Abstract
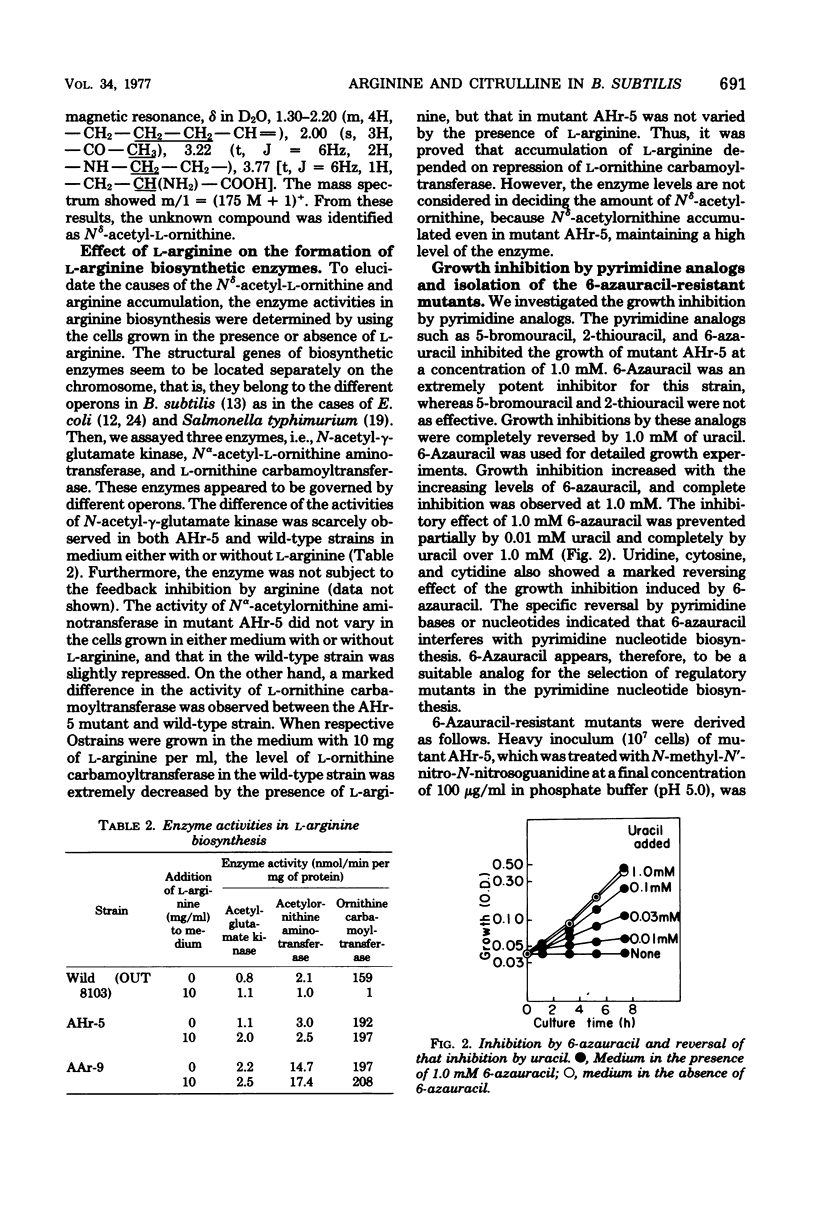
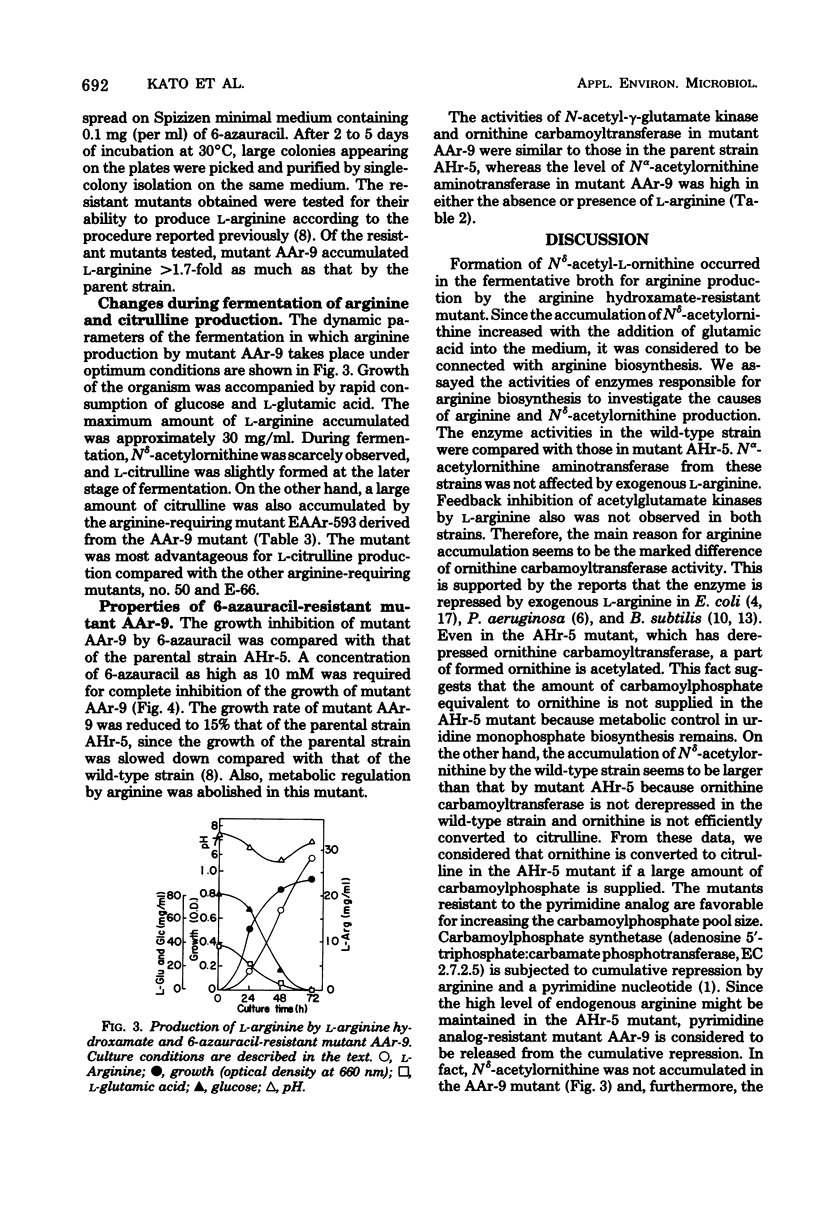
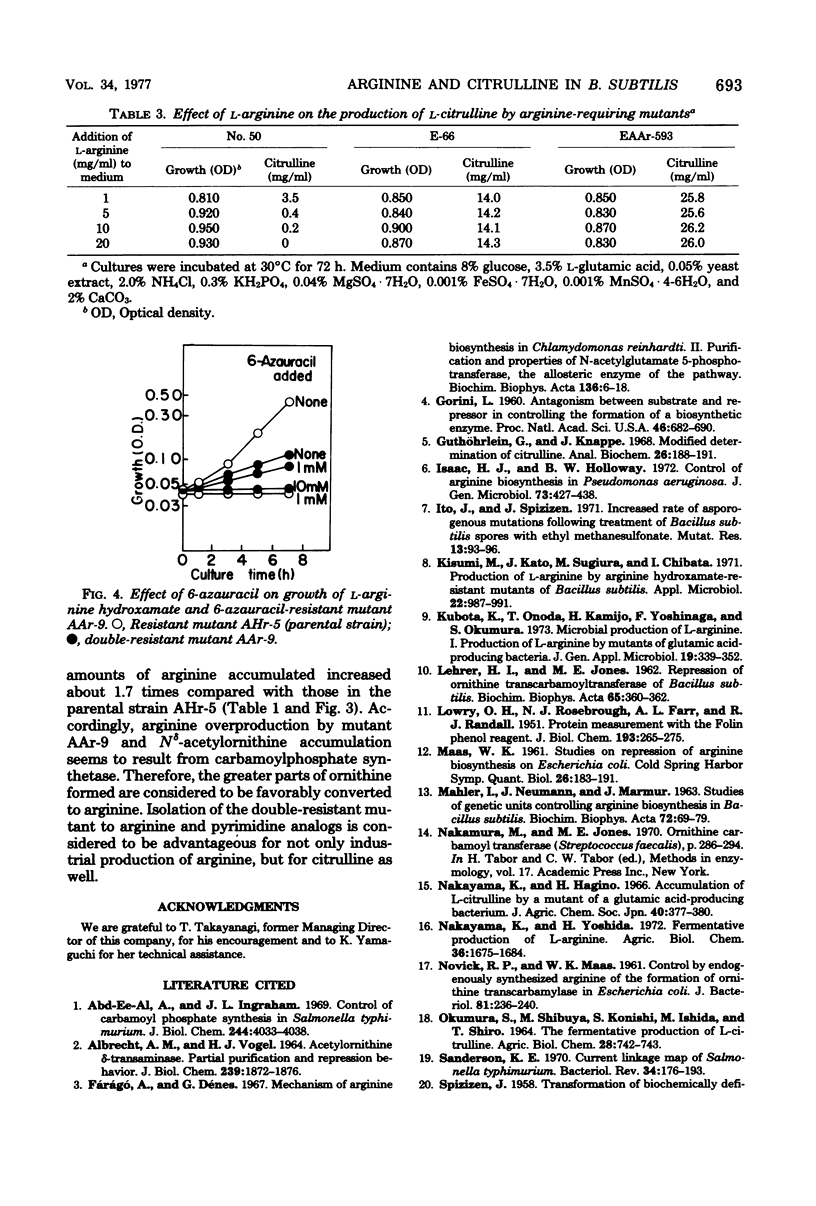
In the arginine producer AHr-5, an L-arginine hydroxamate-resistant mutant of Bacillus subtilis, accumulation of N8-acetyl-L-ornithine increased as the level of L-arginine accumulation increased in the medium containing L-glutamic acid. Ornithine carbamoyltransferase of this strain was genetically derepressed. These results suggested that carbamoylphosphate might be deficient in vivo. With the intention to increase endogenous carbamoylphosphate, pyrimidine analogs inhibiting growth were selected and the mutants resistant to these compounds were derived from the AHr-5 mutant. Of the resistant mutants derived, the 6-azauracil-resistant mutant AAr-9 produced 28 mg of L-arginine per ml, which corresponded to more than twofold the amount produced by the parent strain. Derivation of an arginine-requiring mutant from the double-resistant mutant AAr-9 provides a new advantageous method for the production of L-citrulline. The increase in arginine and citrulline production is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT A. M., VOGEL H. J. ACETYLORNITHINE DELTA-TRANSAMINASE. PARTIAL PURIFICATION AND REPRESSION BEHAVIOR. J Biol Chem. 1964 Jun;239:1872–1876. [PubMed] [Google Scholar]
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Gorini L. ANTAGONISM BETWEEN SUBSTRATE AND REPRESSOR IN CONTROLLING THE FORMATION OF A BIOSYNTHETIC ENZYME. Proc Natl Acad Sci U S A. 1960 May;46(5):682–690. doi: 10.1073/pnas.46.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthöhrlein G., Knappe J. Modified determination of citrulline. Anal Biochem. 1968 Oct 10;26(1):188–191. doi: 10.1016/0003-2697(68)90045-6. [DOI] [PubMed] [Google Scholar]
- Isaac J. H., Holloway B. W. Control of arginine biosynthesis in Pseudomonas aeruginosa. J Gen Microbiol. 1972 Dec;73(3):427–438. doi: 10.1099/00221287-73-3-427. [DOI] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Increased rate of asporogenous mutations following treatment of Bacillus subtilis spores with ethyl methanesulfonate. Mutat Res. 1971 Sep;13(1):93–96. doi: 10.1016/0027-5107(71)90130-8. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Kato J., Sugiura M., Chibata I. Production of L-arginine by arginine hydroxamate-resistant mutants of Bacillus subtilis. Appl Microbiol. 1971 Dec;22(6):987–991. doi: 10.1128/am.22.6.987-991.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHRER H. I., JONES M. E. Repression of ornithine transcarbamoyltransferase of Bacillus subtilis. Biochim Biophys Acta. 1962 Dec 4;65:360–362. doi: 10.1016/0006-3002(62)91059-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- MAHLER I., NEUMANN I. M., MARMUR J. Studies of genetic-units controlling arginine biosynthesis in Bacillus subtilis. Biochim Biophys Acta. 1963 May 28;72:69–79. [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J. Aspects of repression in the regulation of enzyme synthesis: pathway-wide control and enzyme-specific response. Cold Spring Harb Symp Quant Biol. 1961;26:163–172. doi: 10.1101/sqb.1961.026.01.021. [DOI] [PubMed] [Google Scholar]


