Abstract
Details are presented for the construction of a simple precision temperature-controlled chamber for investigating bacterial motile behavior. Independent of original incubation temperature, all species of motile bacteria observed showed a five- to sevenfold increase in average translational velocity (micrometers per second) as the environment temperature was incremented over the range from 10 to 50 degrees C. Temperature jumps downward produced transient tumbling or reciprocal behavior responses, depending on the mode of flagellar distribution, in all species examined. Upward temperature jumps induced accelerated velocities without tumbling or reversal. A partial capacity adaptation to temperature was noted, in that the greatest average translational velocity at any given observation temperature occurred when the organisms were grown at temperatures less than the optimum.
Full text
PDF
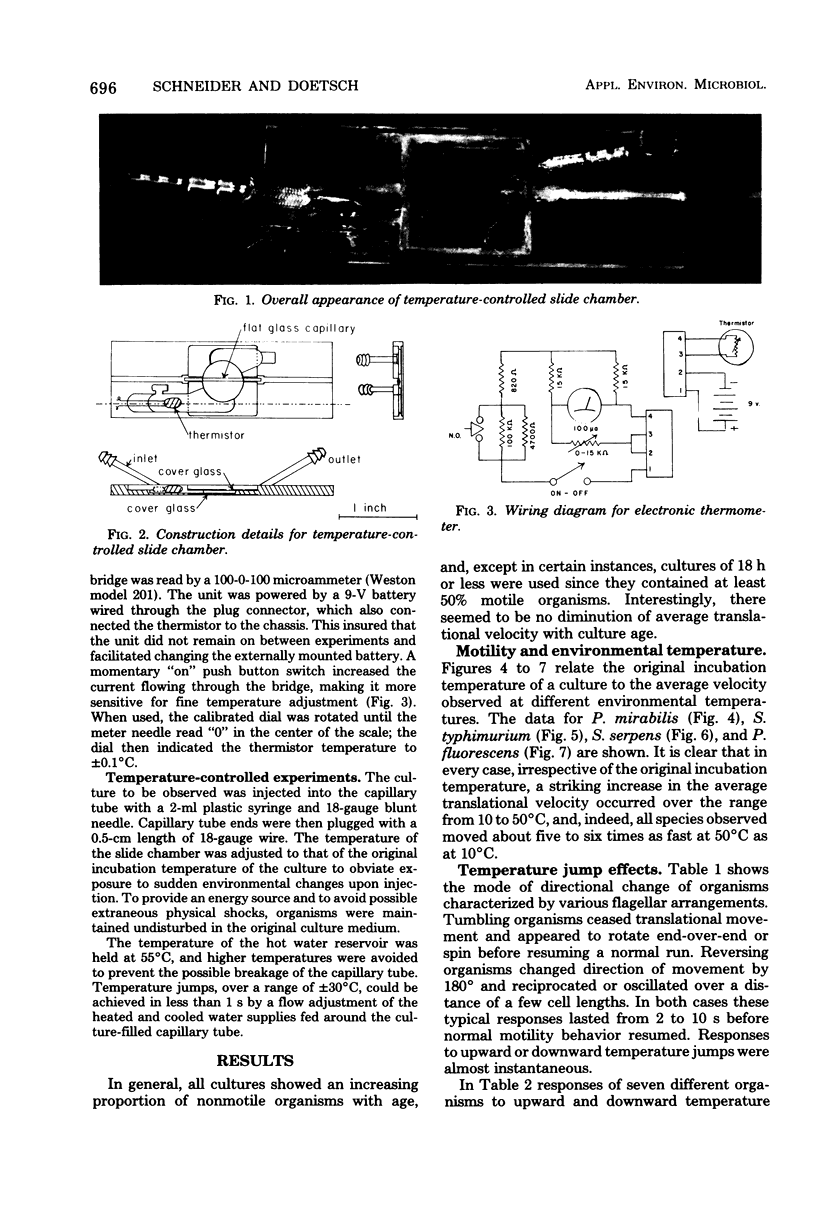
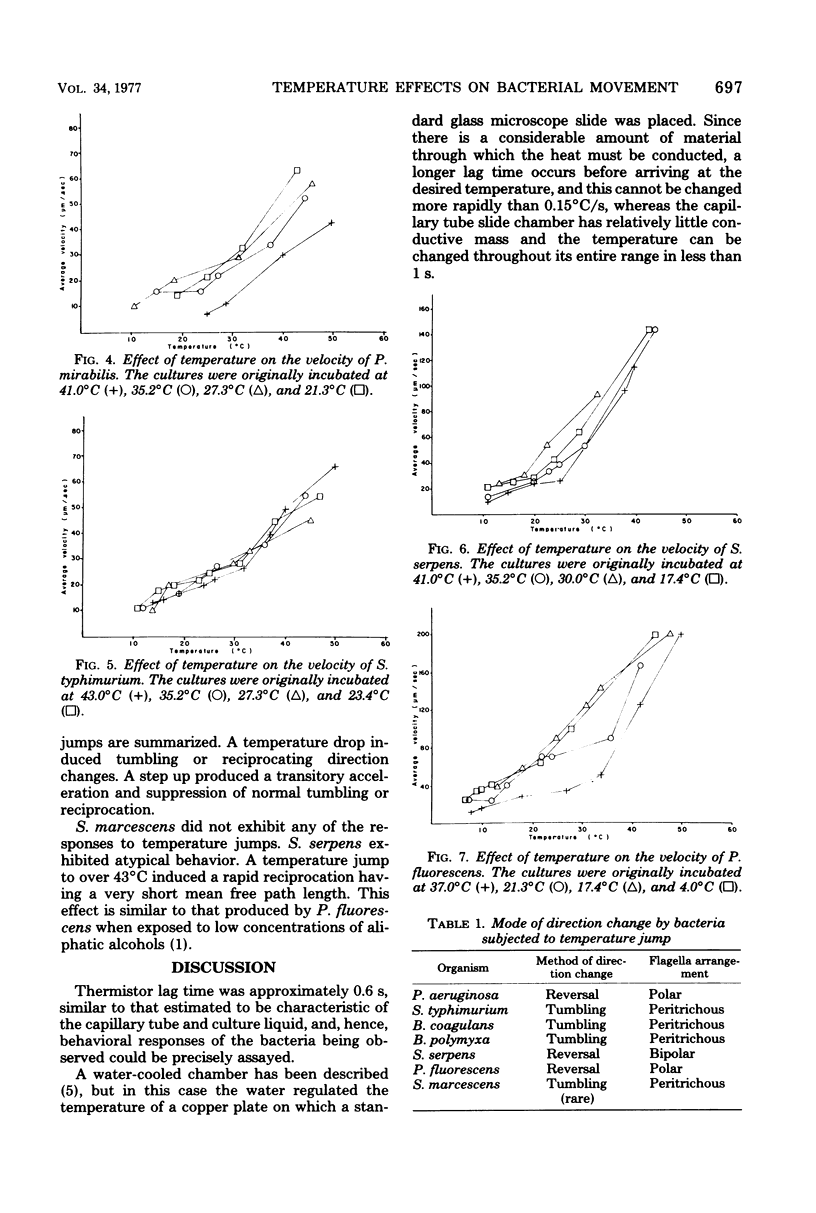
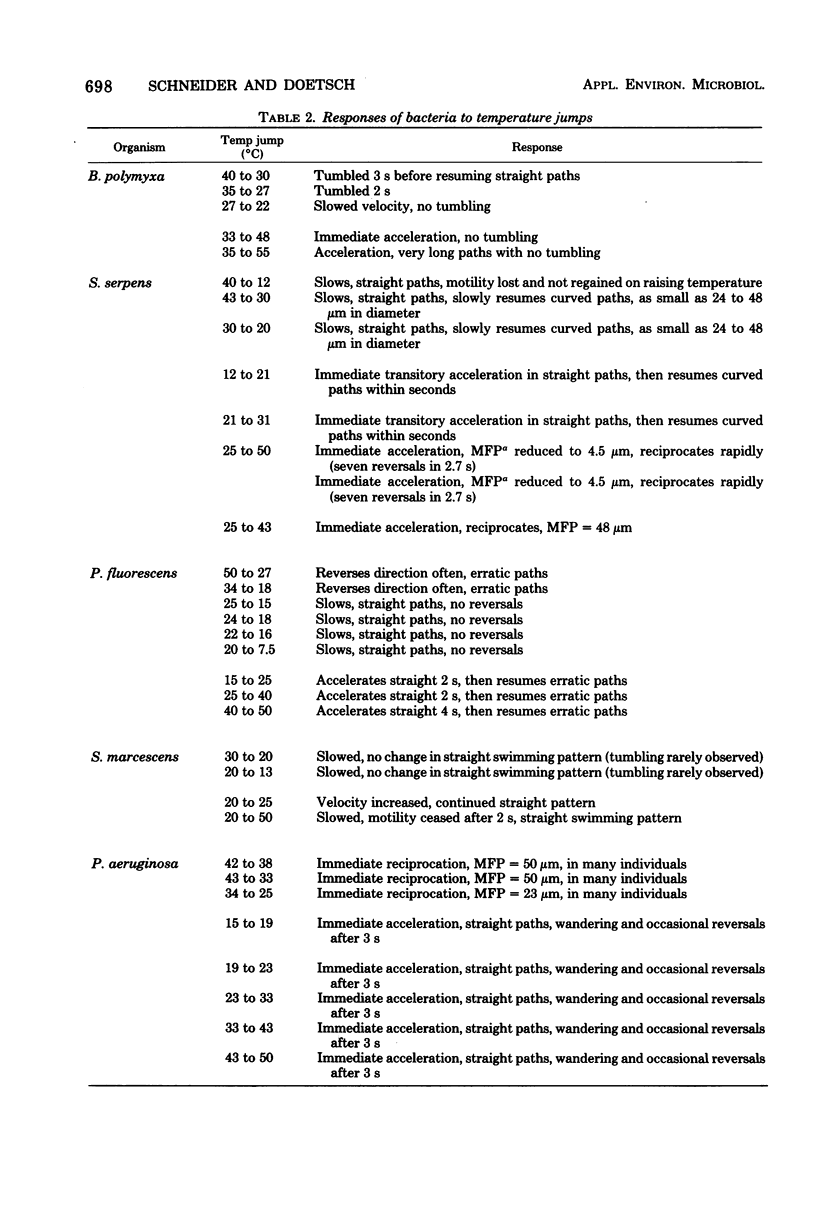


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddle C., Doetsch R. N. Aliphatic alcohols and bacterial motile behavior. Can J Microbiol. 1974 Jul;20(7):1063–1065. doi: 10.1139/m74-165. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Berg H. C. Temporal stimulation of chemotaxis in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1388–1392. doi: 10.1073/pnas.71.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Imae Y., Shioi J. I., Oosawa F. Effect of temperature on motility and chemotaxis of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1039–1046. doi: 10.1128/jb.127.3.1039-1046.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R. Ciliary orientation: controlled by cell membrane or by intracellular fibrils? Science. 1969 Dec 26;166(3913):1633–1635. doi: 10.1126/science.166.3913.1633. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R. Ionic mechanisms controlling behavioral responses of paramecium to mechanical stimulation. Science. 1969 May 23;164(3882):963–965. doi: 10.1126/science.164.3882.963. [DOI] [PubMed] [Google Scholar]
- Schneider W. R., Doetsch R. N. Velocity measurements of motile bacteria by use of a videotape recording technique. Appl Microbiol. 1974 Jan;27(1):283–284. doi: 10.1128/am.27.1.283-284.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada K., Oosawa F. Responses of Paramecium to temperature change. J Protozool. 1972 Feb;19(1):53–57. doi: 10.1111/j.1550-7408.1972.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Tsang N., Macnab R., Koshland D. E., Jr Common mechanism for repellents and attractants in bacterial chemotaxis. Science. 1973 Jul 6;181(4094):60–63. doi: 10.1126/science.181.4094.60. [DOI] [PubMed] [Google Scholar]