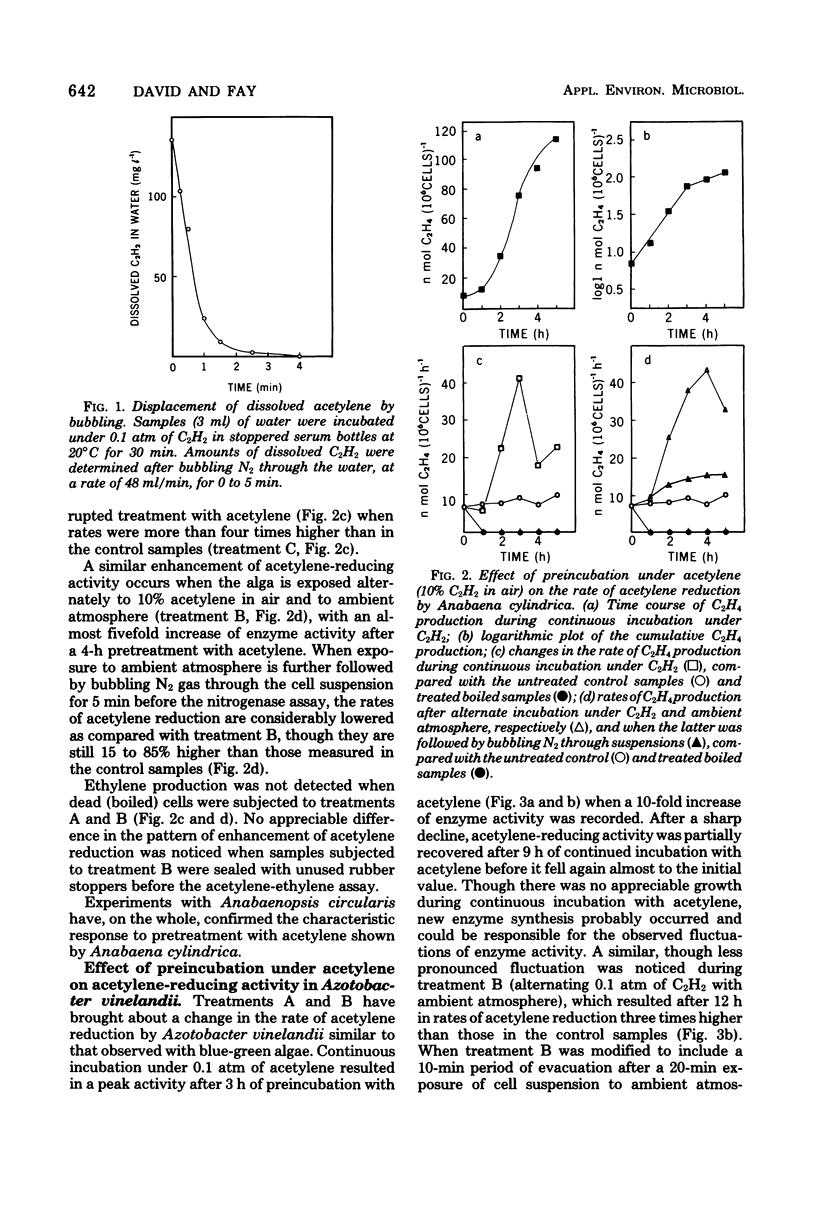
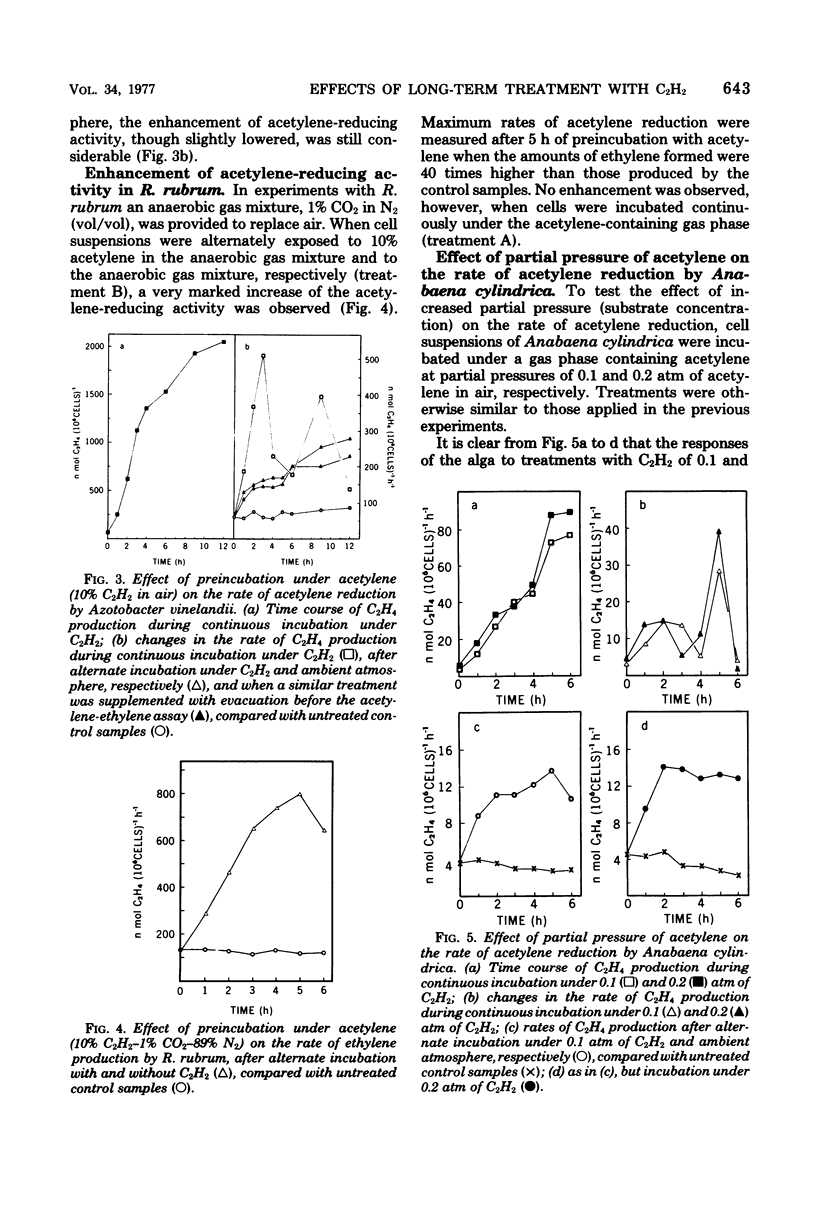
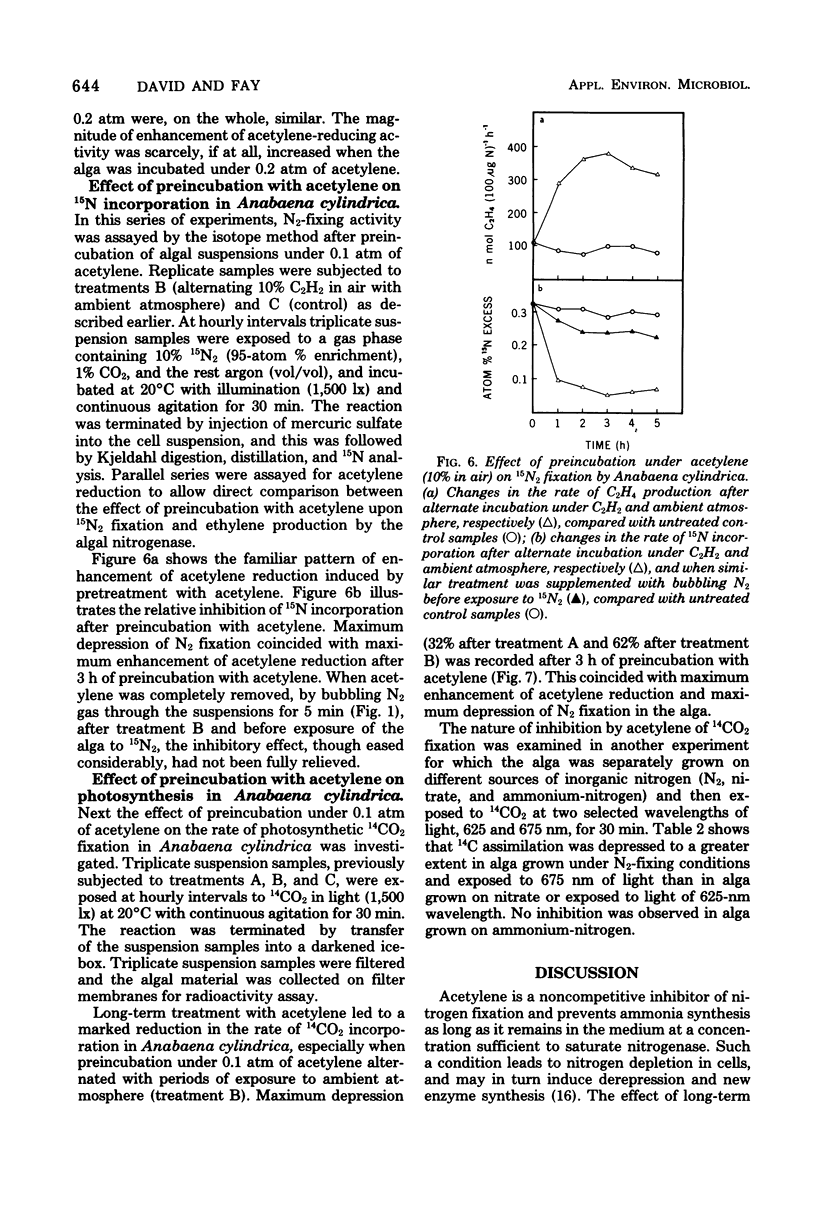
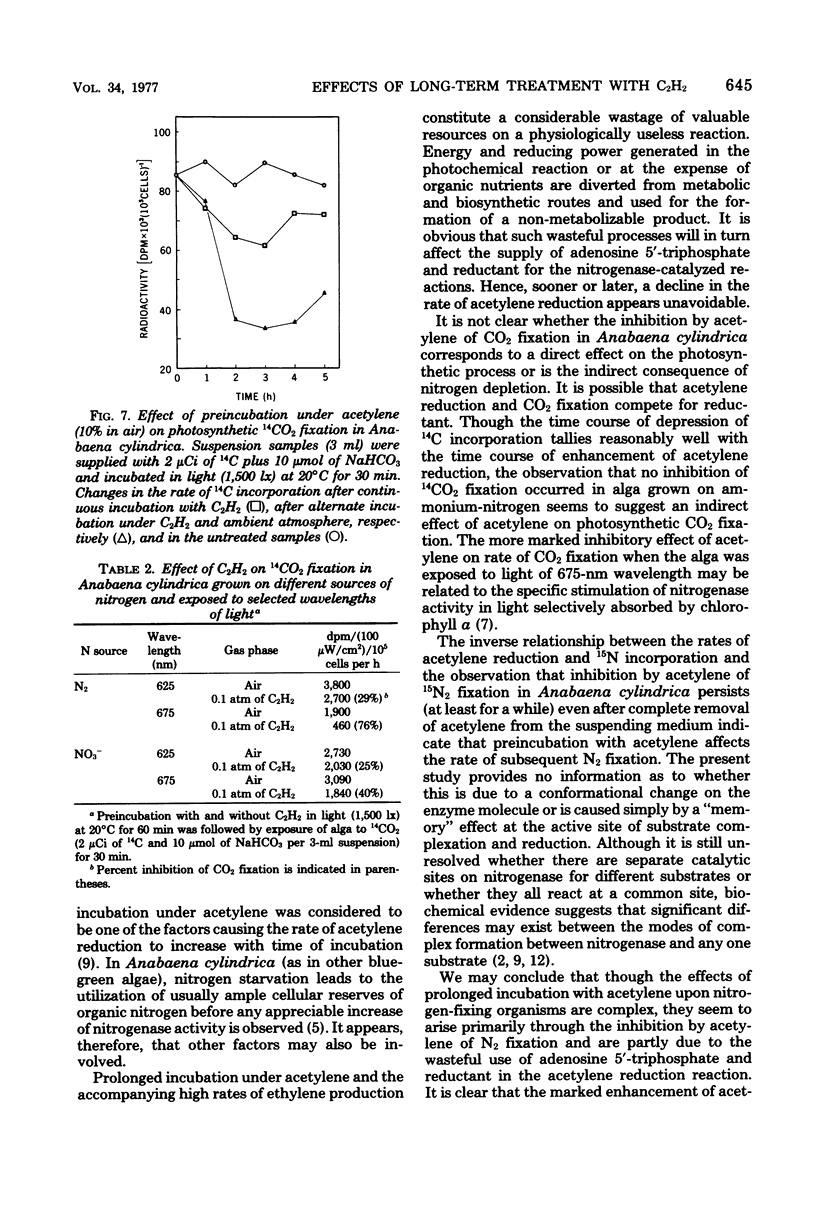
Long periods of experimental incubation with acetylene led to a multifold enhancement of acetylene-reducing activity in Anabaena cylindrica, Anabaenopsis circularis, Rhodospirillum rubrum, and Azotobacter vinelandii. Rates of acetylene reduction showed a gradual increase and reached a peak after 2 to 6 h of continuous incubation under acetylene. Thereafter, enzyme activity rapidly declined. A similar enhancement of ethylene production was observed when pretreatment with acetylene was interrupted periodically by a brief exposure to ambient (or oxygen-free) atmosphere without acetylene although the decline of acetylene-reducing activity was less rapid. Pretreatment with acetylene depressed photosynthetic 14CO2 fixation and 15N2 incorporation in Anabaena cylindrica. It is concluded that assessments based on long-term experimental incubation with acetylene may grossly overestimate the actual quantities of fixed nitrogen in the field.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Fay P. Photostimulation of nitrogen fixation in Anabaena cylindrica. Biochim Biophys Acta. 1970 Sep 1;216(2):353–356. doi: 10.1016/0005-2728(70)90226-4. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Knight E., Jr ATP-dependent reduction of azide and HCN by N2-fixing enzymes of Azotobacter vinelandii and Clostridium pasteurianum. Biochim Biophys Acta. 1967 May 16;139(1):69–90. doi: 10.1016/0005-2744(67)90114-3. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., Chen C. H., Burris R. H. Inhibition of nitrogenase-catalyzed reductions. Biochim Biophys Acta. 1973 Jan 18;292(1):256–270. doi: 10.1016/0005-2728(73)90270-3. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Shanmugam K. T., Valentine R. C. Molecular biology of nitrogen fixation. Science. 1975 Mar 14;187(4180):919–924. doi: 10.1126/science.238283. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- de Vasconcelos L., Fay P. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Mikrobiol. 1974 Mar 28;96(4):271–279. [PubMed] [Google Scholar]


