Abstract
Gonadotropin-regulated testicular helicase (GRTH)/DDX25 is an essential post-transcriptional regulator of spermatogenesis. In GRTH null mice severe apoptosis was observed in spermatocytes entering the metaphase of meiosis. Pro- and anti-apoptotic factors were found to be under GRTH regulation in comparative studies of spermatocytes from wild type and GRTH–/– knock-out (KO) mice. KO mice displayed decreased levels of Bcl-2 and Bcl-xL (anti-apoptotic factors), an increase in Bid, Bak, and Bad (pro-apoptotic), reduced phospho-Bad, and release of cytochrome c. Also, an increase on Smac, a competitor of inhibitor apoptotic proteins that release caspases, was observed. These changes caused an increase in cleavage of caspases 9 and 3, activation of caspase 3 and increases in cleavage products of PARP. The half-life of caspase 3 transcripts was markedly increased in KO, indicating that GRTH had a negative role on its mRNA stability. IκBα, which sequesters NF-κB from its transcriptional activation of pro-apoptotic genes, was highly elevated in KO, and its phospho-form, which promotes its dissociation, was reduced. The increase of HDAC1 and abolition of p300 expression in KO indicated a nuclear action of GRTH on the NF-κB-mediated transcription of anti-apoptotic genes. It also regulates the associated death domain pathway and caspase 8-mediated events. GRTH-mediated apoptotic regulation was further indicated by its selective binding to pro- and anti-apoptotic mRNAs. These studies have demonstrated that GRTH, as a component of mRNP particles, acts as a negative regulator of the tumor necrosis factor receptor 1 and caspase pathways and promotes NF-κB function to control apoptosis in spermatocytes of adult mice.
Gonadotropin-regulated testicular helicase (GRTH)3 is a multifunctional RNA helicase that is an essential post-transcriptional regulator of spermatid development and the completion of spermatogenesis. It is a male-specific protein expressed in rat, mouse, and human testis (1–5) that contains 483 amino acids and shares the nine conserved signature motifs found in members of the DEAD box family of RNA helicases. This helicase displays ATP binding and hydrolysis, RNA binding, and RNA unwinding activities (1).
GRTH is regulated by gonadotropin/androgen in Leydig cells and germ cells of the testis (1, 2) where its expression is both cell- and stage-specific. It is highly expressed in pachytene and metaphase spermatocytes and round spermatids, where it regulates the expression of crucial proteins in sperm maturation including H4, HMG2, TP1, TP2, PGK2, ACE, and protamines 1 and 2 (3, 4). As a component of messenger ribonucleoprotein particles, GRTH participates in transport of mRNAs to cytoplasmic sites for storage of mRNAs prior to their translation at specific times during spermatogenesis. Also, through its association with polyribosomes, GRTH regulates the translation of messages encoding spermatogenic factors (4). GRTH null mice are sterile and lack sperm because of the failure of round spermatids to elongate, resulting in complete arrest at step 8 of spermiogenesis (3). Wild type and heterozygous males are fertile and have normal sperm development. There is also a major decrease in size of the chromatoid body in GRTH null mice, consistent with the marked reduction of nuclear-cytoplasmic transport of messages relevant to spermiogenesis, presumably for storage in these organelles (3, 4).
In spermatocytes entering the metaphase of meiosis before the appearance of round spermatids, striking apoptosis (30%/cell/tubule) is observed in GRTH null mice as revealed by deoxynucleotidyltransferase-mediated dUTP nick end labeling assay (3). The degree of apoptosis is related to the reduction of GRTH protein expressed in germ cells, because apoptotic cells are reduced in heterozygous mice (8%/cell/tubule) and are sparse in wild type animals. The DNA repair proteins, Rad 51 and Dmc1, which are necessary to maintain chromosomal integrity, are increased in GRTH null mice. Despite the severe apoptosis, sperm development proceeds through stages 1–8 of round spermatids until the arrest point. In this case, activation of survival mechanisms and/or the partial protection from other helicases (i.e. PL10/DDX3 and DBP5/DDX19) could permit the progression of the remaining meiotic viable cells to haploid germ cells. In this study we provide evidence for a central role of GRTH in the control of germ cell apoptosis in adult mice.
EXPERIMENTAL PROCEDURES
Animals—Wild type (C57BL/6-SV129J) (Charles River) and GRTH–/– male mice (3) were housed in pathogen-free, temperature- and light-controlled conditions (20 °C), with an alternating light-dark cycle with 14 h of light and 10 h of darkness). All of the animal studies were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. The animals were killed by asphyxiation with CO2 and decapitated. The testes were removed and decapsulated for the purification of germ cells.
Purification of Spermatocytes—Testicular germ cells were prepared by collagenase/trypsin dispersion and purified by centrifugal elutriation (2). After collagenase dispersion, seminiferous tubules were minced and incubated in medium 199 containing 0.1% bovine serum albumin, 0.1% trypsin (Sigma), and 17 μg/ml DNase (Sigma) for 15 min in a rotary water bath (80 rpm, 35 °C). After the addition of soybean 0.04% trypsin inhibitor, the sample was filtered through a 300-, 90-, or 40-μm mesh screen and glass wool, and the cells were pelleted and resuspended in elutriation buffer containing 2 μg/ml DNase. The spermatocytes were subsequently separated and purified by centrifugal elutriation using Beckman Avanti 21B centrifuge with elutriator rotor model J 5.0 as described previously. The first two fractions (fractions 1 and 2) were collected with flow rates of 31.5 and 41.4 ml/min at 3000 rpm, and two additional fractions (fractions 3 and 4) were obtained with flow rates of 23.2 and 40 ml/min at 2000 rpm. Fraction 4 containing pachytene spermatocytes at a purity of ∼86% was used for protein and RNA analyses.
Western Blot Analysis—Protein samples were extracted from germ cells using Mammalian Protein Extraction Reagent (Pierce) in the presence of a mixture of protease inhibitors (Roche Applied Sciences). The extracts (25 μg) were subjected to separation on a 4–20% SDS-PAGE gel and transferred to nitrocellulose membrane for Western analysis with specific GRTH peptide rabbit polyclonal antibody (amino acids 465–477) (2) purified by protein A-Sepharose (Amersham Biosciences) or antibodies obtained from commercial sources including anti-rabbit antisera: Bak, TNFα, TNF-R1, TRADD, caspase 8, HDAC1, HDAC2, HDAC3, HDAC4, p300, (Santa Cruz), Bid, Bad, Bcl-2, phospho-Bad, caspase 3, caspase 9, PARP, XIAP, p65, NF-κB (p105/50), RIP, TNF receptor-associated factor 2, IKKα/β, Erk1/2, phospho-Erk1/2, cytochrome c, AKT, and IκBα (Cell signaling), acetyl p65 (Abcam), Hsp10 (Sigma); anti-goat anti-serum FADD (Santa Cruz) and anti-Bcl-xL (monoclonal, Sigma), Smac (monoclonal; Santa Cruz), p53 and phospho-IκBα (monoclonal, Cell signaling), and PKAc (monoclonal; BD Biosciences). Anti-rabbit β-tubulin (Santa Cruz Biotechnology) or β-actin antisera (monoclonal; Sigma) were used for normalization. The antibodies were used at a dilution of 1:500 to 1:1000, and the appropriate second antibody was employed (goat anti-rabbit and anti-mouse IgG horseradish peroxidase at 1:10,000; donkey anti-goat IgG horseradish peroxidase 1:5000). Immunosignals were detected at different exposure times using the supersignal chemiluminescence system (Pierce). The intensity of each protein band from Western blots was scanned by densitometry (GS-800 calibrated densitometer; Bio-Rad) in the linear range of optical density and quantitated by a software package (Quantity One version 4.2.1). The values obtained from quantitation of optical densities of Western blot signals were normalized by endogenous β-actin or tubulin, which were constant in WT and KO. KO values were expressed as percentages of WT (100%). Each comparative evaluation was repeated at least three times from independent samples of purified spermatocytes obtained from at least 20 wild type or KO mice.
Caspase 3 Activity Assay—Protein samples (25 μg) extracted from total germ cells collected by centrifugation (1000 × g, 4 °C, 10 min) after collagenase/trypsin dispersion were used to determine endogenous caspase 3 activity using caspase 3 cellular activity assay kit (number 235419; Calbiochem).
Co-immunoprecipitation and RT-PCR Analysis—Testicular extracts (1 mg) prepared from WT and GRTH–/– mice by homogenization were initially subjected to preclearing by incubation with 40 μl of protein A-agarose (50% of slurry) and 2 μg of normal rabbit or mouse IgG in the immunoprecipitation assay buffer with gentle agitation. The recovered supernatant was incubated with GRTH antiserum (2 μg) for 2 h at 4°C in the presence of 1× protease inhibitor mixture (Roche Applied Sciences) to co-immunoprecipitate the GRTH-RNP complex. 50 μl of protein A-agarose in 50% slurry was added, and the incubation was continued for overnight at 4 °C. Protein A-precipitated GRTH–RNP complex was recovered by brief centrifugation followed by washing three times with assay buffer. The RNA from the complexes was extracted by phenol:chloroform:isoamyl alcohol (25:24:1, v/v; Invitrogen) and subjected to RT-PCR analysis. First strand cDNA reverse transcribed by using a Super Script III first strand synthesis kit (Invitrogen) was further amplified by real time PCR with specific primer sets for the genes of interest.
Real Time PCR Quantification of mRNA—Total RNA from either whole cell or cytoplasmic fractions of purified spermatocytes obtained from adult mouse testes (GRTH+/+ and GRTH–/–) were isolated using an RNeasy mini kit (Qiagen). The cell fractions were prepared using nuclear and cytoplasmic extraction reagents kit (Pierce) in the presence of a mixture of protease (Roche Applied Sciences) and phosphatase inhibitors (Pierce). Prior to reverse transcription reaction, total RNA was treated with DNase I to remove any possible co-purified genomic DNA. 1 μg of RNA was reverse transcribed using a SuperScript III first strand synthesis system (Invitrogen) containing a mixture of oligo(dT)20. The first strand cDNA was used as a template in real time PCR with SYBR Green Master Mix and an ABI 7500 sequence detection system (Applied Biosynthesis). The cycling program was set as follows: denature at 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 1 min. Specific primers for gene of interests were designed accordingly (supplemental Table S1). The specificity of the PCR products was verified by melting curve and agarose gel analyses. The results presented are from three individual experiments, in which each sample was assayed in triplicate, normalized to the level of β-actin mRNA, and expressed as a percentage of wild type.
mRNA Stability of Caspase 3—Spermatocytes of WT and GRTH–/– mice were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum for 3 h followed by incubation with 10 μg/ml actinomycin D for 0–10 h. Total RNA was isolated at specific times after treatment, reverse transcribed, and quantitated by real time PCR as described above.
Statistical Analysis—The significance of the differences in the expression of proteins and mRNAs between groups (wild type and KO) was determined by nonparametric Kruskall Wallis followed by Dunn's multiple-comparison test using Prism statistical software version 4.
RESULTS
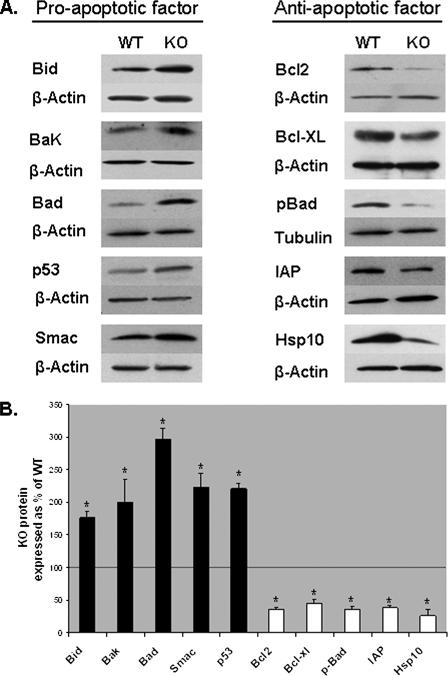
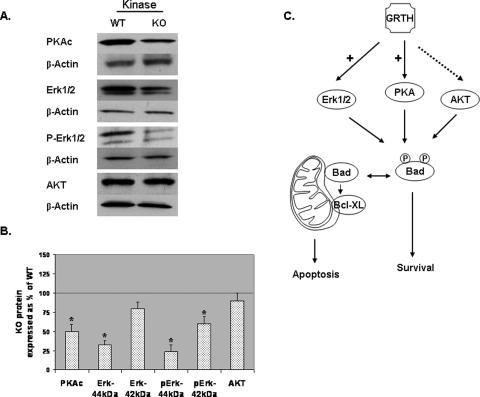
Effect of GRTH on Cellular Apoptotic Factors—To study the effects of GRTH in apoptosis, we initially examined the protein expression of anti-apoptotic and pro-apoptotic factors in purified spermatocytes from WT and KO mice by Western blot analysis. Our results showed a significant increase in the levels of some pro-apoptotic factors in spermatocytes of KO mice compared with WT including Bid, Bad, Bak, Smac, and p53 (Fig. 1). In contrast, the levels of anti-apoptotic proteins such as Bcl-2, Bcl-xL, Phospho-Bad (pBad), IAP, and Hsp10 were significantly reduced in KO. In addition, reduction of the protein levels of key enzymes of two independent kinase pathways reported to phosphorylate Bad including the PKA catalytic subunit (PKAc), mitogen-activated protein kinase (Erk1/2), and phospho-Erk1/2 (pErk1/2) were observed in the null mice (Fig. 2). AKT levels were not significantly changed. These results demonstrated that GRTH have a general effect on mitochondria controlled apoptotic pathways. GRTH suppresses pro-apoptotic proteins such as Bid, Bad, Bak, Smac, and p53, whereas it increases the expression of anti-apoptotic proteins such as Bcl-xL, Bcl-2, and others (summary and Fig. 2C).
FIGURE 1.
Western analysis of pro- and anti-apoptotic factors expression in spermatocytes of WT and GRTH KO mice. A, protein expression analysis of Bcl-2 protein family members (Bcl-2, Bcl-xL, Bid, Bak, Bad, and phospho-Bad (pBad)), Smac, p53, and Hsp10, IAP utilizing specific antibodies. B, signals from three independent experiments were quantified and normalized by β-actin or tubulin. The KO values are presented as percentages of WT. WT, 100% (thin black line). The values are the means ± S.E. *, p < 0.05.
FIGURE 2.
Western blot analysis of protein kinase expression representative of three independent pathways in WT and KO spermatocytes. A, analysis of expression of PKAc, Erk1/2, phospho-Erk1/2 (P-Erk1/2), and AKT. B, signals from three independent experiments were quantified and normalized by β-actin. The KO values are presented as percentages of WT. WT, 100% (thin black line). The values are the means ± S.E. *, p < 0.05. C, summary diagram of GRTH effect in the apoptotic pathway. +, required for optimal protein expression. Dotted arrow, no effect.
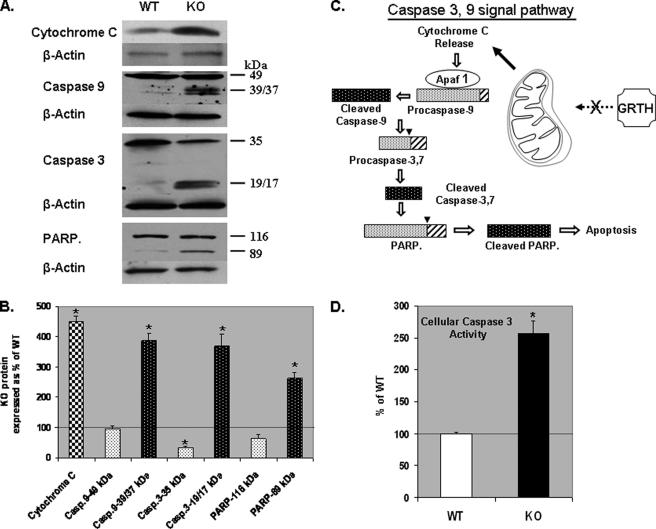
Effect of GRTH on Caspase Signal Pathway—Cleaved products of caspase 9, caspase 3, and PARP were minimally detectable in spermatocytes of WT mice, but significant increases in active cleaved products were observed in KO mice compared with WT (Fig. 3, A and B) (200–300% increases in cleaved products 39/37 kDa, caspase 9; 19/17 kDa, caspase 3; and 89 kDa, PARP). Uncleaved products of caspase 9 (49 kDa) and PARP (116 kDa) were not significantly different from WT. In contrast, uncleaved caspase 3 (35 kDa) was reduced to 30% of WT. Moreover, a 250% increase in the levels of caspase 3 cellular activity was found in KO mice compared with WT (Fig. 3D).
FIGURE 3.
Evaluation of cleavage products of members of caspase 3 and 9 signal pathway in WT and KO mice. A, Western analysis of caspase (Casp.) 3/9, PARP, and their associated cleavage products. B, signals were quantified and normalized by β-actin from three independent experiments. The KO values are presented as percentages of WT. WT, 100% (thin black line). The values are the means ± S.E. *, p < 0.05. C, summary diagram of the effects of GRTH in the caspase 3 and 9 signal pathway. D, endogenous cellular caspase 3 activity in the germ cells of WT and GRTH KO. The KO values are presented as percentages of WT. WT, 100% (thin black line). The values are the means ± S.E. of three independent experiments in triplicate. *, p < 0.05.
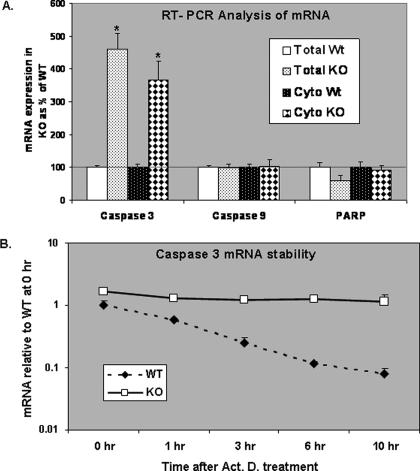
Effect of GRTH on Caspase 3 Transcript Stability—We also determined the steady-state levels of mRNA transcripts of the caspase pathway in WT and GRTH KO mice by real time PCR analysis. Caspase 3 mRNA transcript levels in total cell extract and cytosolic fraction of spermatocytes were 500% in KO compared with WT, whereas those of caspase 9 and PARP were unchanged (Fig. 4A). The increases observed on caspase 3 transcripts in KO mice are related to changes in mRNA stability because the half-life of caspase 3 mRNA transcripts was 1 h in WT, whereas these transcript levels were stable for at least 10 h in KO (Fig. 4B).
FIGURE 4.
Effect of GRTH on caspase 3 transcript stability. A, RT-PCR analysis of mRNA level of caspase 3 and 9 and PARP in cytoplasm (Cyto) and whole cell extracts (Total) of spermatocytes from wild type (Wt) and GRTH KO mice. The KO values are presented as percentages of WT. WT, 100% (thin black line). The values are the means ± S.E. of three independent experiments in triplicate. *, p < 0.05. B, spermatocytes prepared from WT or GRTH KO mice were incubated with 10 μg/ml actinomycin D (Act. D) for 1–10 h. The mRNA samples were analyzed by RT-PCR using specific caspase 3 primers.
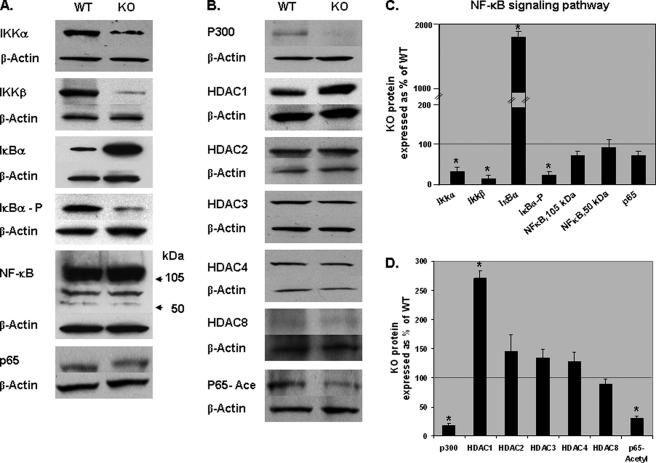
Effect of GRTH on NF-κB-mediated Anti-apoptotic Pathway—Because we observed major reductions of anti-apoptotic factors expression in the GRTH null mice and given the major role of NF-κB in the stimulation of transcription of anti-apoptotic genes, we investigated the role of GRTH on NF-κB mediated anti-apoptotic pathway in spermatocytes of WT and KO mice (Fig. 5). We observed a major increase in the levels of IκBα and a profound decrease in phospho-IκBα (p-IκBα) levels in KO spermatocytes compared with WT (Fig. 5, A and C). The latter may have resulted from the major reduction of IKKα/β kinase catalytic subunits detected. In contrast, levels of NF-κB protein species (NF-κB1 and p65) were unchanged (Fig. 5, A and C). In addition, acetylation of NF-κB required for its transcriptional activity could be curtailed by the nearly absence of p300 and increase in HDAC1 (Fig. 5, B and D). Other members of the HDAC I and II subgroups were minimally increased (HDAC2, HDAC3, and HDAC4) or unchanged (HDAC8).
FIGURE 5.
Effect of GRTH on NF-κB-mediated anti-apoptotic pathway. A and B, Western analysis of protein samples prepared from spermatocytes of adult WT and GRTH KO mice. C and D, signals from three independent experiments were quantified and normalized by β-actin. The KO values are presented as percentages of WT. WT, 100% (thin black line). The values are the means ± S.E. of three independent experiments in triplicate. *, p < 0.05.
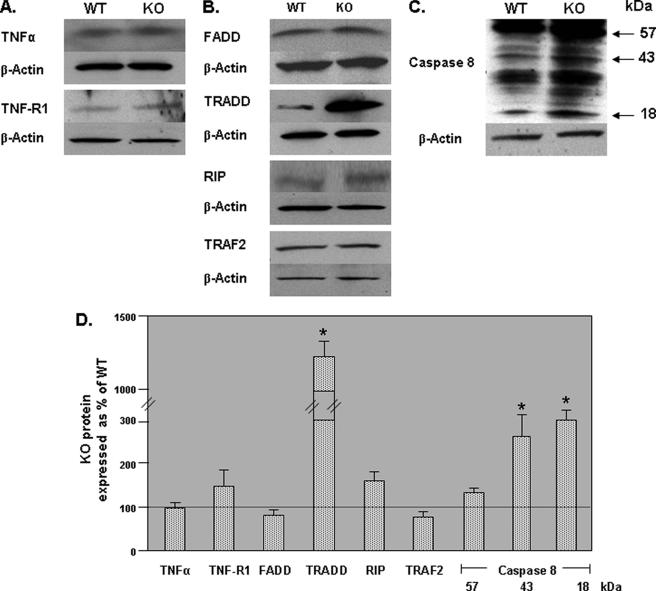
Effect of GRTH on TNFα/TNF-R1-mediated Apoptotic Pathway—Because the NF-κB pathway could be also regulated by signaling resulting from activation of TNF-R1 by TNFα via the death domain associated adaptor protein complex, we investigated the expression of components of this pathway in the GRTH null mice. Only significant increases in TNF-R1-associated adaptor protein TRADD was observed in the absence of GRTH (Fig. 6, B and D). No obvious change in the levels of the receptor, its binding ligand (Fig. 6, A and D) and other TNF-R1-associated factors including FADD protein, receptor-interacting protein (RIP), and TNF receptor-associated factor 2 were observed (Fig. 6, B and D). Activation of caspase 8 indicated by increases in the 18- and 43-kDa cleaved products from the 57-kDa precursor was observed in KO mice (Fig. 6, C and D). These results suggest the participation of the death receptor signaling in the germ cell apoptosis observed in GRTH null mice.
FIGURE 6.
Effect of GRTH on TNFα/TNF-R1 mediated apoptotic pathways. A–C, Western analysis of factors associated in TNFα/TNF-R1 signaling pathway. A, ligand binding at the cell surface. B, receptor associated cytoplasmic factors. C, caspase 8. D, signals from three independent experiments were quantified and normalized by β-actin. KO values are presented as percentages of WT. WT, 100% (thin black line). The values are the means ± S.E. *, p < 0.05.
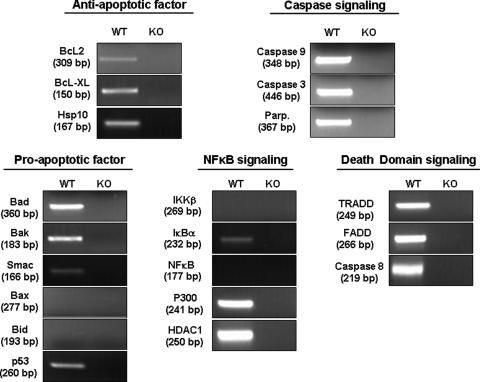
GRTH Associated with a Subset of Apoptotic Factors mRNA Transcript as GRTH-mRNP—We previously demonstrated that the export and translational functions of GRTH as a component of mRNP are required for the expression of gene products relevant to spermiogenesis (3, 4).). Thus, it was of interest to determine the association of GRTH with messages involved in pro- and anti-apoptotic pathways through isolation of GRTH-mRNA complexes and characterization of mRNAs candidates of apoptotic and anti-apoptotic factors (Fig. 7). GRTH was found to associate selectively with certain pro-apoptotic factors including Bad, Bak, Smac, and p53, and anti-apoptotic factors Bcl-2, Bcl-xL, and Hsp10. It also associated with caspase 9, caspase 3, and PARP mRNAs. GRTH associated with NF-κB cytoplasmic inhibitor IκBα and nuclear regulators, acetyltransferase p300, and HDAC1, whereas no association with NF-κB was observed. GRTH also formed RNP particles with death domain-associated cytoplasmic adaptor proteins mRNAs including TRADD and FADD and the caspase 8 message in this signaling pathway. GRTH association with certain pro-apoptotic or anti-apoptotic transcripts could cause the reduction of the pro-apoptotic pathway and prevalence of anti-apoptosis through its silencing/degradation or translation/transport functions in spermatocytes.
FIGURE 7.
RT-PCR analysis of immunoprecipitated testicular GRTH complexes with cellular pro- and anti-apoptotic mRNAs. KO testicular extracts were used as negative controls. Specific sets of primers for genes of interests were designed accordingly (supplemental Table S1). A representative example of three independent experiments is provided.
DISCUSSION
Apoptosis during spermatogenesis of the pubertal testis is crucial to achieve normal mature germ cells at adulthood (6, 7). Although apoptosis is a major event in the prepubertal mouse testis during the first spermatogenic wave and affects spermatogonia and zygotene-pachytene spermatocytes (8–13), only sporadic apoptosis is observed in the adult testis. This difference suggests that factor(s) residing in the late spermatocytes might suppress apoptosis in the mature rodent testis. However, the control mechanism(s) of this tightly regulated process are largely unknown.
The striking apoptosis observed in the spermatocytes of adult GRTH null mice testis indicated the important role of this helicase in determining the survival and apoptotic fate of adult germ cells (3). By comparing the global expression of pro- and anti-apoptotic protein profiles in spermatocytes of wild type and GRTH null mice, we have demonstrated that lack of GRTH caused increases in most of the pro-apoptotic factors and decreases of anti-apoptotic factors. A potential synergism of death receptor, NF-κB, and mitochondrial pathways leading to massive germ cell apoptosis caused by the absence of GRTH could be envisioned. Our findings underscore the importance of GRTH to promote cell survival by acting as a negative regulator of these apoptotic signaling pathways. In the case of caspase 3, an accelerated turnover rate of its mRNA was governed by GRTH to prevent cell death. In the NFκB pathway, both the upstream cytoplasmic regulator IKK and the nuclear co-activator p300 could affect NFκB-mediated signaling and transcriptional activation of anti-apoptotic genes to prevent cell death. In the death receptor signaling, a significant up-regulation of TRADD was observed in KO mice compared with the minimally detectable level present in WT. This suggests a specific impact of GRTH on TRADD-mediated signaling, which is further supported by the activation of caspase 8. In contrast, minimal or no changes of other death domain-associated adaptor proteins were observed, suggesting the specificity of GRTH regulation within this pathway.
Our findings suggest that the lack of GRTH might disrupt mitochondria integrity (14) by reduction of its membrane-bound anti-apoptotic proteins and increases in pro-apoptotic Bad, Bak, and Bid, which promote the release of cytochrome c. In the unphosphorylated state, Bad is targeted to the mitochondrial surface where it binds Bcl-xL, preventing the anti-apoptotic activity of this protein and leading to events causing cell death. Phosphorylation at serines 112, 136, and 155 causes dissociation of Bad from Bcl-xL (15–18). The marked reduction of Bad phosphorylation observed in KO mice (Fig. 1) could contribute to induction of the apoptotic cascade and further suggested that the cells destined to degenerate could not survive in the absence of GRTH. Phosphorylation of Bad is known to be associated with the cell survival mechanism that raises the threshold at which the mitochondria release cytochrome c in response to the apoptotic stimuli (19–21). Our studies have shown down-regulation of enzymes from two independent kinase pathways (PKA and mitogen-activated protein kinase) in GRTH null mice. Because the message of the mitochondrial PKA-anchoring protein AKAP1 was profoundly decreased in a microarray gene expression analysis (not shown), PKA could be reduced or absent at mitochondrial sites to counterbalance the apoptotic process (15). This could also affect the cellular environment necessary for cell survival in GRTH null mice. It is possible that GRTH is required to maintain cellular kinases for other functions, specifically to support requisite levels of its cytoplasmic phospho-form (61 kDa) (4).
In this study, the absence of GRTH caused the release of cytochrome c from mitochondria into the cytosol, and the increase of factors that induced caspase cleaved products from caspase 9/3 and PARP known to induced DNA fragmentation in the apoptotic process (22, 23). The higher level of caspase 3-binding factor, Smac, and the diminished level of binding competitor (IAP), might relieve the inhibitory effect of the IAPs on caspase and trigger the observed increases of its protease activity in the GRTH null mice. Hsp10, a known anti-apoptotic factor (24) that stabilizes mitochondrial cross membrane potential, thus inhibiting caspase 3 activity and suppressing PARP, was also markedly decreased. The inhibitory effect of GRTH on caspase 3 was further revealed by the marked increase in its mRNA and the cleaved protein products observed in its absence. GRTH is an integral component of messenger ribonuclear protein particles associated with most apoptotic mRNA transcripts (Fig. 7). By associating with GRTH, caspase 3 undergoes rapid degradation, and apoptosis is kept in check, in contrast with its prolonged stability in the absence of GRTH (Fig. 4). Caspase 3 degradation presumably occurs at cytoplasmic sites such as chromatoid bodies or its precursors (25) through small interfering RNA pathways. We have previously suggested that GRTH could function as a component of mRNP to transport relevant messages into chromatoid bodies for their post-translational regulation (3–5). In contrast only changes in activity were observed for caspase 9 and PARP as indicated by their cleavage products (Fig. 3).
NF-κB dimers in the cytoplasm translocate to the nucleus and stimulate the transcription of anti-apoptotic genes (26–28). In the presence of IκBα, such dimers are sequestered, and NF-κB transfer to the nucleus is prevented, leading to apoptosis. Phosphorylation of IκBα causes its ubiquitination and proteosomal degradation, thus increasing cell survival (29). In GRTH mice, the increased levels of IκBα and reduction of its phosphorylation could cause sequestration of NF-κB dimers in the cytoplasm. Accumulation of IκBα and the marked reduction of its phospho-form, possibly caused by the lower levels of IKKα and IKKβ, could decrease its proteosomal degradation. This would prevent NF-κB transfer to the nucleus and reduce the transcription of anti-apoptotic factors. Furthermore, acetylation of NF-κB, which is required for its activation of gene transcription (30, 31), could be compromised because of the almost complete absence of p300 in GRTH KO mice. In addition, the increases in tumor suppressor p53 observed in the GRTH KO mice might facilitate association of NF-κB with HDAC1 (32), which was highly up-regulated in the absence of GRTH. This will consequently impair IKKα/β recruitment to promoter regions of NF-κB-regulated genes. IKKα, by interacting with HAT co-activators, is recruited to promoter regions of NF-κB-regulated genes and contributes to gene expression through phosphorylation of histone H3 (33, 34). Thus, a lack of GRTH may impair the nuclear events associated with NF-κB activation of anti-apoptotic genes Bcl-2 and Bcl-xL. GRTH has dual roles in regulating NF-κB activity at cytoplasmic and nuclear sites. These findings indicate that GRTH could be required for IKK expression to accelerate IκBα degradation through phosphorylation to free NF-κB from inhibition, allowing its dimers to translocate to nuclear sites. In addition, IKK recruitment to promoter regions of NF-κB-regulated anti-apoptotic genes may contribute its transcriptional function.
We also determined whether the involvement of the extrinsic pathway initiated by the cell surface death receptor in the downstream apoptotic events was observed in GRTH null mice. TNFα has been suggested to promote cell survival during spermatogenesis (35). In other systems (i.e. immune, hepatic, and nervous system), recruiting the death domain of TNF-R1-associated adaptor proteins, TRADD, FADD, and RIP upon TNFα stimulation can trigger either cell survival through the activation of NF-κB pathway or cell death via caspase 8, caspase 10, or Jun N-terminal kinase signaling cascades (27). Activation of NF-κB requires the joint recruitment of adaptor protein complexes to the activating IKK complex, which phosphorylates the NF-κB inhibitor IκB. However, in GRTH null mice the observed increase solely in TRADD expression indicates that this pathway is not operative to maintain the NF-κB survival route (Fig. 6). This is consistent with our findings of a marked reduction of IKKs, the phospho-form of IκB, and the inactivation of NF-κB function discussed above. Furthermore, the apoptosis observed in these mice is unlikely to be mediated through the Jun N-terminal kinase signaling pathway triggered by RIP because only minor increases of RIP were found in our study (Fig. 6). Because we did not observe any change in either Fas (data not shown) or FADD, which is known to activate the caspase 8 cascade (36), the significant increases in the active subunits of p18 from pro-caspase 8 suggest that TRADD could activate the caspase 8 cascade by recruiting FADD to trigger the release of mitochondrial factors and activation of downstream effector caspases 3, resulting in cell death. Unlike the study showing that TNFα can promote germ cell survival through its cognate receptor (35), GRTH seems to act as a regulator at the level of its downstream death receptor-associated adaptor protein TRADD in the TNFα-associated pathway. Whether this isolated change could induce activation of the pathway remains to be determined. It is possible that the TRADD message associated with GRTH as a mRNP complex is unstable or that its translation is inhibited by GRTH, and it is consequently inactive in the death domain signaling of apoptosis in wild type testis.
These observations suggest that when GRTH interacts with a subset of mRNAs of pro-apoptotic factors, these might be either down-regulated or silenced through the small RNA degradation pathway (5). Conversely, GRTH could favor export of nuclear anti-apoptotic messages and subsequent translational events during spermatogenesis in the wild type mice (4, 5). In regard to the species whose mRNA does not interact with GRTH, this will require further investigation.
In conclusion, these studies have clearly demonstrated that GRTH acts as a negative regulator in the apoptotic fate of germ cells at both cytoplasmic and nuclear sites (summary diagram is in Fig. 8). Acting as a component of RNP particles, with a panel of messages for pro- and anti-apoptotic genes, GRTH could directly or indirectly regulate their respective gene degradation and/or translation required for germ cell survival. It might also have a specific inhibitory action on the transport of messages of pro-apoptotic gene from the nucleus to the cytoplasm. GRTH could indirectly regulate nuclear events through the maintenance of adequate expression of co-activator p300 and IKKs for acetylation of NF-κB and recruitment of IKKα to promoter sites to increase NF-κB transcriptional activity. The participation of tumor necrosis factor-mediated downstream death domain TNF-R1/TRADD signaling in the KO mice further demonstrates that GRTH has an essential regulatory role in two opposing sets of pro- and anti-apoptosis in spermatocytes of post-pubertal mice. This study provides new insights into the regulatory mechanism of cell programming death in male germ cells and highlights the central role of GRTH in the prevention of apoptosis in the adult male gonad.
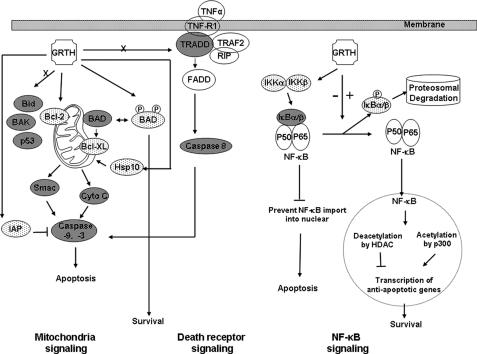
FIGURE 8.
Summary of GRTH impact in apoptotic pathways in germ cells. GRTH regulates subsets of pro-apoptotic and anti-apoptotic factors involved in mitochondria, death receptor, and NF-κB signaling pathways in germ cells apoptosis. Left, GRTH acts as a negative regulator to prevent antiapoptotic factor expression (×) during spermatogenesis. Absence of GRTH caused increased expression of anti-apoptotic factors (solid oval) and suppression of pro-apoptotic factors (stippled oval) in the mitochondria controlled apoptotic pathway with subsequent cytochrome c release, and downstream caspase 3/9 activation (middle). Regarding the TNFα/TNF-R1 signaling pathway, the lack of GRTH increased the TNF-R1-associated adaptor protein TRADD expression could cause the downstream caspase 8 activation (solid oval). Other cytoplasmic adaptor proteins are not apparently involved in the GRTH regulatory action (open oval). Right, NF-κB pathway. GRTH could have impact in NF-κB action at both cytoplasmic and nuclear levels. In the cytoplasm, lack of GRTH (–) caused the decrease in IKKα/β kinase complex expression and IκB phosphorylation; consequently its proteasomal-dependent degradation is curtailed. This favors nonphosphorylate IκBα/β association with NF-κB, which reduces its import to the nucleus and presumably the transcriptional activation of anti-apoptotic genes. Lack of GRTH could also alter the acetylation status of NF-κB in the nucleus by increases in HDAC and decreases in acetylase p300 expression, resulting in the decrease in anti-apoptotic gene transcription and apoptosis. Presence of GRTH (+) promotes cell survival. P, phosphorylation.
Supplementary Material
This work was supported, in whole or in part, by the National Institutes of Health (Intramural Research Program of the NICHD). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
Footnotes
The abbreviations used are: GRTH, gonadotropin-regulated testicular helicase(s); KO, knock-out; TNF, tumor necrosis factor; TNF-R, TNF receptor; HDAC, histone deacetylase; PARP, poly(ADP-ribose)polymerase-1; Erk, extracellular signal-regulated kinase; FADD, FAS-associated death domain; WT, wild type; RT, reverse transcription; PKA, cAMP-dependent protein kinase; RIP, receptor-interacting protein; TRADD, TNF-R1-associated death domain.
References
- 1.Tang, P. Z., Tsai-Morris, C. H., and Dufau, M. L. (1999) J. Biol. Chem. 274 37932–37940 [DOI] [PubMed] [Google Scholar]
- 2.Sheng, Y., Tsai-Morris, C. H., and Dufau, M. L. (2003) J. Biol. Chem. 278 27796–27803 [DOI] [PubMed] [Google Scholar]
- 3.Tsai-Morris, C. H., Sheng, Y., Lee, E., Lei, K. J., and Dufau, M. L. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng, Y., Tsai-Morris, C. H., Gutti, R., Maeda, Y., and Dufau, M. L. (2006) J. Biol. Chem. 281 35048–35056 [DOI] [PubMed] [Google Scholar]
- 5.Dufau, M. L., and Tsai-Morris, C. H. (2007) Trends Endocrinol. Metab. 18 314–320 [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez, I., Ody, C., Araki, K., Garcia, I., and Vassalli, P. (1997) EMBO J. 16 2262–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaha, C. (2007) Soc. Reprod. Fertil. Suppl. 63 173–186 [PubMed] [Google Scholar]
- 8.Mori, C., Nakamura, N., Dix, D. J., Fujioka, M., Nakagawa, S., Shiota, K., and Eddy, E. M. (1997) Dev. Dyn. 208 125–136 [DOI] [PubMed] [Google Scholar]
- 9.Lee, J., Richburg, J. H., Younkin, S. C., and Boekelheide, K. (1997) Endocrinology 138 2081–2088 [DOI] [PubMed] [Google Scholar]
- 10.Boekelheide, K., Fleming, S. L., Johnson, K. J., Patel, S. R., and Schoenfeld, H. A. (2000) Proc. Soc. Exp. Biol. Med. 225 105–115 [DOI] [PubMed] [Google Scholar]
- 11.Jeyaraj, D. A., Grossman, G., and Petrusz, P. (2003) Reprod. Biol. Endocrinol. 1 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahnukainen, K., Chrysis, D., Hou, M., Parvinen, M., Eksborg, S., and Soder, O. (2004) Biol. Reprod. 70 290–296 [DOI] [PubMed] [Google Scholar]
- 13.Morales, A., Mohamed, F., and Cavicchia, J. C. (2007) Anat. Rec. 290 206–214 [DOI] [PubMed] [Google Scholar]
- 14.Donovan, M., and Cotter, T. G. (2004) Biochim. Biophys. Acta 1644 133–147 [DOI] [PubMed] [Google Scholar]
- 15.Harada, H., Becknell, B., Wilm, M., Mann, M., Huang, L. J., Taylor, S. S., Scott, J. D., and Korsmeyer, S. J. (1999) Mol. Cell 3 413–422 [DOI] [PubMed] [Google Scholar]
- 16.Lizcano, J. M., Morrice, N., and Cohen, P. (2000) Biochem. J. 349 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta, S. R., Katsov, A., Hu, L., Petros, A., Fesik, S. W., Yaffe, M. B., and Greenberg, M. E. (2000) Mol. Cell 6 41–51 [PubMed] [Google Scholar]
- 18.Tan, Y., Demeter, M. R., Ruan, H., and Comb, M. J. (2000) J. Biol. Chem. 275 25865–25869 [DOI] [PubMed] [Google Scholar]
- 19.Bergmann, A. (2002) Dev. Cell 3 607–608 [DOI] [PubMed] [Google Scholar]
- 20.Thomson, M. (2002) Cell Mol. Life Sci. 59 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horbinski, C., and Chu, C. T. (2005) Free Radic. Biol. Med. 38 2–11 [DOI] [PubMed] [Google Scholar]
- 22.Shi, Y. (2002) Mol. Cell 9 459–470 [DOI] [PubMed] [Google Scholar]
- 23.Koh, D. W., Dawson, T. M., and Dawson, V. L. (2005) Pharmacol. Res. 52 5–14 [DOI] [PubMed] [Google Scholar]
- 24.Shan, Y. X., Liu, T. J., Su, H. F., Samsamshariat, A., Mestril, R., and Wang, P. H. (2003) J. Mol. Cell Cardiol. 35 1135–1143 [DOI] [PubMed] [Google Scholar]
- 25.Fawcett, D. W., Eddy, E. M., and Phillips, D. M. (1970) Biol. Reprod. 2 129–153 [DOI] [PubMed] [Google Scholar]
- 26.Hayden, M. S., and Ghosh, S. (2004) Genes Dev. 18 2195–2224 [DOI] [PubMed] [Google Scholar]
- 27.Dutta, J., Fan, Y., Gupta, N., Fan, G., and Gelinas, C. (2006) Oncogene 25 6800–6816 [DOI] [PubMed] [Google Scholar]
- 28.Perkins, N. D. (2007) Nat. Rev. Mol. Cell. Biol. 8 49–62 [DOI] [PubMed] [Google Scholar]
- 29.Hacker, H., and Karin, M. (2006) Sci. STKE 2006 re13. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen, L., De Wilde, G., Notebaert, S., Vanden Berghe, W., and Haegeman, G. (2002) Biochem. Pharmacol. 64 963–970 [DOI] [PubMed] [Google Scholar]
- 31.Quivy, V., and Van Lint, C. (2004) Biochem. Pharmacol. 68 1221–1229 [DOI] [PubMed] [Google Scholar]
- 32.Gu, W., Luo, J., Brooks, C. L., Nikolaev, A. Y., and Li, M. (2004) Novartis. Found. Symp. 259 197–205 [PubMed] [Google Scholar]
- 33.Yamamoto, Y., Verma, U. N., Prajapati, S., Kwak, Y. T., and Gaynor, R. B. (2003) Nature 423 655–659 [DOI] [PubMed] [Google Scholar]
- 34.Anest, V., Hanson, J. L., Cogswell, P. C., Steinbrecher, K. A., Strahl, B. D., and Baldwin, A. S. (2003) Nature 423 659–663 [DOI] [PubMed] [Google Scholar]
- 35.Suominen, J. S., Wang, Y., Kaipia, A., and Toppari, J. (2004) Eur. J. Endocrinol. 151 629–640 [DOI] [PubMed] [Google Scholar]
- 36.Beere, H. M. (2005) J. Clin. Investig. 115 2633–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.