Abstract
Herpesviruses such as cytomegaloviruses encode functions that modulate the innate response in diverse ways to counteract host sensing and delay host clearance during infection. The murine cytomegalovirus M45 protein interacts with receptor-interacting protein (RIP) 1 and RIP3 via a RIP homotypic interaction motif. Cell death suppression by M45 requires RIP homotypic interaction motif-dependent interaction with RIP1. This interaction also underlies the cell tropism role of M45 in preventing premature death of endothelial cells during murine cytomegalovirus infection. Thus, M45 is a viral inhibitor of RIP activation that provides a direct cell type-dependent replication benefit to the virus while modulating other biological processes signaling via the RIP1 adaptor such as activation of Toll-like receptor (TLR)3 as well as other mediators of cell death.
Receptor-interacting protein kinase 1 (RIP12 or RIPK1) carries out an adaptor function at the intersection of major cytokine signaling and pathogen pattern recognition pathways to control cell fate and inflammatory responses (1, 2). Pathogens are known to encode functions that counteract a number of inflammatory and death pathways downstream of cellular sensors (3). Programmed cell death, in particular, is a central and evolutionarily conserved component of the intrinsic response to intracellular pathogens. RIP1 is the founding member of a family of serine-threonine protein kinases that are related to the interleukin 1 receptor-associated kinase family (1, 2). RIP1 contains a carboxyl-terminal death domain and a central RIP homotypic interaction motif (RHIM) that mediate protein-protein interactions. The RHIM controls binding with two other RHIM-containing adaptor proteins, RIP3 and Toll/interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon β (TRIF) (4–6). These RHIM-RHIM interactions regulate the behavior of RIP1 (1, 2). The RIP1 death domain interacts with a different set of adaptors and triggers apoptosis (1, 2). Although RIP3 may regulate RIP1 function under some circumstances, mice and cell lines deficient for RIP3 appear normal (7–10), whereas RIP1-deficient mice die soon after birth (11). RIP1 therefore plays an important homeostatic role controlling cell fate in addition to its role in the host cell response to pathogens. TRIF is an adaptor that transduces signals from Toll-like receptor (TLR)3 and TLR4, initiating an interferon response and cell death pathways (12, 13). Ectopic expression of TRIF induces RHIM-dependent apoptosis via RIP1 (4).
Study of these RHIM-containing adaptors has revealed the central role of RIP1 in controlling complex pathways related to decisions of cell survival and death (1, 2). RIP1 is crucial to signaling from death receptors (e.g. tumor necrosis family (TNF) receptor (TNFR1), Fas, and TNF-related apoptosis-inducing ligand receptor) and is recruited to transduce cell survival as well as cell death signals (11, 14). These functions are independent of protein kinase activity and possibly the direct consequence of a caspase-8-generated RIP1 cleavage product (containing the death domain and RHIM but lacking the kinase domain (15–17)). In addition, RIP1 may mediate both necrosis and autophagy, functions where its kinase domain may be critical (2, 18–20). Significantly less is known about the role of RIP3 in any of these pathways, although this kinase is thought to regulate RIP1. Pathogens are not known to directly modulate either RIP1 or RIP3 activity, although such interactions would influence innate immune signaling and cell fate (3).
The M45 gene of murine cytomegalovirus (MCMV) encodes a cell type-specific suppressor of cell death (21). This herpesvirus-conserved homolog of the large subunit of ribonucleotide reductase (RR1) lacks enzymatic activity (22) but is a cell tropism determinant required to inhibit premature cell death during infection of endothelial and macrophage cell lines (21). M45 promotes replication in cultured cells and facilitates viral replication in mice (22). Recently, M45 was identified as a physiologically relevant RIP1 binding partner by mass spectroscopy (23). The study also showed that the full-length M45 retained cell death suppression activity independent of viral replication. Although showing that M45 inhibited NF-κB activation, the study did not pursue detailed mapping of the M45-RIP1 interaction or the mechanism of cell death suppression. Here, we demonstrate that M45 is a RHIM-containing adaptor that interacts with cellular RIP1 and RIP3. RHIM-dependent interactions underlie M45-mediated protection from cell death in several contexts including the natural setting of viral infection. Thus, M45 is the first virus-encoded adaptor that intersects with and modulates RHIM-dependent events in cells.
EXPERIMENTAL PROCEDURES
Cell Lines, Transfection, and Infection—NIH3T3 fibroblasts (ATCC number CRL-1658) (24), SVEC4–10 (ATCC number CRL-2181) (21), and 293T cells (25) were transfected with Superfect (Qiagen), Effectene (Qiagen), and Polyfect (Qiagen), respectively, according to the manufacturer's protocols. Bacmid-derived parental virus MCMV-GFP (strain Smith-ATCC) and MCMVΔ45 (26) were propagated and used for infections as described (21).
Plasmid Construction—M45 open reading frame and mutants were PCR-amplified from the MCMV K181 bacmid (a gift of Alec Redwood, University of Western Australia, Nedlands, Australia; GenBank™ accession AM886412). Truncation mutants were generated by amplifying nucleotides 1–831 (aa 1–277) or nucleotides 832–3522 (aa 278–1174). M45mut-RHIM and M45-(1–277)mutRHIM were generated by overlap extension PCR replacing M45 sequence amino acid (aa) 61–64 (IQIG to AAAA). All PCR fragments were cloned into the PstI site of pCMV-TAG 5A (Stratagene), resulting in an in-frame carboxyl-terminal c-Myc epitope tag. Expression vectors encoding CrmA, FADD-DN, Bcl2, RIP1, RIP2, RIP3, RIP4, and TRIF have been described (4).
Immunofluorescence—NIH3T3 cells were seeded on coverslips, transfected with 1 μg of expression plasmid, and fixed 18 h later in 4% paraformaldehyde, PBS for 20 min. Cells were permeabilized with 0.5% Triton X-100, PBS and blocked for 1 h using blocking buffer (0.1% bovine serum albumin, 5% donkey serum, PBS). Cells were stained for 1 h with rabbit polyclonal anti-c-Myc (1:1000; Upstate Biotechnology) and mouse monoclonal anti-RIP1 (1:100; BD Biosciences) antibodies diluted in blocking buffer. Samples were then washed four times in PBS and incubated for 1 h with Alexa Fluor 488-conjugated donkey anti-mouse and Alexa Fluor 594-conjugated donkey anti-rabbit (1:1000; Molecular Probes) antibodies diluted in blocking buffer. Samples were washed four times in PBS and mounted on slides with Gel-Mount (Biomeda Corp).
Immunoprecipitation and Immunoblotting—293T cells in 10-cm dishes were co-transfected with 4 μg of each plasmid (8 μg total), harvested in 500 μl of lysis buffer containing 1% Nonidet P-40 16–18 h after transfection and subjected to a combined immunoprecipitation/immunoblot (IP/IB) analysis to detect interactions (27). Lysates were cleared by centrifugation and immunoprecipitated with 30 μl of anti-FLAG(M2)-agarose (Sigma). Precipitates were washed four times, separated by electrophoresis (SDS-PAGE), transferred to nylon membrane, and immunoblotted with rabbit anti-c-Myc peroxidase and mouse anti-FLAG(M2) peroxidase-conjugated antibodies (Sigma). Blots were detected with ECL (Amersham Biosciences).
Cell Death Assays—NIH3T3 cells in 6-well dishes were transfected with 2 μg of expression vectors mixed with 0.2 μgof pEGFP-C1 (Clontech). At 24 h after transfection, cells were treated with 25 ng/ml murine TNF-α (Peprotech) and 10 μg/ml cyclohexamide (CHX; Sigma) for 6 h and then examined by fluorescence microscopy. GFP-positive cells in 8–9 random fields/sample were scored for hallmarks of apoptosis, and the percentage of survival was calculated (total GFP-positive cells less apoptotic GFP-positive cells × 100) in each of three independent experiments. 293T cells in 6-well dishes were co-transfected with 1 μg of vector, M45, M45 derivatives, or FADD-DN, 1 μg of RIP3 or TRIF, and 0.2 μg of pEFGP-C1. 18 h after transfection, cells were scored, and the percentage of survival was calculated as above for three independent transfections. SVEC4–10 cells were transfected with 1 μg of M45 or M45 derivatives and 0.5 μg pDsRed-Monomer-N1 (Clontech). At 36 h after transfection, cells were infected with either WT MCMV-GFP or mutant MCMVΔ45. 24 h after infection, samples were washed four times with PBS, fixed in 4% paraformaldehyde, and scored for DsRed+ cells.
RESULTS
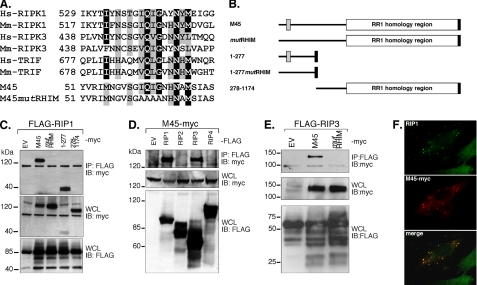
M45 Interacts with RIP1 and RIP3 via a RHIM—We evaluated the aa sequence of MCMV M45, a cell type-specific cell death suppressor (21), for motifs that might provide insight into function. A short stretch (aa 61–69) of M45 contained a (I/V)Q(I/L/V)GXXNXM motif characteristic of the RHIMs of RIP1, RIP3, and TRIF (Fig. 1A). This segment of M45 is located near the amino terminus (Fig. 1B), a region outside the herpesvirus-conserved RR1 homology located in the carboxyl-terminal region. We prepared expression vectors that encoded full-length M45 (M45WT) and a mutant (M45mutRHIM) with four alanine substitutions disrupting the putative RHIM (Fig. 1A). An identical strategy has been used before to characterize interactions of other RHIM proteins (5, 6). Because M45 is naturally produced during infection as a full-length protein that is cleaved after aa 277 (22), we also prepared vectors representing polypeptides predicted to be derived from the amino-terminal region (aa 1–277) and the carboxyl-terminal RR1 region (aa 278–1174) following cleavage. We also constructed M45-(1–277)mutRHIM, encoding aa 1–277 with the RHIM mutated.
FIGURE 1.
M45 RHIM-dependent interactions. A, amino acid sequence of RHIM-containing regions of human (Hs) and mouse (Mm) proteins RIP1 (RIPK1), RIP3 (RIPK3), and TRIF aligned with MCMV M45 and a mutant (mutRHIM) prepared for these studies. B, schematic diagram of MCMV full-length M45 and mutants used in these studies. The amino-terminal RHIM is depicted by a gray box, and the carboxyl-terminal c-Myc epitope tag is depicted by a black box. The mutation shown in panel A is depicted by the absence of a gray box in mutRHIM and 1–277mutRHIM. C, autoradiograph resulting from IP/IB analysis of M45-RIP1 interaction. 293T cells were transfected with c-Myc-tagged M45 or mutants and FLAG-tagged RIP1. The top panel shows the result of cell lysates subjected to anti-FLAG-immunoprecipitation of RIP1 and anti-c-Myc immunoblot analysis (IP:FLAG and IB:Myc), with results for empty vector (EV), M45WT (M45), M45mutRHIM (mutRHIM), M45 amino-terminal portion (1–277), and M45 carboxyl-terminal portion (278–1174). The lower panels depict expression controls: whole cell lysate (WCL) immunoblotted with anti-c-Myc antibody (WCL and IB:myc) and the WCL immunoblotted with anti-FLAG antibody. D, autoradiograph resulting from IP/IB analysis of M45-RIP3 interaction. 293T cells were transfected with M45WT (M45-myc) and FLAG-tagged RIP1, RIP2, RIP3, RIP4, or TRIF. E, 293T cells were transfected with c-Myc-tagged M45 or M45mutRHIM and FLAG-tagged RIP3. Top panels (IP:FLAG and IB:myc) in panels D and E were performed, with the control panels below, as described for panel C. Protein molecular size is shown on the left of the lanes. F, M45 co-localization with endogenous RIP1 in murine cells. NIH3T3 cells were transfected with M45-Myc and processed for immunofluorescence analysis to detect RIP1 (green; top panel), cMyc (red; middle panel) and merged (bottom panel) images.
To follow up on the reported natural interaction between M45 and RIP1 detected in infected cells (21) and to determine whether the M45 interaction with RIP1 was RHIM-dependent, we performed IP/IB analyses from 293T cell extracts after transfection with c-Myc epitope-tagged M45 and M45 mutant expression vectors together with FLAG epitope-tagged RIP1 (5, 6). Initially, RIP1 was shown to immunoprecipitate WT (140 kDa) but not mutRHIM M45 (Fig. 1C, top panel). RIP1 interaction was detected with the M45-(1–277) region (40 kDa) but not with the carboxyl-terminal RR1 region (278–1174). Control experiments showed that each of the constructs was expressed efficiently in cell extracts, that RIP1 immunoprecipitated efficiently, and that cell lysates contained comparable amounts of proteins (Fig. 1C, lower panels, and data not shown). These results suggest that natural MCMV M45 may also interact with RIP1 in a RHIM-dependent fashion. An attempt to investigate RIP1mutRHIM was complicated by interaction with endogenous RIP1 via known death domain-dependent interactions (28) (data not shown).
To determine whether M45 interacts with other RHIM proteins, IP/IB analyses were performed with FLAG epitope-tagged RIP1, RIP2, RIP3, RIP4, and TRIF (4). RHIM-containing RIP1 and RIP3 immunoprecipitated M45. RHIM-lacking RIP kinases (RIP2 and RIP4) did not interact with M45 (Fig. 1D, top panel). M45 and each of the RIPs were expressed and immunoprecipitated efficiently (Fig. 1D, lower panels, and data not shown); TRIF expression levels were not sufficient to investigate RHIM-dependent protein-protein interactions (data not shown). To further evaluate the M45-RIP3 interaction, IP/IB analyses were performed with FLAG epitope-tagged RIP3 and M45 or M45mutRHIM. RIP3 was found to immunoprecipitate M45 but not M45mutRHIM (Fig. 1E, top panel). M45WT and M45mutRHIM were expressed to similar levels in cell extracts, and RIP3 expression was consistent between samples (Fig. 1E, lower panels). These data suggest that M45 may interact with RIP3 as well as RIP1 in a RHIM-dependent fashion.
To determine whether M45 interacted with endogenous RIP1, as reported (21), we co-localized transfected c-Myc epitope-tagged M45WT and endogenous RIP1 in NIH3T3 cells (Fig. 1E). Both proteins were associated with distinct cytoplasmic aggregates that were found in M45-expressing cells.
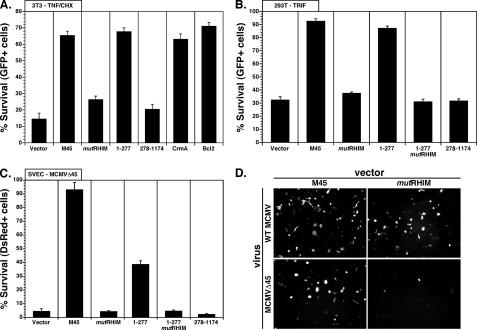
M45 Prevents Cell Death Induced by TNF—To address the role of M45 RHIM-dependent interactions in cell death suppression, we investigated the RHIM dependence of M45 suppression of TNF-mediated cell death in transfected NIH3T3 cells. M45WT, but not M45mutRHIM, protected cells treated with TNF and CHX from death (Fig. 2A), suggesting that M45 suppresses death in a RHIM-dependent manner. Importantly, M45mutRHIM failed to protect, but the amino-terminal RHIM-containing region of M45 (aa 1–277) protected cells (Fig. 2A) as efficiently as M45. The RR1 region (aa 278–1174) failed to protect. The levels of protection with either full-length M45WT or M45-(1–277) were similar to control inhibitors CrmA and Bcl2 (4). These data indicate the M45 amino-terminal fragment is sufficient to protect cells from induced cell death, and that protection was RHIM-dependent whether assessed with full-length or amino terminus.
FIGURE 2.
M45 RHIM-dependent protection from cell death. A, TNF-induced cell death assay in NIH3T3 cells following transfection with EGFP vector alone (vector) or together with M45WT or mutants (mutRHIM, 1–277, 1–277mutRHIM, 278–1174), or together with CrmA or Bcl-2 expression vector. Cells were treated for 6 h with TNF and CHX, and GFP+ cells were scored microscopically for survival. Data are presented as the percentage of survival of total GFP+ cells per field and are representative of three independent experiments. B, 293T cells were transfected with TRIF, EGFP, vector control, and M45WT or M45 mutants. 18 h after transfection, GFP+ cells were scored microscopically for survival. Data are presented as the percentage of survival of total GFP+ cells per field and are representative of three independent experiments. C, SVEC4–10 murine endothelial cells were transfected with DsRed and vector control and M45WT or M45 mutants. 36 h after transfection, cells were infected at multiplicity of infection = 10 with MCMV-GFP (WT) or MCMVΔ45 (M45-deficient). 24 h after transfection, survival of DsRed expressing cells was determined. Data are presented as the percentage of survival ± S.E. of Δ45 infection when compared with WT MCMV infection for each transfected M45 mutant and are representative of two independent experiments. D, representative fields from panel C.
M45 Prevents RIP1-dependent Death—To induce a RIP1 RHIM-dependent apoptosis, we expressed TRIF directly in 293T cells (4) and assessed the ability of M45WT and M45 derivatives to prevent cell death. M45WT blocked TRIF-induced cell death under these conditions (Fig. 2B). The amino-terminal region (aa 1–277) protected at levels similar to full-length protein. Either was as efficient as a dominant negative form of FADD, which acts downstream in this pathway (data not shown) (4). RHIM mutants of either the full length of the amino-terminal M45 species or the carboxyl-terminal region of M45 (aa 278–1174) were no more active than the vector control in protection. An identical pattern was also observed when cells were exposed to RIP3; however, overall levels of death were less dramatic (data not shown), as expected from previous reports (4). This experiment demonstrates that M45 efficiently counteracts RIP1-dependent death and that this activity is RHIM-dependent. Thus, M45 acts as a viral inhibitor of RIP activation.
M45 RHIM-dependent Suppression of Premature Endothelial Cell Death during Viral Infection—We investigated the RHIM dependence of M45-mediated suppression of natural virus-induced endothelial cell death, a hallmark activity of M45 (21). SVEC4–10 cells were transfected with M45WT or M45 derivatives, and cell survival was assessed 24 h after infection using equivalent doses of either WT MCMV or Δ45 mutant. Suppression of cell death was assessed by counting DsRed+ (surviving) cells. Cell death induced by M45 mutant virus was suppressed efficiently by expression of M45 but not M45mutRHIM (Fig. 2, C and D). The proportion of DsRed+ surviving infected cells in WT MCMV-infected cultures was not influenced by prior transfection of M45WT or M45 derivatives; however, the proportion of DsRed+ cells in M45 mutant virus-infected cultures was strongly influenced by the M45 species. Full-length M45 increased survival of MCMVΔ45-infected cells dramatically, such that the levels were comparable with those of WT MCMV (Fig. 1C). The M45-(1–277) region also rescued mutant virus-infected cells, albeit less efficiently than M45WT. Both mutRHIM constructs, as well as the carboxyl-terminal RR1 region of M45, failed to rescue. These data show that M45-mediated protection from cell death during MCMV infection of endothelial cells is RHIM-dependent under conditions of natural infection.
DISCUSSION
RIP1 interacts with adaptors and effectors in multiple signaling pathways controlling cell survival and cell death signaling (1, 2). We have shown that MCMV M45 modulates RIP1 activity via RHIM-dependent interactions. M45 is the fourth known RHIM-containing protein, joining a family of cellular adaptors (RIP1, RIP3, and TRIF). Additionally, M45 is the first identified viral RHIM-containing immunomodulatory protein.
As a vIRA, M45 prevents RHIM-dependent activation and downstream events mediated by RIP1 (and possibly RIP3). When expressed in cells or when expressed during MCMV infection (23), M45 appears to interact with RIP1 and modulate RIP1-dependent biological activities. This strongly suggests that RIP1 is a natural target of M45-mediated modulation. Given the evidence that all other RHIM-containing proteins retain the ability to interact (4), we cannot exclude a modulatory role for M45 in the biological activity of RIP3 or TRIF. Experiments to study M45-TRIF interactions were confounded by low expression levels and will require further investigation. Selective interaction with RIP1 and RIP3 would implicate M45 in modulating a specific subset of RHIM-dependent signaling events in cells.
RHIM-containing adaptor proteins control a diverse array of inflammatory and death signals that are generated by sensors such as TLR3 and TLR4, as well as the cytokine, TNF. TLR3 (29) and TNF (30–33) pathways are important in the host response to MCMV, albeit in complex interlaced host sensing and clearance pathways. Control of these pathways may contribute to the importance of M45 as a replication function in endothelial cells and macrophages (21). Although our current study does not provide any evidence as to why both of these cell types are so sensitive to premature death, we speculate that levels of upstream pathogen sensors present in cells, or induced by viral infection, are likely to play some role. In addition, our evidence is compatible with M45 being one viral protein that participates in modulating the TNF response in MCMV-infected macrophages (34), due to the importance of RIP1 in the TNF pathway. The RHIM-dependent interactions we have identified certainly provide MCMV with a potent suppressor of cell death during infection of endothelial cells and macrophages or suppression of extrinsic cell death via TNF.
M45 has been shown to be post-translationally processed to release the aa 278–1174 carboxyl-terminal portion as a separate gene product (22). Although the mechanism by which M45 is processed remains unclear, the consistency of its processing suggests specific proteolysis (19). The RHIM of M45 is contained within the unique amino-terminal region and is sufficient to confer protection from cell death, suggesting that the amino-terminal RHIM-containing portion may be a form that functions independently from full length. Therefore, proteolytic processing of M45 upon infection of endothelial cells and macrophages may be involved in M45 RHIM-dependent modulation of RIP1 activity and protection from cell death. Although the consequence of full-length and protease-cleaved M45 together during infection remains to be determined, it is interesting to note that the function of RIP1 is modulated by proteolytic cleavage as well. The caspase-8-dependent cleavage of RIP1 suppresses survival signaling and promotes TNF-driven apoptosis (16). Although MCMV encodes a viral inhibitor of caspase-8 activation, the M36 gene product (35), expression of this modulatory protein follows virus entry into cells and would not influence signaling to RIP1 immediately post entry. M45 accumulates during viral infection (22) and is packaged into virions (36), probably as a tegument protein. Thus, M45 is delivered to cells upon viral entry, and the immediate delivery of a RHIM-containing modulator of RIP1 activation into cells would allow an immediate disruption of the intrinsic antiviral response.
The recent identification of M45 as a RIP1 binding partner (23) did not completely define specific interactions controlling cell death suppression. The study relied on a combination of cell death suppression and NF-κB activation assays and showed that the carboxyl-terminal RR1 domain blocked NF-κB activation. The relationship of the impact on NF-κB activation and the RHIM-dependent M45-RIP1 interaction in cell death suppression remain to be determined. Regardless of NF-κB activation, the M45 RHIM-dependent interactions with RIP1 demonstrated here underlie the role of this viral protein as a cell death suppressor. Our mapping of the M45 RHIM to the amino-terminal aa 1–277 suggests that this region is sufficient for M45 cell death suppression. Furthermore, the RHIM of RIP1 is critical for activity of full-length M45 or the amino terminus. Any role for the carboxyl-terminal RR1 portion of M45 in cell death suppression remains speculative and unsupported by the data we present here.
M45 is a cell death suppressor required for protection from virus-induced cell death in endothelial and macrophage cell lines immediately upon entry (21). Although the human cytomegalovirus homolog of M45, UL45, has not been ascribed an analogous function (37), other modulators of innate immune signaling are delivered to cells with HCMV virions. One example, the UL83 gene product (pp65), is a tegument protein encoded by the virus that modulates interferon regulatory protein 3 (38). Although the MCMV homologs of UL83 have not yet been evaluated, it seems likely that MCMV and HCMV encode additional proteins that modulate multiple pathways in the intrinsic and innate responses immediately upon infection.
We show that through targeting RIP1, M45 disrupts death receptor signaling pathways. We also demonstrate that M45 directly blocks cell death signals relayed to RIP1 via the RHIM in RIP3 and the TLR3/4 adaptor, TRIF, suggesting that M45 serves as a general inhibitor of RHIM-mediated signaling. Cell survival is crucial to viral infections. Strategies to evade cellular sensors acting on intermediate players such as RIP1 and IRF-3 have obvious benefits to the virus in facilitating pathogenesis.
Acknowledgments
We gratefully acknowledge A. Redwood for providing bacmid-derived MCMV (strain K181) and W. Brune for providing bacmid-derived MCMV-GFP and MCMVΔ45 (strain Smith).
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 AI 030363 (to E. S. M.) and T32 HL069769 (to J. W. U.) from the USPHS. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: RIP, receptor-interacting protein; RHIM, RIP homotypic interaction motif; MCMV, murine cytomegalovirus; TLR, Toll-like receptor; TIR, Toll/interleukin-1 receptor; TRIF, TIR domain-containing adaptor inducing interferon β; TNF, tumor necrosis factor; GFP, green fluorescent protein; aa, amino acids; FADD, Fas-associated protein with death domain; PBS, phosphate-buffered saline; IP/IB, immunoprecipitation/immunoblot; CHX, cyclohexamide; WT, wild type; WCL, whole cell lysate; DsRed, red fluorescent protein.
References
- 1.Meylan, E., and Tschopp, J. (2005) Trends Biochem. Sci. 30 151–159 [DOI] [PubMed] [Google Scholar]
- 2.Festjens, N., Vanden Berghe, T., Cornelis, S., and Vandenabeele, P. (2007) Cell Death Differ. 14 400–410 [DOI] [PubMed] [Google Scholar]
- 3.Roy, C. R., and Mocarski, E. S. (2007) Nat. Immunol. 8 1179–1187 [DOI] [PubMed] [Google Scholar]
- 4.Kaiser, W. J., and Offermann, M. K. (2005) J. Immunol. 174 4942–4952 [DOI] [PubMed] [Google Scholar]
- 5.Meylan, E., Burns, K., Hofmann, K., Blancheteau, V., Martinon, F., Kelliher, M., and Tschopp, J. (2004) Nat. Immunol. 5 503–507 [DOI] [PubMed] [Google Scholar]
- 6.Sun, X., Yin, J., Starovasnik, M. A., Fairbrother, W. J., and Dixit, V. M. (2002) J. Biol. Chem. 277 9505–9511 [DOI] [PubMed] [Google Scholar]
- 7.Sun, X., Lee, J., Navas, T., Baldwin, D. T., Stewart, T. A., and Dixit, V. M. (1999) J. Biol. Chem. 274 16871–16875 [DOI] [PubMed] [Google Scholar]
- 8.Yu, P. W., Huang, B. C., Shen, M., Quast, J., Chan, E., Xu, X., Nolan, G. P., Payan, D. G., and Luo, Y. (1999) Curr. Biol. 9 539–542 [DOI] [PubMed] [Google Scholar]
- 9.Pazdernik, N. J., Donner, D. B., Goebl, M. G., and Harrington, M. A. (1999) Mol. Cell Biol. 19 6500–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton, K., Sun, X., and Dixit, V. M. (2004) Mol. Cell Biol. 24 1464–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelliher, M. A., Grimm, S., Ishida, Y., Kuo, F., Stanger, B. Z., and Leder, P. (1998) Immunity 8 297–303 [DOI] [PubMed] [Google Scholar]
- 12.Hoebe, K., Du, X., Georgel, P., Janssen, E., Tabeta, K., Kim, S. O., Goode, J., Lin, P., Mann, N., Mudd, S., Crozat, K., Sovath, S., Han, J., and Beutler, B. (2003) Nature 424 743–748 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto, M., Sato, S., Hemmi, H., Hoshino, K., Kaisho, T., Sanjo, H., Takeuchi, O., Sugiyama, M., Okabe, M., Takeda, K., and Akira, S. (2003) Science 301 640–643 [DOI] [PubMed] [Google Scholar]
- 14.Micheau, O., and Tschopp, J. (2003) Cell 114 181–190 [DOI] [PubMed] [Google Scholar]
- 15.Kim, J. W., Choi, E. J., and Joe, C. O. (2000) Oncogene 19 4491–4499 [DOI] [PubMed] [Google Scholar]
- 16.Lin, Y., Devin, A., Rodriguez, Y., and Liu, Z. G. (1999) Genes Dev. 13 2514–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinon, F., Holler, N., Richard, C., and Tschopp, J. (2000) FEBS Lett. 468 134–136 [DOI] [PubMed] [Google Scholar]
- 18.Holler, N., Zaru, R., Micheau, O., Thome, M., Attinger, A., Valitutti, S., Bodmer, J. L., Schneider, P., Seed, B., and Tschopp, J. (2000) Nat. Immunol. 1 489–495 [DOI] [PubMed] [Google Scholar]
- 19.Vandenabeele, P., Vanden Berghe, T., and Festjens, N. (2006) Science's STKE 2006 pe44. [DOI] [PubMed] [Google Scholar]
- 20.Yu, L., Alva, A., Su, H., Dutt, P., Freundt, E., Welsh, S., Baehrecke, E. H., and Lenardo, M. J. (2004) Science 304 1500–1502 [DOI] [PubMed] [Google Scholar]
- 21.Brune, W., Menard, C., Heesemann, J., and Koszinowski, U. H. (2001) Science 291 303–305 [DOI] [PubMed] [Google Scholar]
- 22.Lembo, D., Donalisio, M., Hofer, A., Cornaglia, M., Brune, W., Koszinowski, U., Thelander, L., and Landolfo, S. (2004) J. Virol. 78 4278–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack, C., Sickmann, A., Lembo, D., and Brune, W. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 3094–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning, W. C., Stoddart, C. A., Lagenaur, L. A., Abenes, G. B., and Mocarski, E. S. (1992) J. Virol. 66 3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick, A. L., Smith, V. L., Chow, D., and Mocarski, E. S. (2003) J. Virol. 77 631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brune, W., Nevels, M., and Shenk, T. (2003) J. Virol. 77 11633–11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upton, J. W., van Dyk, L. F., and Speck, S. H. (2005) Virology 341 271–283 [DOI] [PubMed] [Google Scholar]
- 28.Varfolomeev, E. E., Boldin, M. P., Goncharov, T. M., and Wallach, D. (1996) J. Exp. Med. 183 1271–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabeta, K., Georgel, P., Janssen, E., Du, X., Hoebe, K., Crozat, K., Mudd, S., Shamel, L., Sovath, S., Goode, J., Alexopoulou, L., Flavell, R. A., and Beutler, B. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 3516–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavic, I., Polic, B., Crnkovic, I., Lucin, P., Jonjic, S., and Koszinowski, U. H. (1993) J. Gen. Virol. 74 2215–2223 [DOI] [PubMed] [Google Scholar]
- 31.Orange, J. S., Salazar-Mather, T. P., Opal, S. M., and Biron, C. A. (1997) J. Virol. 71 9248–9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dommelen, S. L., Sumaria, N., Schreiber, R. D., Scalzo, A. A., Smyth, M. J., and Degli-Esposti, M. A. (2006) Immunity 25 835–848 [DOI] [PubMed] [Google Scholar]
- 33.Zhou, J., Zhang, M., and Atherton, S. S. (2007) Investig. Ophthalmol. Vis. Sci. 48 1691–1700 [DOI] [PubMed] [Google Scholar]
- 34.Popkin, D. L., and Virgin, H. W. (2003) J. Virol. 77 10125–10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick, A. L., Skaletskaya, A., Barry, P. A., Mocarski, E. S., and Goldmacher, V. S. (2003) Virology 316 221–233 [DOI] [PubMed] [Google Scholar]
- 36.Kattenhorn, L. M., Mills, R., Wagner, M., Lomsadze, A., Makeev, V., Borodovsky, M., Ploegh, H. L., and Kessler, B. M. (2004) J. Virol. 78 11187–11197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn, G., Khan, H., Baldanti, F., Koszinowski, U. H., Revello, M. G., and Gerna, G. (2002) J. Virol. 76 9551–9555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abate, D. A., Watanabe, S., and Mocarski, E. S. (2004) J. Virol. 78 10995–11006 [DOI] [PMC free article] [PubMed] [Google Scholar]