Abstract
Nucleotide excision repair proteins have been implicated in genetic recombination by experiments in Saccharomyces cerevisiae and Drosophila melanogaster, but their role, if any, in mammalian cells is undefined. To investigate the role of the nucleotide excision repair gene ERCC1, the hamster homologue to the S. cerevisiae RAD10 gene, we disabled the gene by targeted knockout. Partial tandem duplications of the adenine phosphoribosyltransferase (APRT) gene then were constructed at the endogenous APRT locus in ERCC1− and ERCC1+ cells. To detect the full spectrum of gene-altering events, we used a loss-of-function assay in which the parental APRT+ tandem duplication could give rise to APRT− cells by homologous recombination, gene rearrangement, or point mutation. Measurement of rates and analysis of individual APRT− products indicated that gene rearrangements (principally deletions) were increased at least 50-fold, whereas homologous recombination was affected little. The formation of deletions is not caused by a general effect of the ERCC1 deficiency on gene stability, because ERCC1− cell lines with a single wild-type copy of the APRT gene yielded no increase in deletions. Thus, deletion formation is dependent on the tandem duplication, and presumably the process of homologous recombination. Recombination-dependent deletion formation in ERCC1− cells is supported by a significant decrease in a particular class of crossover products that are thought to arise by repair of a heteroduplex intermediate in recombination. We suggest that the ERCC1 gene product in mammalian cells is involved in the processing of heteroduplex intermediates in recombination and that the misprocessed intermediates in ERCC1− cells are repaired by illegitimate recombination.
Eukaryotic cells defective in nucleotide excision repair (NER) are characterized by their inability to repair lesions such as pyrimidine dimers, bulky DNA adducts that are believed to distort the DNA helix (1, 2), and interstrand crosslinks (3). The role of NER in the repair of interstrand crosslinks, in particular, is believed to proceed by a pathway involving recombination between damaged and undamaged copies of DNA duplexes (1). In humans, defects in NER cause the genetic disease xeroderma pigmentosum (XP), which is associated with a high incidence of skin cancer and, in many cases, developmental and neurological abnormalities (1, 2). Some of the gene products involved in NER have been identified as components of the cellular transcription machinery (1, 2), but it is not known what other biological roles NER gene products might play in mammalian cells. In this study, we investigate the role of the NER gene ERCC1 in recombination and gene stability.
The Ercc1 protein, in a multiprotein complex with the XPF gene product (4–8), forms an endonuclease that cleaves on the 5′ side of damaged DNA during NER (4, 6, 7). Another protein, the XPG gene product, is responsible for cleavage on the 3′ side of the damaged DNA (7–11). In vitro, these complexes are also capable of recognizing structures such as DNA bubbles and loops that might be found in heteroduplex intermediates in homologous recombination (4, 5, 8–11). In Saccharomyces cerevisiae, mutations in the RAD1 and RAD10 genes, which encode the 5′ structure-specific excision repair endonuclease, have variable effects on mitotic recombination (12–17) and severely reduce recombination between sequences containing terminal nonhomologies (18, 19). Mice with engineered disruptions of the ERCC1 gene are defective for DNA repair as expected, but also exhibit a more complex set of phenotypes (20, 21). In mammalian cells, though, a role for NER proteins in recombination has not yet been determined.
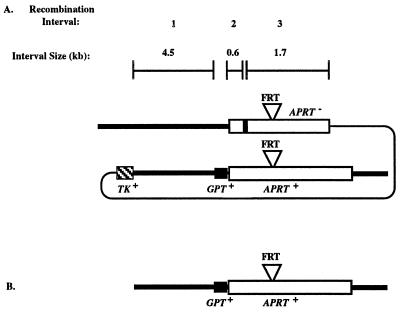
To investigate the influence of the ERCC1 gene product on homologous recombination in mammalian cells, we constructed tandemly duplicated adenine phosphoribosyltransferase (APRT) genes in ERCC1+ and ERCC1− hamster cell lines (Fig. 1A). Because the tandem duplications in both cell lines were constructed at the endogenous APRT locus, we can directly compare measurements of recombination made in the presence and absence of the ERCC1 gene product (22). To detect the full spectrum of events associated with recombination, we designed these experiments as a loss-of-function assay in which the parental APRT+ tandem duplication could give rise to APRT− cells by homologous recombination (both crossovers and gene conversions), illegitimate recombination (all forms of gene rearrangements: deletions, duplications, inversions, and translocations), and small mutations (base changes and frameshifts) (22, 23).
Figure 1.
Gene structures of the APRT locus and a possible recombination intermediate recognized by NER. (A) Tandemly duplicated APRT recombination substrate. In this and other figures, the heavy lines represent chromosomal sequences and the thin line represents plasmid backbone. The APRT gene is represented by an open box, the GPT gene by a solid box, and the TK gene by a crosshatched box. The FRT sequences located in intron 2 are represented by the inverted triangle above both APRT gene copies. The exon 2 mutation that destroys the EcoRV site is denoted by a heavy vertical line at the 5′ end of the upstream APRT copy; at other positions (see Table 2) the heavy line represents undefined APRT point mutations. The sizes of the three recombination intervals defined by the TK gene, GPT gene, and exon-2 mutation are shown above the APRT map. (B) Single copy APRT gene in cell lines used for measuring spontaneous rates of point mutations, deletions, and gene rearrangements.
MATERIALS AND METHODS
Vectors and Gene Targeting.
The targeted disruption of the hamster ERCC1 gene in RMP41 cells, which carry a single copy of the native APRT gene with a yeast FLP recombination target (FRT) recognition site in intron 2 (24), was carried out by using a replacement vector, pSL1, which contains a NEO expression cassette interrupting exon 5 of ERCC1 (25). Recombination substrates (Fig. 1A) were constructed at the native APRT gene in ERCC1− and ERCC1+ RMP41 cells by using the yeast FRT/FLP recombinase site-specific recombination system and the FRT-containing targeting vector pGS73 (24).
Fluctuation Tests and Drug Selections.
Fluctuation tests were done by seeding independent parallel cultures of 50–100 cells and expanding to 2–4 × 107 cells before replating for drug selections (22). The cultures were plated in 0.3 μM 1-(2-deoxy-2-fluoro-β-d]-arabinofuranosyl-5-iodouracil (FIAU) for TK− selections, 0.4 mM 8-azaadenine for APRT− selections, and 0.3 μM FIAU + 0.4 mM 8-azaadenine for TK−APRT− selections, as described (22). Recombination rates (per cell per generation) in each experiment were calculated by the method of the median (26).
Analysis of Recombination Products.
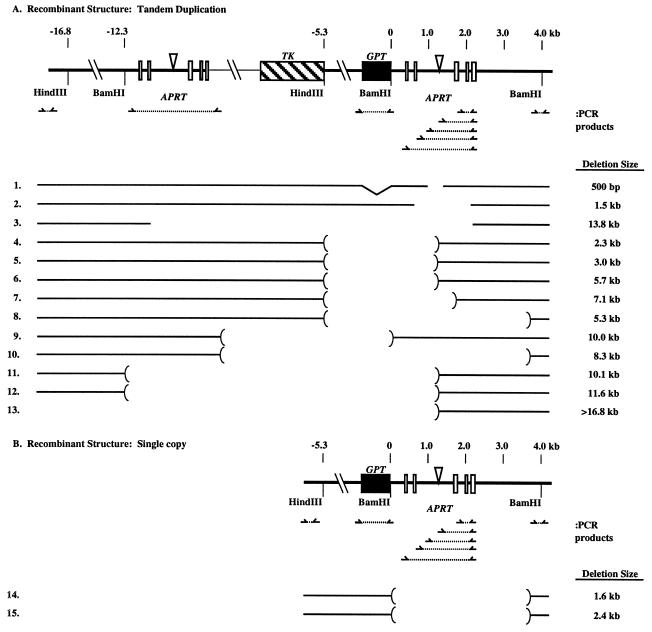
One colony from each parallel culture and each drug selection was picked for further analysis as described (22). Cell DNAs from each colony were used to determine the gene structures of the APRT recombinants by PCR and Southern analysis. Locations of PCR primers used in these experiments are indicated in Fig. 2; their sequences are available on request. The significance level for the difference in crossover with gene conversion products between ERCC1− and ERCC1+ cells was calculated by a Fisher’s exact test (27).
Figure 2.
Molecular structure of APRT− gene deletions and rearrangements recovered from ERCC1− cells. APRT exons are indicated as open boxes. Numbering in kilobase pairs (kb) for these maps is relative to the BamHI site 5′ of the downstream APRT copy (map position 0). The PCR primers used to map the extent of deletions and rearrangements are shown below the APRT maps. The open areas between the brackets for each gene structure represent regions that did not yield PCR products; thus the bracketed regions indicate the interval in which deletion and rearrangement junctions map. The sizes of the deletions as estimated from Southern analysis are indicated at the right. Deletions whose endpoints were determined precisely by sequencing across PCR products are indicated without brackets. A straight line under GPT for both tandem duplication and crossover recombinant structures indicates GPT is present (by PCR); an indentation under the GPT gene indicates it is absent, which may indicate conversion to the wild-type APRT sequence. Rearrangement 1 is missing the GPT gene, perhaps because of gene conversion, but it also has other rearrangements that are apparent from Southern analysis.
RESULTS
Generation of ERCC1− Cells and Experimental Rational.
The ERCC1− cell line used here was generated by targeted insertion of a neomycin (NEO) expression cassette into exon 5 of the hamster ERCC1 gene as described for a closely related Chinese hamster ovary cell line (25). In these Chinese hamster ovary cells, the ERCC1 gene is present as a single copy (25). The ERCC1-targeted insertion initially was produced in RMP41 cells, which carry a single copy of the native APRT gene that contains the yeast FRT recognition site in intron 2 (24). Tandemly duplicated APRT genes (Fig. 1A) then were constructed in ERCC1− and ERCC1+ RMP41 cell lines by site-specific recombination with an APRT targeting vector by using the yeast FRT/FLP recombination system (24). The targeted NEO insertion was judged to eliminate the Ercc1 protein activity by three criteria: (i) no ERCC1 transcript was detected by Northern analysis, (ii) no Ercc1 protein was detected by Western analysis, and (iii) the cells were mitomycin c sensitive, as expected for the loss of Ercc1 activity (ref. 25, data not shown).
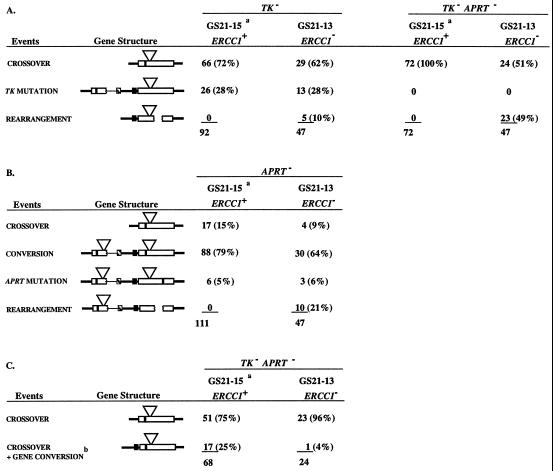
The tandem duplications in the ERCC1+ cell line (GS21–15) and in the ERCC1− cell line (GS21–13) share 6.8 kb of homology that is divided into three intervals defined by two sequence differences (the GPT gene and the APRT-inactivating exon-2 mutation in the EcoRV site) between the upstream and downstream copies (Fig. 1A). Homologous recombination between the duplicated segments yields crossovers and gene conversions. Crossovers, which eliminate one copy of the repeat and the intervening plasmid sequences, were selected in two ways: crossovers in any of the three intervals were selected as TK− cells by plating in medium containing 1-(2-deoxy-2-fluoro-β-d]-arabinofuranosyl-5-iodouracil (FIAU) (22); crossovers in interval 3 were selected as TK−APRT− cells by plating in medium containing FIAU and 8-azaadenine (22). Gene conversions, which involve the unidirectional transfer of the exon-2 EcoRV mutation from the upstream APRT copy to the downstream copy, were selected as APRT− cells by plating in 8-azaadenine (22). In wild-type cells, gene conversions typically outnumber crossovers by 5–10 to 1, accounting for about 80% of all events that lead to an APRT− phenotype (22, 23).
Recombination in ERCC1− Cells.
Rates at which TK−, TK−APRT−, and APRT− cells were generated in the ERCC1+ and ERCC1− cell lines were measured by fluctuation analysis (26) and are shown in Table 1. Rates in the ERCC1− cell line were 2- to 4-fold higher than in the ERCC1+ cell line. When adjusted to reflect only homologous recombination events (by subtracting out the proportion of events that were caused by point mutations and deletions; see below), the rates in the ERCC1− cell line were minimally increased, about 1.5- to 2.5-fold, over those in the ERCC1+ cell line (Table 1). These changes in rate are comparable to results in Drosophila, where mutations in the mei-9 gene, the fly homologue of XPF (RAD1) (28), have been shown to cause a small elevation of mitotic recombination (29). In S. cerevisiae, however, mutations in RAD1 or in RAD10 have been shown to reduce mitotic recombination 2- to 10-fold (13, 15–17), have no effect on recombination (14), or moderately stimulate recombination 3- to 4-fold (12).
Table 1.
Recombination and mutation rates
| Recombination rates (× 10−7)*
| ||
|---|---|---|
| Cell lines
|
||
| Cells | GS21-15, ERCC1+ | GS21-13, ERCC1− |
| TK− | 15 ± 3 (11) | 48 ± 23 (29) |
| TK− APRT− | 1.4 ± 0.5 (1.4) | 6 ± 3 (3.4) |
| APRT− | 14 ± 3 (11) | 31 ± 25 (19) |
| Mutation rates (× 10−7)† | ||
| Cell lines | ||
| Cells | GS782, ERCC1+ | GS96, ERCC1− |
| APRT− | 1.8 ± 0 | 13 ± 8 |
These rates represent the average ± SD of three fluctuation tests. Rates adjusted to reflect only homologous recombination events (by subtracting out the proportion of events caused by point mutations and rearrangements) are indicated in parenthesis. Because the recombination rates from the ERCC1+ cell line GS21-15, which contains the identical APRT gene structure as GS21-13, are the same as previously reported results (22), we have included the previously reported rates in these data.
These rates represent the average ± SD of two fluctuation tests for GS7B2 and three fluctuation tests for GS96.
Gene Rearrangements in ERCC1− Cells.
To determine if the products of the various selections were similar in the ERCC1+ and ERCC1− cell lines, we isolated independent colonies and characterized the structures of their APRT loci by PCR and Southern analysis. As shown in Table 2, the proportions of colonies that fell into various classes were very similar in ERCC1+ and ERCC1− cell lines with one striking exception: gene rearrangements were prominent among the products from ERCC1− cells, but were absent among the products from ERCC1+ cells. Because the ERCC1+ cell line yielded no rearrangements in 275 products (less than 0.4%), whereas the ERCC1− cell line gave 38 rearrangements among 141 analyzed products (27%), rearrangements were increased more than 50-fold in the ERCC1− cell line.
Table 2.
Recombination products
Recombination products from ERCC1+ cell lines are the combined results from experiments done here by using the cell line GS21-15 and previously reported results (22).
This is a subclass of the crossover events obtained from the TK− APRT− selections summarized in A. Of the 72 TK−APRT− recombinants derived from ERCC1+ cells with a crossover structure, 68 were tested for the accompanying gene conversion of GPT. All 24 TK− APRT− recombinants derived from ERCC1− cells were analyzed for the accompanying gene conversion of GPT.
To identify the specific types of rearrangements formed in these experiments, we analyzed 15 in detail by using both PCR and Southern analysis. Their molecular structures, shown in Fig. 2, indicate that deletions are the predominant rearrangement; only rearrangement 1 is more complex, because it had other bands in addition to the predicted Southern pattern. Deletions involving the tandem duplication are shown in Fig. 2A; deletions associated with crossovers, are shown in Fig. 2B. The combination of PCR and Southern analysis allowed us to map approximate locations for the deletions (Fig. 2, brackets) and to estimate their size (Fig. 2, Right). Rearrangement 1 and deletions 2 and 3 were amplified by PCR primers that spanned the deletion junctions and sequenced. The DNA sequences at the deletion junctions shared 1–4 bp of homology similar to other well characterized APRT and mammalian cell deletions and rearrangements (23, 30–33).
Gene Stability in ERCC1− Cells.
The prevalent deletions in the ERCC1− cell line might reflect an abnormality in the recombination process associated with the absence of the ERCC1 gene product, or alternatively they could have arisen independently of recombination as a general effect on gene stability caused by abnormal NER. To distinguish between these alternatives, we isolated from the ERCC1− cell line GS21–13 a derivative, GS96, that contained a single copy of the APRT+ gene with an adjacent GPT gene (Fig. 1B). In the absence of the second copy of the APRT gene, the homologous recombination events detected in the above experiments could not occur; however, other events, including deletions and small mutations, would be detectable as APRT− cells. The rate at which APRT− cells were generated in the ERCC1− cell line GS96 was about 7-fold higher than in the corresponding ERCC1+ cell line (GS7B2), which contains an identical APRT gene structure (Table 1). Consistent with this observation, the rates at which TK− and APRT− mutant cells arose in the ERCC1− cell line GS21–13 were increased 2- to 3-fold (Tables 1 and 2), not significantly different from GS96. (Because the overall rates of appearance of TK− and APRT− cells in GS21–13 were increased 2- to 3-fold and the proportion of TK− and APRT− mutant cells remained the same relative to the GS21–15, the overall rates of TK− and APRT− mutations increased 2- to 3-fold.)
Significantly, the higher rate measured in GS96 was not accompanied by an increase in deletions or rearrangements: among 49 independent colonies from the ERCC1− cell line, 48 were point mutants and only one was a rearrangement. These numbers fit with the general expectation of a 10:1 ratio of point mutations to deletions at the APRT locus in wild-type Chinese hamster ovary cells (30, 31). They also agree with other studies that show that deletions in cell lines with a single copy of APRT were stimulated less than 2-fold in Chinese hamster ovary cell lines with two different ERCC1− mutant alleles (refs. 30, 34, and 35; data not shown). Collectively, these studies (refs. 30, 34, and 35; data not shown) and ours indicate that ERCC1 deficiency does not have a substantial effect on gene stability as assessed by deletions and other rearrangements.
DISCUSSION
As a component of a structure-specific endonuclease, the Ercc1 protein is expected to play two roles in DNA metabolism: (i) cleavage on the 5′ side of damaged DNA in NER (6, 9) and (ii) by analogy to the S. cerevisiae RAD1/RAD10 homologs, removal of nonhomologous tails from invading single strands during recombination (18, 19). The 50-fold increase in deletions among the products of intrachromosomal recombination in ERCC1−-deficient cells suggests that the Ercc1 protein may have an additional role in recombination and gene stability. The possibility that the deletions were generated as a consequence of a genome-wide instability induced by the ERCC1 deficiency was ruled out by showing that deletions were not prevalent in ERCC1− cells that carry a single copy of the APRT gene: an observation consistent with previous studies that used two different ERCC1− mutant alleles (30, 34, 35). Because deletions were increased only in ERCC1− cells that contained tandemly duplicated APRT genes, it is likely that excision repair proteins are involved in the recombination process itself.
The absence of an increase in deletions in ERCC1− cells with a single copy of APRT also suggests, indirectly, that nonidentical copies of the APRT locus may be necessary to trigger the formation of deletions. For mammalian cells it is estimated that about 80% of mitotic recombination events between tandemly duplicated copies occurs by recombination between sister chromatids (36). In the absence of a tandem duplication, recombination between the single APRT copies on sister chromatids presumably still occurs; such recombination events normally would not be detected because there would be no genetic consequence if the copies were identical. If the recombination process were rendered abnormal, however, by the absence of the Ercc1 protein, detectable deletions should be generated. The low incidence of deletions in the ERCC1−, single-copy APRT cell line argues that the Ercc1 protein may interact with the recombination machinery only when the recombining genes are not identical.
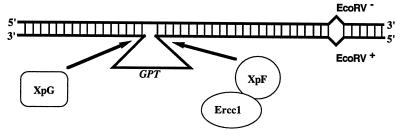
A candidate substrate for NER that might arise during recombination between nonidentical, tandemly duplicated genes is the heteroduplex indicated in Fig. 3. We have argued previously (22) that this heteroduplex is common in recombination at the APRT locus because of the prevalence of recombination products that are crossovers associated with a gene conversion; in ERCC1+ cell lines this class accounts for 25% of the TK−APRT− crossovers (see Table 2). This class is thought to arise by repair at one or both mismatches in the heteroduplex with a crossover downstream of the EcoRV mismatch (22). Significantly, this class of products appears to be substantially reduced (P < 0.05) in ERCC1− cells; only one of 24 crossovers (4%) had an associated gene conversion (Table 2). The reduction in crossovers associated with a gene conversion is similar to results observed in RAD1− S. cerevisiae cells, where this class of products was significantly reduced (13).
Figure 3.
A recombination heteroduplex intermediate that could be a substrate for the Ercc1/XpF endonuclease. The heteroduplex DNA was created by annealing the top DNA strand from the upstream APRT gene to the bottom strand from the downstream APRT gene so that the heteroduplex spans the GPT gene (see Fig. 1). This heteroduplex creates an ≈800-bp deletion loop similar to in vitro substrates processed by purified NER proteins, whose putative sites of action are indicated.
How might the intermediate in Fig. 3 lead to deletions? One possibility is that the heteroduplex is misprocessed in cells lacking the Ercc1/XpF endonuclease activity. In vitro experiments that used purified proteins have shown that similar substrates can be bound and cleaved by the Ercc1/XpF complex and by the XpG protein (4–11). In ERCC1− cells, XpG-mediated cleavage of the loop in the heteroduplex intermediate shown in Fig. 3 would leave a nicked DNA duplex with an ≈800-bp single-stranded tail. In the absence of further processing by the Ercc1/XpF complex, this abnormal structure could serve as an entry point for proteins that resolve the structure by illegitimate recombination; alternatively, the single-strand tail could infiltrate elsewhere in the genome and form structures that lead to deletions or other gene rearrangements (23, 37, 38). If misprocessing of the heteroduplex intermediate by the NER machinery is the cause of the observed deletions, then deletion breakpoints might be expected to encompass GPT, as appears to be the case: 11 of 15 deletions have breakpoints that bracket the GPT gene (Fig. 2A, deletions 3–13). Elimination of the mismatched heteroduplex, by using tandemly duplicated APRT genes without the GPT gene, might be expected to decrease the frequency of deletions, as would a second mutation in the XPG gene, which might prevent misprocessing of the heteroduplex. We currently are pursuing these experimental approaches and others to clarify the nature of detected interaction between the NER and recombination machinery in mammalian cells.
The high incidence of deletions recovered in our recombination experiments was not observed in similar experiments that used RAD1− or RAD10− S. cerevisiae (12–17). In part, this difference is likely to be a consequence of experimental design. Our experiments used a loss-of-function recombination substrate, which allowed detection of homologous recombination, rearrangements, and mutations. By contrast, the gain-of-function substrates used in S. cerevisiae were designed to select specifically for homologous recombination events, and thus were blind to rearrangements and mutations (12–17). Even if loss-of-function substrates had been used in S. cerevisiae, however, rearrangements still might not have been observed because illegitimate recombination rarely is detected in S. cerevisiae except in RAD52-defective cells or in cells where no homolog is available for repair by homologous recombination (39–41). Because illegitimate recombination is very active in mammalian cells (32, 33), the consequences of ERCC1 deficiency may be fundamentally different than in S. cerevisiae.
Our results, and those from in vitro studies that used purified proteins, suggest that NER is the mammalian cell pathway that processes bulky DNA adducts and DNA structures containing bubbles and loops greater than about 14 bp (11), whereas mismatch repair is responsible for single base mismatches and structures containing bubbles and loops fewer than 14 bp (42–44). Cells defective for mismatch repair often display an increased polymorphism in dinucleotide repeat lengths (45–51). It is believed that slippage of these sequences during DNA synthesis and the resulting out-of-register mispairing, which goes unrepaired in mismatch defective cells, lead to the observed sequence expansions and contractions. Other sequences with the potential to generate DNA structures such as H-DNA, stem-loop structures, and DNA structures associated with some triplet repeat sequences (52–54), also may generate substrates for processing by NER. In that context, it is interesting to note that triplet repeats do not show instability in human cell lines deficient in mismatch repair (55). We currently are testing the influence of NER on the stability of these candidate sequences by introducing them into the APRT gene in ERCC1+ and ERCC1− cells.
Our results suggest that the ERCC1 gene product helps to maintain genome integrity by ensuring the correct processing of intermediates in homologous recombination. The loss of Ercc1 activity, or loss of other excision repair activities, may contribute to carcinogenesis by promoting deletions and chromosomal rearrangements, similar to the deletions observed here. NER may be especially important in recombination events that take place between partially diverged members of gene families (56). If the Ercc1 protein played the same role in meiotic recombination, it would help to maintain genome stability across generations. Interestingly, mutations in the Drosophila mei-9 gene, which encodes an XPF homologue, affect both mitotic and meiotic recombination (29, 57–59). Mei-9 mutants exhibit an increased incidence of chromosomal instability (29, 60, 61), increased frequency of postmeiotic segregation (58), and lowered rate of meiotic recombination (58, 59), all of which could be explained by misprocessing of heteroduplex intermediates in homologous recombination. It will be interesting to see if NER proteins also play a similar role in meiotic recombination in mammals.
Acknowledgments
We thank Barbara Santi for her excellent technical assistance, and Mark Brenneman and Peter Glazer for comments on the manuscript. This investigation was supported by National Institutes of Health grants to J.H.W. (GM38219) and to R.S.N. (CA36361). R.L.R. is supported by a Rosalie B. Hite fellowship.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: APRT, adenine phosphoribosyltransferase; NER, nucleotide excision repair; XP, xeroderma pigmentosum; FRT, FLP recombination target.
References
- 1.Friedberg E C, Walker G C, Seide W. DNA Repair and Mutagenesis. Washington D.C.: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 2.Chu G, Mayne L. Trends Genet. 1996;2:187–192. doi: 10.1016/0168-9525(96)10021-4. [DOI] [PubMed] [Google Scholar]
- 3.Jachymczyk W J, von Borstol R C, Mowat M R A, Hastings P J. Mol Gen Genet. 1981;182:196–205. doi: 10.1007/BF00269658. [DOI] [PubMed] [Google Scholar]
- 4.Bessho T, Sancar A, Thompson L H, Thelan M P. J Biol Chem. 1997;272:3833–3837. doi: 10.1074/jbc.272.6.3833. [DOI] [PubMed] [Google Scholar]
- 5.Park C H, Bessho T, Matsunaga T, Sancar A. J Biol Chem. 1995;270:22657–22660. doi: 10.1074/jbc.270.39.22657. [DOI] [PubMed] [Google Scholar]
- 6.Sijbers A M, de Laat W L, Ariza R R, Biggerstaff M, Wei Y F, Moggs J G, Carter K C, Shell B K, Evans E, de Jong M C, Rademakers S, de Rooij J, Jaspers N G, Hoeijmakers J H, Wood R D. Cell. 1996;86:811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 7.Mu D, Hsu D S, Sancar A. J Biol Chem. 1996;271:8285–8294. doi: 10.1074/jbc.271.14.8285. [DOI] [PubMed] [Google Scholar]
- 8.Mu D, Park C H, Matsunaga T, Hsu D S, Reardon J T, Sancar A. J Biol Chem. 1996;270:2415–2418. doi: 10.1074/jbc.270.6.2415. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga T, Mu D, Park C H, Reardon J T, Sancar A. J Biol Chem. 1995;270:20862–20869. doi: 10.1074/jbc.270.35.20862. [DOI] [PubMed] [Google Scholar]
- 10.O’Donovan A, Davies A A, Moggs J G, West S C, Wood R D. Nature (London) 1994;371:432–435. doi: 10.1038/371432a0. [DOI] [PubMed] [Google Scholar]
- 11.Matsunaga T, Park C H, Bessho T, Mu D, Sancar A. J Biol Chem. 1996;271:11047–11050. doi: 10.1074/jbc.271.19.11047. [DOI] [PubMed] [Google Scholar]
- 12.Huang K N, Symington L S. Mol Cell Biol. 1994;14:6030–6045. doi: 10.1128/mcb.14.9.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein H L. Genetics. 1988;120:367–377. doi: 10.1093/genetics/120.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rattray A J, Symington L S. Genetics. 1995;139:45–56. doi: 10.1093/genetics/139.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiestl R H, Prakash S. Mol Cell Biol. 1988;8:3619–3626. doi: 10.1128/mcb.8.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiestl R H, Prakash S. Mol Cell Biol. 1990;10:2485–2491. doi: 10.1128/mcb.10.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas B J, Rothstein R. Genetics. 1989;123:725–738. doi: 10.1093/genetics/123.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishman-Lobell J, Haber J E. Science. 1992;258:480–484. doi: 10.1126/science.1411547. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov E L, Haber J E. Mol Cell Biol. 1995;15:2245–2251. doi: 10.1128/mcb.15.4.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mcwhir J, Selfridge J, Harrison D J, Squires S, Melton D W. Nat Genet. 1993;5:217–224. doi: 10.1038/ng1193-217. [DOI] [PubMed] [Google Scholar]
- 21.Weeda, G., Donker, I., de Wit, J., Morreau, H., Janssens, R., Vissers, C. J., Nigg, A., van Steeg, H., Bootsma, D. & Hoeijmakers, J. H. J. Curr. Biol. 7, 427–439. [DOI] [PubMed]
- 22.Sargent R G, Merrihew R V, Nairn R, Adair G, Meuth M, Wilson J H. Nucleic Acids Res. 1996;24:746–753. doi: 10.1093/nar/24.4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sargent R G, Brenneman M A, Wilson J H. Mol Cell Biol. 1997;17:267–277. doi: 10.1128/mcb.17.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrihew R V, Sargent R G, Wilson J H. Somat Cell Mol Genet. 1995;21:299–307. doi: 10.1007/BF02257465. [DOI] [PubMed] [Google Scholar]
- 25.Rolig R L, Layher S K, Santi B, Adair G M, Gu F, Rainbow A J, Nairn R S. Mutagenesis. 1997;12:277–283. doi: 10.1093/mutage/12.4.277. [DOI] [PubMed] [Google Scholar]
- 26.Lea D E, Coulson C A. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 27.Matson D E. Statistics. St. Louis: Mosby; 1981. [Google Scholar]
- 28.Sekelsky J J, McKim K S, Chin G M, Hawley R S. Genetics. 1995;141:619–627. doi: 10.1093/genetics/141.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker B S, Carpenter A T C, Ripoll P. Genetics. 1978;90:531–578. doi: 10.1093/genetics/90.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phear G, Armstrong W, Meuth M. J Mol Biol. 1989;209:577–582. doi: 10.1016/0022-2836(89)90595-0. [DOI] [PubMed] [Google Scholar]
- 31.Meuth M, Miles C, Phear G, Sargent G. Progr Clin Biol Res. 1990;340A:305–314. [PubMed] [Google Scholar]
- 32.Roth D B, Wilson J H. In: Genetic Recombination. Kucherlapati R, Smith G R, editors. Washington, D.C.: Am. Soc. Microbiol.; 1988. pp. 621–653. [Google Scholar]
- 33.Meuth M. In: Mobile DNA. Berg D E, Howe M M, editors. Washington, D.C.: Am. Soc. Microbiol.; 1989. pp. 833–860. [Google Scholar]
- 34.Adair G M. In: Mammalian Cell Mutagenesis, Banbury Report 28. Moore M M, Marini D M, de Serres F J, Tindall K R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1987. pp. 3–13. [Google Scholar]
- 35.Sage E, Lamolet B, Brulay E, Moustacchi E, Chateauneuf A, Drobetsky E A. Proc Natl Acad Sci USA. 1996;93:176–180. doi: 10.1073/pnas.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bollag R J, Liskay R M. Mol Cell Biol. 1991;11:4839–4845. doi: 10.1128/mcb.11.10.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrihew R V, Marburger K, Pennington S L, Roth D B, Wilson J H. Mol Cell Biol. 1996;16:10–18. doi: 10.1128/mcb.16.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakagami K, Tokinaga Y, Yoshikure H, Kobayashi I. Proc Natl Acad Sci USA. 1994;91:8527–8531. doi: 10.1073/pnas.91.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer K, Brock J A, Bloom K, Moore J K, Haber J E. Mol Cell Biol. 1994;14:1293–1301. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore J K, Haber J E. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugawara N, Haber J E. Mol Cell Biol. 1992;12:563–575. doi: 10.1128/mcb.12.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umar A, Boyer J, Kunkel T A. Science. 1994;266:814–816. doi: 10.1126/science.7973637. [DOI] [PubMed] [Google Scholar]
- 43.Tran H T, Gordenin D A, Resnick M A. Genetics. 1996;143:1579–1587. doi: 10.1093/genetics/143.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fishel R, Ewel A, Lee S, Lescoe M K, Griffith J. Science. 1994;266:1403–1405. doi: 10.1126/science.7973733. [DOI] [PubMed] [Google Scholar]
- 45.Strand M, Early M C, Crouse G F, Petes T D. Proc Natl Acad Sci USA. 1995;92:10418–10421. doi: 10.1073/pnas.92.22.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strand M, Prolla T, Liskay R M, Petes T D. Nature (London) 1993;365:274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- 47.Thibodeau S N, Bren G, Schaid D. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 48.Aaltonen L A, Peltomaki P, Leach F S, Sistonen P, Pylkkanen L, Mecklin J P, Jarvinen H, Powell S M, Jen J, Hamilton S R, Petersen G M, Kinzler K W, Vogelstein B, de la Chapelle A. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 49.Fishel R, Lescoe M K, Rao MR, Copeland NG, Jenkins N A, Garber J, Kane M, Kolodner R. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 50.Ionov Y, Peinado M A, Malkhosyan S, Shibata D, Perucho M. Nature (London) 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 51.Parsons R, Li G M, Longley M J, Fang W H, Papadopoulos N, Jen J, de la Chapelle A, Kinzler K W, Vogelstein B, Modrich P. Cell. 1993;75:1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 52.Blaho J A, Wells R D. Progr Nucleic Acid Res Mol Biol. 1989;37:107–126. doi: 10.1016/s0079-6603(08)60696-0. [DOI] [PubMed] [Google Scholar]
- 53.Sinden R R, Wells R D. Curr Opin Biotechnol. 1992;3:612–622. doi: 10.1016/0958-1669(92)90005-4. [DOI] [PubMed] [Google Scholar]
- 54.Wells R D. J Biol Chem. 1996;271:2875–2878. doi: 10.1074/jbc.271.6.2875. [DOI] [PubMed] [Google Scholar]
- 55.Kramer P R, Pearson C E, Sindon R R. Hum Genet. 1996;98:151–157. doi: 10.1007/s004390050179. [DOI] [PubMed] [Google Scholar]
- 56.Collins F S, Weissman S M. Progr Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter A T C. Proc Natl Acad Sci USA. 1982;79:5961–5965. doi: 10.1073/pnas.79.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker B S, Carpenter A T C. Genetics. 1972;71:255–286. doi: 10.1093/genetics/71.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carpenter A T C, Sandler L. Genetics. 1974;76:453–475. doi: 10.1093/genetics/76.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker B S, Gatti M, Carpenter A T C, Pimpinelli S, Smith D A. Basic Life Sci. 1980;15:189–208. doi: 10.1007/978-1-4684-3842-0_13. [DOI] [PubMed] [Google Scholar]
- 61.Gatti M. Proc Natl Acad Sci USA. 1979;76:1377–1381. doi: 10.1073/pnas.76.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]