Abstract
Neurotransmitter:sodium symporters (NSS)1 mediate sodium-dependent reuptake of neurotransmitters from the synaptic cleft and are targets for many psychoactive drugs. The crystal structure of the prokaryotic NSS protein, LeuT, was recently solved at high resolution; however, the mechanistic details of regulation of the permeation pathway in this class of proteins remain unknown. Here we combine computational modeling and experimental probing in the dopamine transporter (DAT) to demonstrate the functional importance of a conserved intracellular interaction network. Our data suggest that a salt bridge between Arg-60 in the N terminus close to the cytoplasmic end of transmembrane segment (TM) 1 and Asp-436 at the cytoplasmic end of TM8 is stabilized by a cation-π interaction between Arg-60 and Tyr-335 at the cytoplasmic end of TM6. Computational probing illustrates how the interactions may determine the flexibility of the permeation pathway, and mutagenesis within the network and results from assays of transport, as well as the state-dependent accessibility of a substituted cysteine in TM3, support the role of this network in regulating access between the substrate binding site and the intracellular milieu. The mechanism that emerges from these findings may be unique to the NSS family, where the local disruption of ionic interactions modulates the transition of the transporter between the outward- and inward-facing conformations.
Upon neuronal stimulation, neurotransmitters are released into the synaptic cleft where they activate both pre- and postsynaptic receptors. The duration of action of the transmitters is tightly controlled by integral membrane transport proteins situated in the presynaptic nerve terminal or on the surface of surrounding glial cells where they mediate rapid sequestering of the transmitters from the synaptic cleft. Neurotransmitter:sodium symporters (NSS)5 (also called Na+/Cl--dependent transporters) constitute the major class of these transport proteins and include the transporters for dopamine (DA), serotonin, norepinephrine, glycine, and γ-aminobutyric acid (GABA) (1-4). NSS proteins operate by coupling transport of Na+ down its concentration gradient with “uphill” transport of substrate. Moreover, NSS proteins are characterized by co-transport of Cl- (5). Transporters in this family have received particular attention as targets for many drugs including antidepressants, antiepileptics, and psychostimulants, such as cocaine and amphetamines (1-4).
Little is known about the structural dynamics that underlie the function of NSS proteins. Presumably, the transporters follow an alternating access model in which the binding site is alternately exposed to the extracellular (“outward facing” conformation) and intracellular environments (“inward facing” conformation) (6, 7). It is envisioned that binding of substrate together with sodium and chloride to the outward facing conformation elicits a conformational change that shifts the transporter to the inward facing conformation, allowing release of substrate and co-transported ions to the intracellular environment. Notably, such a mechanism implies the existence of two “gates,” one external and one internal, capable of occluding access to the substrate binding site from the extracellular or intracellular environments, respectively.
The first insight into the three-dimensional structure of this class of proteins was achieved recently by crystallization of the prokaryotic NSS member, LeuT (8-10). The structure revealed a conformation likely representing an intermediate between the “outward” and “inward” facing conformations (8). At the cytoplasmic side, the structure suggested the existence of a tight network of interactions that might serve as an intracellular gate (Fig. 1). Specifically, an ionic interaction was found between Arg-51.26 in the N terminus close to the cytoplasmic end of transmembrane segment (TM) 1 and Asp-3698.74 at the cytoplasmic end of TM8 (generic numbers of residues in superscript, see “Experimental Procedures”). This interaction is one of three that are mutually stabilized, including the cation-π interaction between Arg-5 and Tyr-2686.68, and the hydrogen bonding between Arg-5 and Ser2676.67 (Fig. 1 and Ref. 8). Furthermore, the side chain of Tyr-268 also interacts with Gln-3618.66 and Ile-1874.62, and altogether these residues form the network illustrated in Fig. 1, D and E. The residues in this network are all highly conserved among NSS proteins and thus likely of critical importance for transporter function (Fig. 1 and Ref. 11). In agreement, prior to the availability of the LeuT structure we had mutated in DAT the tyrosine (Tyr-335 in DAT) and aspartic acid (Asp-436 in DAT), and obtained evidence for a pivotal role of these residues (12, 13); however, we were at that time unable to interpret the data in a relevant structural context.
FIGURE 1.
Ionic interactions among conserved residues in NSS transporters interconnect the N terminus/TM1 region with IL3/TM6 and TM8. a, alignment of the sequences of N-terminal/TM1, IL3, and TM8 in LeuT, hDAT, hNET, hSERT, hGAT1 hGlyT1b (glycine transporter 1b), and the bacterial transporter Tyt1. Residues corresponding to Arg-51.26, Ser-2676.67, Tyr-2686.68, and Asp-3698.74 in LeuT are highlighted in red. The conserved acidic residues surrounding positions 1.26 and 8.74 are highlighted in blue, whereas the most conserved residue (1.50 and 8.50) within a TM is in bold. b, two-dimensional schematic representation of DAT. Residues in the intracellular interaction network are highlighted. c, view parallel to the membrane plane of the three-dimensional structure of LeuT in which the broken line identified the interconnected region shown in greater detail in panel c. d and e, detail from the encircled region in panel b, turned ∼20° from the perspective shown in that figure, for LeuT (d) and a homology model of DAT (e). The ionic interactions are identified. Note that the aligned serines at position 6.67 (Ser-267 and Ser-334) in the compared structures are shown with their backbone because the interactions involve the -C = O moiety.
Here, in the context of the recent LeuT structure and a DAT homology model, we perform a detailed analysis of the conserved network in DAT. The experimental work is focused on the putative salt bridge between Arg-60 (Arg-5 in LeuT) and Asp-436 (Asp-369 in LeuT). From a series of single and double mutations, we show evidence that the salt bridge is indeed present also in DAT. Moreover, analysis of dynamic models and computational normal mode analyses of the protein suggests together with experimental efforts that this interaction network is critical for stabilizing the outward facing conformation of the transporter and for regulating conformational transitions in the translocation cycle.
EXPERIMENTAL PROCEDURES
Materials—All chemicals were from Sigma unless stated otherwise.
Indexing of Residues—A generic numbering scheme for amino acid residues in NSS proteins has been proposed to facilitate direct comparison of positions between the individual members of the family (11, 14). According to this scheme, the most conserved residue in each transmembrane segment has been given the number 50, and each residue is numbered according to its position relative to this conserved residue. For example, 1.55 indicates a residue in TM1 five residues carboxyl-terminal to the most conserved residue in this TM (Trp1.50). For DAT, the most conserved residues in each transmembrane segment is as follows (generic number being indicated in superscript): TM1, Trp-841.50; TM2, Pro-1122.50; TM3, Tyr-1563.50; TM4, Cys-2434.50; TM5, Pro-2735.50; TM6, Gln-3176.50; TM7, Ser-3667.50; TM8, Phe-4128.50; TM9, Phe-4579.50; TM10, Phe-47810.50; TM11, Pro-52911.50; and TM12, Pro-57312.50. Compared with the generic numbering scheme defined in Ref. 11, we extend the TM index into the immediate conserved loop regions, specifically TM1 to the NH2 terminus, TM6 to IL3, and TM8 to IL4.
Site-directed Mutagenesis—Synthetic cDNAs encoding the human DAT (synDAT) and E2C (a DAT construct where Cys-90 and Cys-306 are mutated to alanines) were subcloned into pcDNA3 (Invitrogen) (13). All mutations were generated by the QuikChange™ method (adapted from Stratagene, La Jolla, CA). All mutations were confirmed by DNA sequencing.
Cell Culture and Expression—COS7 cells were grown as described and transiently transfected with the indicated constructs using the calcium phosphate precipitation method (13).
[3H]DA Uptake Measurements—Uptake assays were performed as described (13) using 2,5,6-[3H]DA (9-13 Ci/mmol) (Amersham Biosciences). Briefly, transfected COS7 cells were plated in either 24-well dishes (105 cells/well) or 12-well dishes (3 × 105 cells/well) coated with polyornithine (Sigma). The uptake assays were carried out 2 days after transfection for 5 min at room temperature in uptake buffer (25 mm HEPES, 130 mm NaCl, 5.4 mm KCl, 1.2 mm CaCl2, 1.2 mm MgSO4, 1 mm l-ascorbic acid, 5 mm d-glucose, and 1 μm of the catechol-O-methyltransferase inhibitor Ro 41-0960 (Sigma), pH 7.4). The indicated non-labeled compounds were added to the cells prior to initiation of uptake by addition of 40 nm [3H]DA. Nonspecific uptake was determined with 1 mm DA. Note that the Zn2+ experiments were done in constructs without the HA tag because introduction of the HA tag mutates His-193, which is one of the key coordinates in DAT Zn2+ binding sites (15).
[3H]DA Uptake Assay with MTSET Preincubation—Two days after transfection, the cells (3 × 104/well in a 12-well dish) were washed once in 500 μl of uptake buffer. The cells were subsequently incubated with 0.5 mm MTSET ([2-(trimethylammonium)ethyl]methane thiosulfonate) (Toronto Research Chemicals, Toronto, Canada) at room temperature for 10 min. The stock MTSET solution in H2O was freshly prepared and diluted 10-fold directly into a total volume of 500 μl of uptake buffer. After incubation, the cells were washed twice in 500 μl of uptake buffer before initiation of [3H]DA uptake performed as described above. The effect of Zn2+ on MTSET reactivity was investigated by addition of 10 μm of a ZnCl2 solution just before adding MTSET.
ELISA for Quantification of Cell Surface Expression—Two days after transfection, cells were washed twice with phosphate-buffered saline and fixed in 4% paraformaldehyde. After 30 min blocking of the unspecific site with phosphate-buffered saline supplemented with 5% fetal calf serum, anti-HA antibody, coupled to the horseradish peroxidase (80 milliunits/ml, clone 3F10, Roche, Basel, Switzerland), was applied for 30 min at room temperature in the same buffer. After intense washes with phosphate-buffered saline, the antibody was detected and quantified instantaneously by chemiluminescence using Supersignal ELISA femto maximum sensitivity substrate (Pierce) and a Wallac Victor2 luminescence counter (PerkinElmer Life Science). All experiments were performed at least in triplicate.
Data Calculations and Statistical Analysis—Uptake data were analyzed by nonlinear regression analysis using Prism 4.02 from GraphPad Software, San Diego, CA. The IC50 values used in the estimation of Km were calculated from means of pIC50 values and S.E. interval from pIC50 ± S.E (16). The Ki values were calculated from the IC50 values using the equation,
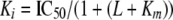 |
(Eq.1) |
in which L = concentration of [3H]DA. All values in the figures are provided as mean ± S.E. For comparisons betweens two groups, t test (two-tailed) was performed.
LeuT Model Construction and Normal Mode Analysis—The construction and equilibration of a full-length LeuT model in a 1-palmitoyl-2-oleol-sn-glycero-3-phosphocholine lipid bilayer is described in detail elsewhere.6 Briefly, the simulation systems with the LeuT molecule immersed in an explicit representation of the water/lipid bilayer/water environment were constructed with VMD (17) and equilibrated with nanoscale molecular dynamics (18), following a procedure modified from a recent description (19). The transporter is imbedded in a membrane patch of 204 1-palmitoyl-2-oleol-sn-glycero-3-phosphocholine molecules, 101 on the periplasmic side and 103 on the cytoplasmic side. The entire system includes around 78,000 atoms, with a final dimension around 87 × 87 × 98 Å3. A Tyt1 homology model was constructed with Modeler (20) based on the LeuT structure, and equilibrated in the lipid bilayer as described above for LeuT. The conformation shown in supplemental Fig. S1 was obtained at the end of 12 ns of free equilibration.
The mutants were constructed with PyMOL (DeLano Scientific, Palo Alto, CA) using its backbone-dependent rotamer library (21). Side chain rotamers were chosen to avoid steric clashes with the rest of the transporter model without changes in the backbone. The resulting models are R5A and Y268A of LeuT, which are aligned with the corresponding R60A and Y335A mutations in DAT. We also built R193A of LeuT, a residue facing lipid, as a control. The normal mode analysis within the elastic network model (16) was carried out on the ElNemo server (22).
RESULTS
A Conserved Intracellular Interaction Network—A recent comprehensive sequence alignment (11) highlighted a marked conservation in the N terminus/TM1, intracellular loop 3 (IL3), and TM8 of NSS proteins (relevant parts shown in Fig. 1a). The residues include Arg1.26, Ser6.67, Tyr6.68, and Asp8.74 that are conserved among most NSS members (except in Tyt1, see below). In the LeuT structure (8), these residues (Arg-51.26, Ser-2676.67, Tyr-2686.68, and Asp-3698.74) together with Ile-1874.62 and Gln-3618.66 form a local network of interactions, interconnecting the N terminus/TM1 to IL3 and TM8 (Fig. 1, c and d). Notably, this network could coordinate the opening and closing of what can be considered an intracellular “gate” (8). The corresponding network in a LeuT-based DAT model involves residues Arg-601.26, Val-2594.62, Ser-3346.67, Tyr-3356.68, and Asp-4368.74 (Fig. 1, b and e). In this model, the Tyr-3356.68 side chain is also stabilized by the carbonyl group of Glu-4288.66 that aligns with Gln-3618.66 in LeuT, but is conserved as a glutamate in most other NSS proteins (Fig. 1, b, d, and e).
In the interaction network, an ionic interaction between the arginine (Arg-5 in LeuT, Arg-60 in DAT) and aspartate (Asp-369 in LeuT, Asp-436 in DAT) is of essential importance (Fig. 1, d and e). The salt bridge is complemented by a cation-π interaction of the arginine with the tyrosine (Tyr-268 in LeuT, Tyr-335 in DAT) that requires a specific dihedral angle preference for the arginine side chain and brings IL3 into the network of interactions with the N terminus and TM8. Of note, a similar cation-π interaction is found in the viral protein VP39 (Protein Data Bank code 1V39) (23) where the interaction between arginine and tyrosine generates mutual stabilization of the interaction between arginine and aspartate (24).
In a comparative molecular dynamics (MD) study for the known structure of LeuT, we attempted to estimate the stabilizing effect of the cation-π interaction by examining the impact of mutating the tyrosine to alanine (Y268A). In the MD trajectories of wild type (WT), Arg-5 maintained its interaction with Ser-267 and Asp-369 (Fig. 2a, top panel), whereas in Y268A, Arg-5 interacted with either Ser-267 or Asp-369, but not with both, and the entire local network was more mobile (Fig. 2a, bottom panel). This suggests that the cation-π interaction between Tyr-268 and Arg-5 positions Arg-5 in the interaction network to enable its effective interaction with both Ser-267 and Asp-369. In addition, the hydrogen bond between Tyr-268 and Gln-361 within the intracellular network could contribute to stabilizing TM8 relative to the N terminus. Note also that Y268A loses its connection to Gln-361, a residue located only one turn below the substrate binding site, whereas Ala-358 and Ile-359 are in direct contact with substrate. Altogether, this in silico analysis supports the hypothesis that the region is important for substrate release, as destabilization of the cation-π and salt bridge interactions appeared to change the structure and flexibility of the local environment surrounding the substrate binding site.
FIGURE 2.
Comparative MD study in LeuT of WT compared with Y268A. a, time evolution of the distances between Arg-51.26 and Asp-3698.74 or Ser-2676.67 in a MD trajectory of WT (top) and Y268A mutant (bottom). Note the constant H-bonding distance maintained throughout the 20-ns trajectory for both interactions in the WT, and the fluctuations in and out of H-bonding distance demonstrating the flexibility of this region in the mutant (Y268A). At any time after the first 2.5 ns, only one of the distances corresponds to a hydrogen bond, and the alternation in H-bonds distances between Arg-5 and the two ionic neighbors in several time intervals (∼9-13 ns). b, the degree of mobility of the residues in the ionic patch is illustrated by the superposition of side chain positions explored during the entire 20-ns MD simulation trajectories for the WT compared with the Y268A mutant. Note the wider range of conformations experienced by the side chains in the mutant compared with the compact bundles in the WT.
The Role of Arg-60 in the Corresponding Interaction Network in DAT—Previously, we showed in DAT that mutation of Tyr-335 to alanine (Y335A) dramatically reduced transport capacity and lowered the Km value for [3H]dopamine ([3H]DA) transport (12). The mutation also changed the effect of Zn2+ at the endogenous high-affinity Zn2+ binding site in DAT; whereas binding of Zn2+ to this site causes non-competitive inhibition of transport in the WT DAT, Zn2+ stimulates [3H]DA transport in Y335A (12, 15, 25). Because Zn2+ is likely to stabilize the transporter in its outward facing conformation (13), this phenotype was proposed to result from a change in the conformational equilibrium in Y335A toward the inward facing conformation; hence, Zn2+ stimulated transport by partially normalizing the equilibrium between the inward and outward facing conformations (13).
Because the tyrosine (Tyr-335) appears to stabilize the Arg-60/Asp-436 salt bridge via a cation-π interaction, we reasoned that disruption of the salt bridge would produce a similar phenotype as that of Y335A. Accordingly, we mutated Arg-60 to alanine, and for comparison we analyzed in parallel the previously described D436A (13). The mutations were made both in the WT DAT background and in a modified DAT containing an HA antibody tag inserted into the second extracellular loop 2. In agreement with previously published results (26), this tag did not affect uptake properties of the transporter, i.e. Km and Vmax for [3H]DA uptake in HA-WT was 1.61 μm (S.E. interval 1.34-1.93) and 4310 ± 736 fmol/min/105 cells (n = 8), respectively, versus 1.14 μm (S.E. interval 1.04-1.24) and 3547 ± 177 fmol/min/105 cells (n = 3) for non-tagged WT DAT. Insertion of the extracellular HA tag permitted quantification of DAT surface expression by ELISA; however, introduction of the HA tag removed one of the Zn2+ coordinating residues in the endogenous Zn2+ binding site (His-193) (15, 25) making HA-WT insensitive to Zn2+ modification. Consequently, the uptake properties of R60A and D436A (as well as in all following mutations) were characterized in both backgrounds, whereas ELISA was done in the HA-WT background and the Zn2+ assays were done in the non-tagged WT DAT background. In both backgrounds, R60A and D436A were functional but displayed marked decreases in Vmax for [3H]DA uptake, with the larger decrease observed for R60A (values for HA-tagged constructs are listed in Table 1 with the values for the corresponding non-tagged WT DAT constructs listed in the legend). This decrease in Vmax was accompanied by a substantial decrease (10-20-fold) in the Km values for [3H]DA uptake (Table 1). ELISA experiments on R60A and D436A in the HA-DAT background showed that surface expression of the two mutants was similar to that observed for HA-DAT itself (Fig. 3a). Thus, the decrease in Vmax for [3H]DA uptake in R60A and D436A resulted most likely from impaired function rather than impaired surface targeting (Vmax normalized to surface expression shown in Fig. 3b).
TABLE 1.
[3H]DA uptake properties measured in COS7 cells transiently expressing HA-tagged WT DAT (HA-WT) or the indicated HA-tagged mutants
The Vmax values for [3H]DA uptake were normalized for surface expression as determined by ELISA experiments and are expressed as mean ± S.E. in % of HA-WT. The Vmax for WT-HA was 4310 ± 736 fmol/min/105 cells (n = 8). The Km values were calculated from the observed IC50 value found by non-linear regression analysis of [3H]DA uptake assays. The S.E. interval for each Km value is indicated and was calculated from the pKI ± S.E. Data are from at least three independent experiments each performed in triplicate. The Km values (in μm) and Vmax values (in fmol/min/105 cells), respectively, for [3H]DA uptake in the corresponding non-HA-tagged WT DAT were as follows: WT, 1.14 (1.04-1.24) and 3547 ± 177; R60A, 0.05 (0.04-0.06), and 52.3 ± 8.2; D436A, 0.13 (0.10-0.17) and 355 ± 13; R60D, 0.08 (0.06-0.10), and 74 ± 8.2; D436R, 0.13 (0.12-0.14) and 338 ± 13; R60D + D436R, 0.07 (0.06-0.09) and 184 ± 9; R60D + D436A, 0.09 (0.09-0.10) and 50 ± 3.8; E61A, 1.27 (1.18-1.36) and 2788 ± 623; E61A + D436R, 0.04 (0.02-0.06) and 68.8 ± 8.8; E437A, 0.63 (0.57-0.70) and 1850 ± 450; D436A + E437A, 0.07 (0.05-0.12) and 31.7 ± 3.2; R60D + E61R, 0.11 (0.10-0.12) and 425 ± 55; R60A + E61R, 0.08 (0.04-0.19) and 293 ± 62.
| Construct | Vmax/surface expression | Km for DA |
|---|---|---|
| % of WT HA | μm | |
| HA-WT | 100 | 1.61 (134-1.93) |
| HA-R60A | 1.23 ± 0.03 | 0.08 (0.06-0.10) |
| HA-D436A | 8.30 ± 0.56 | 0.18 (0.14-0.22) |
| HA-R60D | 1.60 ± 0.09 | 0.10 (0.07-0.15) |
| HA-D436R | 9.13 ± 1.20 | 0.24 (0.18-0.31) |
| HA-R60D + D436R | 2.89 ± 0.44 | 0.11 (0.08-0.15) |
| HA-R60D + D436A | 0.67 ± 0.21 | 0.08 (0.04-0.14) |
| HA-E61A | 88.7 ± 8.52 | 1.78 (1.49-2.13) |
| HA-E61A + D436R | 2.12 ± 0.56 | 0.07 (0.05-0.09) |
| HA-E437A | 45.4 ± 9.24 | 0.90 (0.68-1.20) |
| HA-D436A + E437A | 1.91 ± 0.22 | 0.13 (0.10-0.16) |
| HA-R60D + E61R | 7.10 ± 0.37 | 0.06 (0.04-0.09) |
| HA-R60A + E61R | 8.28 ± 0.76 | 0.15 (0.14-0.17) |
FIGURE 3.
Reduction and Zn2+ enhancement of DA uptake upon mutation of Arg-601.26 or Asp-4368.74 into alanines in DAT. a, similar levels of surface expression of HA-DAT-R60A and HA-DAT-D436A as indicated by ELISA. Data are mean ± S.E. (n = 7 each done in triplicate) with the WT Vmax/expression value being set at 100% in all experiments. b, Vmax for [3H]DA uptake related to the transporter cell surface expression as quantified by ELISA on COS7 cells transiently transfected with the indicated DAT-HA construct. Data are mean ± S.E. (n = 4 each done in triplicate) with the WT Vmax/expression value being set at 100% in all experiments. c, effect of Zn2+ on [3H]DA uptake in WT DAT (▪) compared with the effect in mutants R60A (Δ) and D436A (□). The data are shown as relative uptake in % of [3H]DA uptake in the absence of Zn2+ (mean ± S.E., n = 3). The measurements were done in transiently transfected COS7 cells.
The effect of Zn2+ on [3H]DA uptake in R60A and D436A in the Zn2+-sensitive WT DAT background agreed with our prediction in that [3H]DA uptake was markedly enhanced, albeit with a relative larger effect on R60A than on D436A (Fig. 3c). As for Y335A (12), the effect was maximal with ∼10 μm Zn2+. At higher concentrations, the effect gradually decreased resulting in a bell-shaped dose-response curve. This decrease at high Zn2+ concentrations corresponds most likely to the low affinity phase of the WT DAT inhibition curve and, thus, can be attributed to nonspecific effects of Zn2+ (12, 13, 15). Altogether, similar phenotypes of the three mutations support the participation of Arg-60 together with Tyr-335 and Asp-436 in the interaction network described above.
Charge Reversal of Asp-4368.74 Partially Rescued the Transport Capacity of R60D—To investigate the interaction between Arg-60 and Asp-436 we tested whether reversing the charges at the two loci (R60D + D436R) could lead to at least partial functional rescue. Full rescue would not be expected because the positions of these residues in the interaction network involve them in yet other interactions, so that the repositioned arginine would not benefit from the stabilizing cation-π interaction with Tyr-335 (Fig. 1); however, partial rescue would demonstrate the interdependence of the two residues, consistent with interaction between them. The single mutants, R60D and D436R, like the alanine substitutions of these residues, markedly lowered Km and Vmax values for [3H]DA uptake (Fig. 4a and Table 1). The function of R60D was more impaired than that of D436R, i.e. when corrected for surface expression, Vmax for [3H]DA uptake was 1.6 and 9.1%, respectively, of that observed in HA-DAT (Fig. 4a and Table 1). Note that this difference hints at possible alternative interactions with other residues in the microdomain (see below). The Vmax value for R60D + D435R was also markedly reduced; however, the value (∼2.9% of HA-DAT) was significantly greater than that of R60D but lower than that for D436R (Fig. 4a). This was not observed in the “non-rescuing” mutant (R60D + D436A) in which Vmax was even lower than in R60D (Fig. 4a and Table 1).
FIGURE 4.
Partial rescue of R60D transport capacity by introducing D436R. a, the Vmax value for [3H]DA uptake (normalized to surface expression) is increased in R60D + D436R and decreased in R60D + D436A compared with R60D. The Vmax for [3H]DA uptake was normalized to surface expression as quantified by ELISA on COS7 cells transiently expressing the indicated DAT-HA constructs. Data are mean ± S.E. (n = 4 each performed in triplicate) with WT Vmax/expression value being set at 100% in all experiments. b, effect of 10 μm Zn2+ on [3H]DA uptake for the indicated mutations in WT DAT background transiently expressed in COS7 cells. Data are mean ± S.E. of n = 3 each performed in triplicate. * and **, significantly different values in a paired t test with p < 0.01 and 0.05, respectively, compared with R60D.
In both R60D and D436R, we observed enhancement of uptake by Zn2+ with the most dramatic effect in R60D (Fig. 4b). In R60D + D436R we also observed an enhancing effect of Zn2+; however, the effect was smaller than that in R60D, whereas R60D + D436A showed 6-fold enhancement. According to our working hypothesis, the strength of Zn2+ enhancement represents a read-out of the transporter fraction initially in the inward facing conformation that can be switched by Zn2+ to the outward facing conformation (13). Therefore, the results suggest that mutation in R60D of Asp-436 to arginine (R60D + D436R) but not to alanine (R60D + D436A) lowers the fraction of transporter in the inward facing conformation and distorts less the conformational equilibrium.
Assessing the Conformation of the Mutant Transporters in a Cysteine Reactivity Assay—To obtain a structural read-out for the conformational state of the mutant transporters we employed a conformational assay first developed for NET/SERT (27) and subsequently applied by us to DAT (13). The assay is based on the reactivity of the membrane impermeant, cysteine-reactive, positively charged methanethiosulfonate compound, MTSET, toward a cysteine introduced in position 159 (position 3.53 according to generic nomenclature), which is predicted to be accessible in the outward facing conformation but inaccessible in the inward facing conformation of the transporter (13, 27). This inference is supported by the fact that the aligned position in LeuT is mostly buried in the crystallized conformation, which is characterized by a closed extracellular gate (8). In further agreement, the reactivity of Cys-159 with MTSET applied from the external milieu is decreased in Y335A but increased upon application of Zn2+, consistent with decreased accessibility in the inward facing conformation and partial rescue into an outward facing conformation (13).
We performed the assay in a background in which the only two reduced cysteines on the extracellular face of the transporter (Cys-90 and Cys-306) were mutated (E2C) to eliminate interference from the MTSET reaction with residues other than Cys-159 (15, 23). Because reaction of MTSET with Cys-159 results in transporter inactivation, [3H]DA uptake was used as a functional read-out for MTSET reactivity; hence, in agreement with our previous observations (13), treatment of cells expressing DAT-E2C-I159C with 0.5 mm MTSET caused ∼30% inhibition of uptake (Fig. 5a). This inhibition was reduced in both R60D and D436R, which is taken to indicate that a greater proportion of transporter molecules is in the inward facing conformation with the extracellular gate closed and thus with diminished reactivity of Cys-159. Addition of 10 μm Zn2+ during MTSET pretreatment enhanced inhibition in both R60D and D436R to levels similar to that seen for WT DAT (Fig. 5b), similar to our data previously obtained with Y335A (15). Remarkably, MTSET inhibition of the charge-reversed double mutant R60D + D436R, but not of the non-rescuing control mutant (R60D + D436A), was significantly greater than that in the individual mutants and close to that seen for the WT DAT (Fig. 5a), as would be expected for a mutation bringing the properties closer to WT. This suggests that when the two mutations, R60D and D436R, are combined, WT conformational properties are partially rescued, most likely through the formation of an inverted salt bridge.
FIGURE 5.
Introduction of D436R in R60D increases MTSET accessibility to Cys-1593.53 in TM3. a, inhibition of [3H]DA uptake by MTSET (0.5 mm) in COS7 cells transiently expressing DAT-E2C-I159C or mutations DAT-E2C-I159C/R60D, DAT-E2C-I159C + D436R, DAT-E2C-I159C + R60D + D436R, or DAT-E2C-I159C + R60D + D436A. Values are mean ± S.E. (n = 4 each performed in triplicate). In R60D + D436R the inhibition is significantly different (**, p < 0.01, one-way analysis of variance, Newman-Keuls Comparison post hoc test) from that seen for R60D, D436R, and R60D + D436A. b, same as a with MTSET-induced uptake inhibition measured on the same cells in the presence of 10 μm Zn2+.
Additional Residues in the Ionic Patch between the N Terminus and TM8—Two highly conserved acidic residues (Glu-61 and Glu-437, positions 1.27 and 8.75, respectively, according to generic nomenclature) are situated adjacent to Arg-6 and Asp-436, respectively, creating a patch of charged residues with one positively charged residue surrounded by three negatively charged ones (Figs. 1, a and b, and 6, a and b). The propinquity suggests that these two residues may also play a role in the pattern of ionic interactions between the N terminus and TM8. However, mutation of Glu-61 to alanine had almost no impact on transporter function and the effect of Zn2+ was similar to that in WT DAT (Fig. 6, c and d, and Table 1). Mutation of Glu-437 to alanine decreased the Vmax values for [3H]DA uptake in agreement with previous data (13) (Fig. 6c, Table 1); however, E437A was still inhibited by Zn2+ although to a slightly less extent as compared with WT DAT (Fig. 6d). Thus, neither Glu-61 nor Glu-437 appeared vital to transporter function under “normal” circumstances (Fig. 6a).
FIGURE 6.
Glu-611.27 and Glu-4378.75 are part of an ionic network surrounding the Arg-601.26-Asp-4368.74 salt bridge. a, DAT model showing acidic residues (Glu-611.27, Asp-4368.74, and Glu-4378.75) surrounding Arg-601.26 in the N terminus at the intracellular end of TM1 (purple) and TM8 (blue). The predicted salt bridge between Arg-601.26 and Asp-4368.74 is illustrated by a dashed green line. The other TMs were removed for clarity. b and e, schematics representing the putative interactions between TM1/proximal N terminus (dark gray) and TM8 (light gray) in WT (b) and mutants (e). Acidic residues are represented by a blue letter in a circle, the large labile arginine by a red letter in an ellipse, and the neutral alanine by a black letter in a circle. The hypothetical ionic interactions are represented by a plain and dashed green line, respectively, whereas the putative electrostatic clashes are illustrated by an orange symbol. IP is defined as “interaction potency” between TM1 and TM8 rating the predicted strength of ionic interactions in this area of DAT. +++ is the strongest interaction, + and 0 correspond to a weak interaction or no interaction, respectively. c and f, Vmax values for [3H]DA uptake normalized to cell surface expression in the indicated HA-tagged DAT constructs. Data are % of uptake in HA-WT (mean ± S.E., n = 3-5). d and g, effect of 10 μm Zn2+ on [3H]DA uptake for the indicated mutations in WT DAT background transiently expressed in COS7 cells. Data are % of uptake in the absence of Zn2+ (means ± S.E., n = 3). * and **, significantly different (p < 0.05 and 0.01, respectively, paired t test) compared with WT DAT (c and d) or to D436A or D436R (f and g).
Based on our DAT model we reasoned, however, that the two residues could affect the phenotypes observed upon mutation of Arg-60 and Asp-436. Specifically, although mutation of Asp-436 to alanine removed the main component of the TM1-TM8 interaction, even a weak interaction between Arg-60 and Glu-437 could substitute to a certain extent for the lost interaction between Arg-60 and Asp-436 (Fig. 6, a and b). Mutation of Glu-437 to alanine in the context of D436A supported this notion by showing a more profound effect on Vmax, hence, Vmax normalized for surface expression diminished 4.3-fold for E437A in HA-DAT D436A, whereas Vmax for E437A in HA-DAT background diminished 2.2-fold (Fig. 6, c and f, and Table 1). The enhancing effect of Zn2+ was also increased in D436A + E437A as compared with D436A alone (Fig. 6g), supporting that a higher fraction of transporters have assumed the inward facing conformation in this double mutant because all possible bridges connecting the N terminus with TM8 in this region were eliminated (Fig. 6e).
We next tested Glu-61 for its effect on D436R. The model suggests that mutation of Asp-436 to arginine would generate electrostatic repulsions with Arg-60 but that this might be mitigated by a compensatory interaction between Glu-61 and the arginine inserted in position 436 of D436R (Fig. 6e). Therefore, we mutated Glu-61 to alanine in D436R resulting in E61A + D436R where the possibility for ionic interactions between the N terminus and the cytoplasmic end of TM8 was eliminated (Fig. 6e). As predicted, this mutation in D436R reduced Vmax considerably in contrast to having no effect in WT DAT (Fig. 6, c and f). Moreover, the Zn2+ effect was enhanced in E61A + D436R compared with D436R (Fig. 6g).
If the four charged residues considered here operate as an ionic patch with three negative charges (Glu-61, Asp-436, and Glu-437) and one positive charge (Arg-60), partial functional rescue of R60D might be obtained also by introducing the positive charge in position 61 to restore the ionic interaction between the N terminus and TM8 (Fig. 7a and supplemental Fig. S1)). As predicted, R60D + E61R as well as R60A + E61R (which was generated to exclude that a rescuing effect was due to an ionic interaction between positions 60 and 61) displayed higher Vmax than R60D or R60A, and uptake was only weakly enhanced by Zn2+ (Fig. 7, b and c, Table 1), consistent with functional rescue.
FIGURE 7.
Introduction of E61R in R60D or R60A partially rescues the transport capacity. a, schematics representing the putative network of interactions between the TM1/proximal N terminus (dark gray) and TM8 (light gray) in the indicated DAT mutants (as in Fig. 6). The hypothetical ionic interactions are represented by a dashed green line. b, Vmax values for [3H]DA uptake normalized to cell surface expression in the indicated HA-tagged DAT constructs. c, effect of 10 μm Zn2+ on [3H]DA uptake for the indicated mutations in WT DAT background transiently expressed in COS7 cells. Data are mean ± S.E. (n = 3 each performed in triplicate). ***, significantly different values in a t test with p < 0.001 compared with R60D or R60A.
Normal Mode Analysis—The interaction network is likely to affect the dynamic properties of the whole transporter molecule. Specifically, movements of the N terminus and IL3 relative to the middle region of TM8 that is close to the substrate binding site might be important, and disruption of the network may impair the capability of the transporter to make reversible motions in the gating process. We explored, therefore, the collective motions in the various constructs of the transporter with an analysis of the normal modes (28). LeuT was chosen as the model because application of this analysis to a high resolution structure is likely to be more reliable, whereas the high degree of conservation discussed above suggests that the mechanistic details would be shared by DAT.
We first inspected the low frequency normal modes of WT LeuT and quantified the movements by measuring the changes in Cα-Cα distances between Arg-5 and Gln-361, and Tyr-268 and Gln-361 (see supplemental Fig. S2 for further details). To identify the role of specific interactions in these movements, we compared the results for normal modes calculated for WT with Y268A and R5A, and used R193A, a residue facing lipid, as control (supplemental Fig. 2B). The dynamic details are visualized in the movies provided as supplementary information (supplementary Movies I and II). It is clearly seen from modes 11 and 20 in these movies that R5A and Y268A are more mobile than WT on the intracellular side, whereas WT is significantly more mobile in general (supplementary Movies I and II and supplemental Fig. S2B). By disrupting the interaction between the N terminus and TM8, the R5A mutation renders the extracellular region of the protein less capable of undergoing the dynamic rearrangements coupled to the conformational changes at the intracellular end. Mode 21 of WT exhibits the largest changes of the monitored distances, whereas in R5A and Y268A the motions are markedly different (supplemental Fig. S2B).
DISCUSSION
The analysis underlying this study was made possible by the high-resolution structural information obtained recently for a bacterial member of the NSS family (LeuT) (8-10), which for the first time enabled the construction of reliable molecular models of the mammalian counterparts (11). We focused on a distinctive and conserved residue patch found in the cytoplasmic half of the protein in both the LeuT structure and in the cognate DAT model (Fig. 1). Given the concordance between our previous experimental findings upon mutation of Tyr-335 in DAT (12, 13) and the putative role of the interaction network identified in this patch in the gating of the transporter, we probed computationally the role of this conserved tyrosine in the network. In parallel, we set out to validate experimentally the existence and function of such a network in DAT.
Our comparative MD simulations of WT LeuT and the mutant Y268A indicated that the cation-π interaction (Tyr-268-Arg-5) and the salt bridge (Arg-5-Asp-369) could mutually stabilize each other (Figs. 1C and 2). Based on the hypothesis that Y268A, like the corresponding mutant (Y335A) in DAT, is enriched in the inward-facing conformation (12, 13) and the fact that the Tyr-268-Arg-5 interaction no longer exists in Y268A, we expected the interaction between the N terminus and IL3 to be significantly weakened or disrupted in this mutant. This disruption is likely to propagate to the cytoplasmic end of TM8 through the Arg-5-Asp-369 interaction. This expectation is born out by dynamic analysis, as illustrated by the changes in the corresponding normal modes (supplemental Fig. S2 and supplementary Movies I and II). Thus, we propose a functional role for this network connecting the N terminus, IL3, and TM8, in which alternating conformations account for the opening and closing of the intracellular gate for substrate translocation, in a motion that is propagated through the entire structure to support the corresponding opening/closing changes in the extracellular end of the transporter.
The salt bridge interaction between Arg-60 and Asp-436 in DAT was probed experimentally by interchanging the two residues. This resulted in partial rescue demonstrating the interdependence of the two charged residues consistent with an interaction between them. Note that full rescue would not be expected from our structural models, because the important cation-π interaction would not be restored in the revertant mutants. Charge reversal mutations have been used to validate the existence of salt bridges also in other membrane proteins, and revealed spatial adjacencies even in the absence of three-dimensional structures (29-32). In some cases essentially full functional rescue was observed, as seen, for example, for a salt bridge in the α1b receptor (31), whereas in other studies the observations were more parallel to our findings with only partial functional rescue (29, 30) or more complex phenotypes, as in the GABAB receptor (32).
Altogether, our data strongly support the presence of the interaction network (Fig. 1e) and its impact on the dynamics related to function. Of further interest, mutation of Ser-267 was recently found to cause inverted Zn2+ sensitivity,7 and impaired transport function was seen upon mutation of Glu428 (13), which would disrupt the interaction between Glu-428 and Tyr-335 (Fig. 1e). Our structural models revealed furthermore, that the microenvironment of the mutated residues is enriched in putative alternative interaction partners, such as Glu-61 and Glu-437. These appeared to confer some redundancy to the interaction between TM1 and TM8 by partially compensating for the absence of the stabilizing salt bridge in the Asp-436 mutants but not in the Arg-60 mutants (Fig. 6, a and e). Additional evidence for the importance of this microenvironment of reinforced interactions was provided by the partial “rescuing” of uptake not only upon interchange of Arg-60 and Asp-436 but also upon interchange of Arg-60 and Glu-61.
This “rescue” (R60D + E61R) was more pronounced than that in R60D + D436R, most likely because it preserved an arginine in the key position that allows for stabilization of the reversed charge interaction. This idea is supported by the occurrence of just such a reversed charge combination in one of the bacterial transporters from the NSS family, the tyrosine transporter Tyt1 (33), which possesses an arginine at generic position 1.27 and a glutamate at 1.26 (Fig. 1a). In our MD study of a LeuT-based Tyt1 model, Arg-6 (aligned to Glu61 in DAT) formed not only a salt bridge with Glu-330 (generic position 8.70), which is located one turn above the Asp-436 of DAT, but also a cation-π interaction with Tyr-241 (corresponding to Tyr-335 in DAT and Tyr-268 in LeuT) that was in a different orientation than in LeuT (supplemental Fig. S1) (33). Similarly, we interpret the better rescue seen in DAT R60D + E61R as the result of re-establishing both a salt bridge and a cation-π interaction (between the N terminus and IL3).
Of additional interest, mutation of the residue corresponding to Arg-60 in the homologous GABA transporter-1 (GAT-1) (Arg-44) was shown to impair uptake likely by inhibiting reorientation of the unloaded transporter (34), consistent with a role in closure of the internal gate as suggested here. Furthermore, when the residue following Arg-44 in GAT1, i.e. Asp-45, was deleted, the one-residue shift and the presence of an aspartate in position 43 generated a mutation similar to DAT R60D + E61R. This deletion mutant (del45) was functional (34) and thus exhibited a rescue phenotype similar to what we find for R60D + E61R. Taken together, the similarities between the observations obtained in GAT-1 and the present data obtained in DAT indicate that the role of Arg-601.26 proposed here might be extended to the entire transporter class.
By using a comparative normal mode analysis in the context of the LeuT structure we evaluated the relationship between the dynamic properties of the transporter molecule and the ionic interaction between the N terminus and TM8 in the collective motions of the transporter. The advantage of this approach is that, like conventional time-dependent MD, it is able to identify global structural changes that are critical to substrate translocation, but because it is time independent it is not subject to the same limitations as MD, e.g. high computational cost of long simulations for a large system. Many examples have shown that the collective motions that are functionally important often follow trajectories along one or a few low frequency normal modes (35-37), and that the intrinsic structural flexibility of a protein manifested in the normal modes has evolved to facilitate functionally important conformational transitions (36).
Interpreted on this basis, our results from normal mode analyses point to a pattern of motions that likely represents an allosteric response to the binding of the substrate (supplemental Fig. S2). The comparative analysis of normal modes 11 and 20 was particularly revealing as the motions in mutants and WT are directly comparable (supplementary Movies I and II). As evidenced by the differences between the corresponding modes in WT, R5A, and Y268A, the structural context of the relevant low frequency modes emphasizes the importance that Arg-5, Tyr-268, and the associated network, have for the intrinsic flexibility of the transporter. Importantly, the propagation of motions represented by these modes connects conformational changes in the intracellular portion to the extracellular portion of the protein. The increased mobility of the mutants compared with WT in the intracellular side is associated with a reduction in the ability of the extracellular region to complete the corresponding motions seen in the WT (supplementary Movies I and II). This observation is fully consistent with assigning a functional role to the ionic interaction in the WT related to the transition of the transporter between the outward and inward facing conformations.
Summarized, the present study provides support for a transport mechanism for NSS proteins that appears unique among secondary active ion-coupled transporters. Our mechanistic predictions clearly differ from those involving movements of two symmetrical hairpins reaching from the extracellular and intracellular environments, respectively, that were offered for sodium-coupled glutamate transporters (38). Similarly, our proposed mechanism differs from the “rocker switch” type mechanism proposed for Lac Permease and the glycerol 3-phosphate transporter (39, 40). Although the key role of ionic interactions between the cytoplasmic ends of TM1 and TM8 interactions is clearly demonstrated here, the exact molecular events governing the continuous disruption and reformation of these interactions remains to be determined. It is interesting to speculate that the process might involve sequential protonation and deprotonation, e.g. of Asp-436, but demonstration of this and other mechanistic elements requires future efforts.
Supplementary Material
Acknowledgments
We thank Dorthe Vang Larsen for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant P01 DA 12408. This work was also supported by the Danish Health Science Research Council, the Lundbeck Foundation, the Novo Nordisk Foundation, and the Maersk Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Movies I and II.
Footnotes
The abbreviations used are: NSS, neurotransmitter:sodium symporters; DA, dopamine; DAT, dopamine transporter; WT, wild type; TM, transmembrane segment; IL, intracellular loop; MTSET, [2-(trimethylammonium)ethyl]methane thiosulfonate; GABA, γ-aminobutyric acid; HA, hemagglutinin; MD, molecular dynamics; ELISA, enzyme-linked immunosorbent assay.
L. Shi, M. Quick, Y. Zhao, H. Weinstein, and J. A. Javitch, manuscript in preparation.
Y. Dehnes and J. A. Javitch, unpublished observation.
References
- 1.Chen, N. H., Reith, M. E., and Quick, M. W. (2004) Pflugers Arch. 447 519-531 [DOI] [PubMed] [Google Scholar]
- 2.Gether, U., Andersen, P. H., Larsson, O. M., and Schousboe, A. (2006) Trends Pharmacol. Sci. 27 375-383 [DOI] [PubMed] [Google Scholar]
- 3.Torres, G. E., and Amara, S. G. (2007) Curr. Opin. Neurobiol. 17 304-312 [DOI] [PubMed] [Google Scholar]
- 4.Henry, L. K., Defelice, L. J., and Blakely, R. D. (2006) Neuron 49 791-796 [DOI] [PubMed] [Google Scholar]
- 5.Zomot, E., Bendahan, A., Quick, M., Zhao, Y., Javitch, J. A., and Kanner, B. I. (2007) Nature 449 726-730 [DOI] [PubMed] [Google Scholar]
- 6.Jardetzky, O. (1966) Nature 211 969-970 [DOI] [PubMed] [Google Scholar]
- 7.Rudnick, G. (2006) Handb. Exp. Pharmacol. 175 59-73 [DOI] [PubMed] [Google Scholar]
- 8.Yamashita, A., Singh, S. K., Kawate, T., Jin, Y., and Gouaux, E. (2005) Nature 437 215-223 [DOI] [PubMed] [Google Scholar]
- 9.Zhou, Z., Zhen, J., Karpowich, N. K., Goetz, R. M., Law, C. J., Reith, M. E., and Wang, D. N. (2007) Science 317 1390-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh, S. K., Yamashita, A., and Gouaux, E. (2007) Nature 448 952-956 [DOI] [PubMed] [Google Scholar]
- 11.Beuming, T., Shi, L., Javitch, J. A., and Weinstein, H. (2006) Mol. Pharmacol. 70 1630-1642 [DOI] [PubMed] [Google Scholar]
- 12.Loland, C. J., Norregaard, L., Litman, T., and Gether, U. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 1683-1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loland, C. J., Granas, C., Javitch, J. A., and Gether, U. (2004) J. Biol. Chem. 279 3228-3238 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg, N. R., Beuming, T., Soyer, O. S., Goldstein, R. A., Weinstein, H., and Javitch, J. A. (2003) Eur. J. Pharmacol. 479 3-12 [DOI] [PubMed] [Google Scholar]
- 15.Loland, C. J., Norregaard, L., and Gether, U. (1999) J. Biol. Chem. 274 36928-36934 [DOI] [PubMed] [Google Scholar]
- 16.Tirion, M. M. (1996) Phys. Rev. Lett. 77 1905-1908 [DOI] [PubMed] [Google Scholar]
- 17.Humphrey, W., Dalke, A., and Schulten, K. (1996) J. Mol. Graph. 14 33-38 [DOI] [PubMed] [Google Scholar]
- 18.Phillips, J. C., Braun, R., Wang, W., Gumbart, J., Tajkhorshid, E., Villa, E., Chipot, C., Skeel, R. D., Kale, L., and Schulten, K. (2005) J. Comput. Chem. 26 1781-1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotomayor, M., and Schulten, K. (2004) Biophys. J. 87 3050-3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sali, A., and Blundell, T. L. (1993) J. Mol. Biol. 234 779-815 [DOI] [PubMed] [Google Scholar]
- 21.Dunbrack, R. L., Jr., and Cohen, F. E. (1997) Protein Sci. 6 1661-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suhre, K., and Sanejouand, Y. H. (2004) Nucleic Acids Res. 32 W610-W614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallivan, J. P., and Dougherty, D. A. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 9459-9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slutsky, M. M., and Marsh, E. N. (2004) Protein Sci. 13 2244-2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norregaard, L., Frederiksen, D., Nielsen, E. O., and Gether, U. (1998) EMBO J. 17 4266-4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorkina, T., Miranda, M., Dionne, K. R., Hoover, B. R., Zahniser, N. R., and Sorkin, A. (2006) J. Neurosci. 26 8195-8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen, J. G., and Rudnick, G. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 1044-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui, C., and Bhar, I. (eds) (2006) Normal Mode Analysis: Theory and Applications to Biological and Chemical Systems, Chapman & Hall/CRC, Boca Raton, FL
- 29.Zhou, W., Flanagan, C., Ballesteros, J. A., Konvicka, K., Davidson, J. S., Weinstein, H., Millar, R. P., and Sealfon, S. C. (1994) Mol. Pharmacol. 45 165-170 [PubMed] [Google Scholar]
- 30.Sealfon, S. C., Chi, L., Ebersole, B. J., Rodic, V., Zhang, D., Ballesteros, J. A., and Weinstein, H. (1995) J. Biol. Chem. 270 16683-16688 [DOI] [PubMed] [Google Scholar]
- 31.Porter, J. E., and Perez, D. M. (1999) J. Biol. Chem. 274 34535-34538 [DOI] [PubMed] [Google Scholar]
- 32.Binet, V., Duthey, B., Lecaillon, J., Vol, C., Quoyer, J., Labesse, G., Pin, J. P., and Prezeau, L. (2007) J. Biol. Chem. 282 12154-12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quick, M., Yano, H., Goldberg, N. R., Duan, L., Beuming, T., Shi, L., Weinstein, H., and Javitch, J. A. (2006) J. Biol. Chem. 281 26444-26454 [DOI] [PubMed] [Google Scholar]
- 34.Bennett, E. R., Su, H., and Kanner, B. I. (2000) J. Biol. Chem. 275 34106-34113 [DOI] [PubMed] [Google Scholar]
- 35.Hayward, S., Kitao, A., and Go, N. (1995) Proteins 23 177-186 [DOI] [PubMed] [Google Scholar]
- 36.Ma, J. (2005) Structure 13 373-380 [DOI] [PubMed] [Google Scholar]
- 37.Tama, F. (2003) Protein Pept. Lett. 10 119-132 [DOI] [PubMed] [Google Scholar]
- 38.Boudker, O., Ryan, R. M., Yernool, D., Shimamoto, K., and Gouaux, E. (2007) Nature 445 387-393 [DOI] [PubMed] [Google Scholar]
- 39.Abramson, J., Smirnova, I., Kasho, V., Verner, G., Kaback, H. R., and Iwata, S. (2003) Science 301 610-615 [DOI] [PubMed] [Google Scholar]
- 40.Huang, Y., Lemieux, M. J., Song, J., Auer, M., and Wang, D. N. (2003) Science 301 616-620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









